Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Zhaojun Tian and Version 2 by Camila Xu.
Coal is a fossil fuel with great economic potential, but meanwhile, it is threatened by coal spontaneous combustion during coal production. Gel foam extinguishing agent (gel foam) has promising applications in the prevention and management of mine coal spontaneous combustion.
- coal spontaneous combustion control
- gel foam extinguishing agent
1. Introduction
Coal is a fossil fuel with great economic potential, but meanwhile, it is threatened by coal spontaneous combustion during coal production [1][2][1,2]. Due to the complexity of the coal mining process and the particularity of the environment, coal spontaneous combustion is becoming increasingly frequent [3]. Therefore, coal spontaneous combustion prevention technologies have been widely developed in recent years.
Nowadays, research on preventing and controlling coal spontaneous combustion focuses on three areas. It includes coal natural theory, prevention and control technologies, and field practice. Different technologies, such as grouting, inert gas injection, inhibitor injection, gel injection, foam injection, etc., have been used to control coal spontaneous combustion. Based on the mechanism of action, materials are classified into physical-based, chemical-based, as well as composite retardants [4][5][4,5]. Gel foam fire extinguishing agent (gel foam) is an innovative material that combines the advantages of both gel and foam. It exhibits strong practicality and promising application prospects.
2. Study on Coal Spontaneous Combustion and Prevention Technology
2.1. The Mechanism of Coal Spontaneous Combustion
Coal spontaneous combustion is one of the central causes of fire disasters. Due to the spontaneous combustion of coal, a lot of coal resources in the world’s major coal mining areas such as South Africa, Europe, the United States, India, and China have been burned [6]. Besides that, harmful gases from coal spontaneous combustion pollute the ecosystem and harm human health, like COX, NOX, SO2, etc. [7][8][7,8]. Coal spontaneous combustion has been researched since the 17th century. Various theories have been proposed to explain this phenomenon. Among these, the coal–oxygen composite hypothesis has been recognized by most scholars [9]. Coal spontaneous combustion occurs, which is the result of the joint action between coal and oxygen [10][11][12][10,11,12]. According to the coal–oxygen compound theory, the reaction of reactive groups with oxygen in coal has been investigated [13][14][15][13,14,15]. Experimental investigations have confirmed coal adsorbs oxygen. Meanwhile, the oxygen absorbed would react with the reactive groups of the coal to produce new free radicals, thereby generating a chain of water and gas and releasing heat [16][17][18][16,17,18]. When the heat production rate of coal oxidation is greater than the rate of heat dissipation from the coal to the environment, it will cause the accumulation of heat and make coal oxidation continue, which eventually leads to the spontaneous combustion of coal [19][20][21][19,20,21]. The reaction of radicals during the spontaneous combustion of coal is a chain cycle process.2.2. Technologies of Prevention and Control of Coal Spontaneous Combustion
2.2.1. Grouting
Grouting is one of the traditional technologies in coal mines for extinguishing fires. The mud is made proportionally from water and non-combustible solids, such as yellow mud or fly ash. It was then transported to the coal seam through an injection pipe [22]. According to the distribution of high-temperature zones in the coal seam, a mud injection system is established to produce mud, which is then transported into the coal seam by a pipeline to extinguish the fire [23]. Mud can cover the coal body, filling internal pores and insulating it from oxygen. However, the mud can easily “pull the ditch” and clog the pipe. In addition, it is difficult to pile up mud to a height, and it cannot solve the fire at a height [24]. However, due to the low cost of grouting, it is still one of the main fire prevention and extinguishing measures.2.2.2. Inert Gas
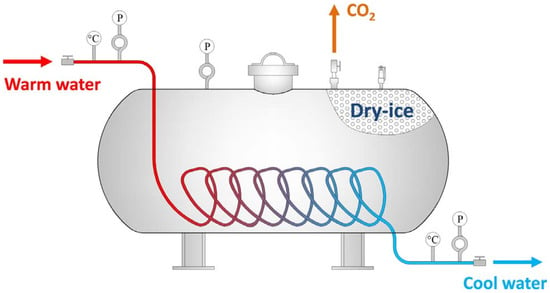
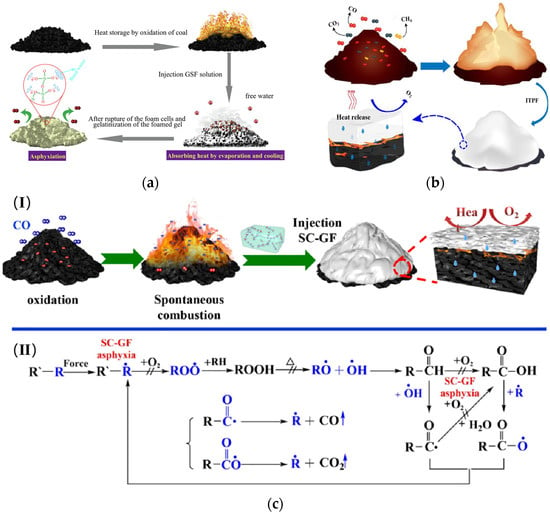
Currently, CO2 and N2 are the main inert gases injected into the fire area. Inert gas can serve the purpose of fire prevention by diluting the oxygen in the fire zone. Inert gas has the advantages of a fast fire extinguishing speed, no pollution, etc. However, due to the severe air leakage in the mining airspace, inert gas cannot be used to extinguish the fire for a long time [25]. It was found that liquid inert gas has a better cooling effect. Currently, liquid inert gas fire prevention technology is being successfully used in many coal mines in ChinaThe liquid inert gas (CO2, N2), loaded into special tanks, is transported to the site by mining vehicles and then connected to pressurized conveying equipment. It is injected into the area along the borehole using a booster or under pressure [26]. However, a lot of heat has to be absorbed during the evaporation of liquid inert gas. In addition, transporting and handling liquid coal dioxide poses a high risk. Under this condition, Liu et al. [27] developed a new equipment called the Dry-ice Phase Transformation Generator (DPTG) (Figure 12). The principle of the DPTG is based on warm water flowing through a copper pipe and exchanging heat with dry ice. The dry ice quickly changes from a solid to a gaseous state.2.2.3. Inhibitor
Physical inhibitors are mainly halogen salt inhibitors, phosphate inhibitors, and ammonium salt inhibitors. The halogen salt type inhibitors mainly include CaCl2, MgCl2, NaCl, etc. [28][29][30][28,29,30]. These can play a better role in the low-temperature stage of coal oxidation. Physical inhibitors are less expensive and are currently the most commonly used in coal mines. Chemical inhibitors are mainly aimed at preventing the reaction between coal and oxygen, such as polyethylene glycol, anthocyanins, and catechin [31][32][31,32]. To offset the disadvantages of a single physical or chemical inhibitor, compound inhibitors offer the advantages of both: They can absorb heat to lower the temperature and effectively break the chain reaction. The compound inhibitors mainly include catechin polyethylene glycol, ascorbic acid–Rosmarinus acid, halogenated salt compound inhibitors, and so on [33][34][33,34]. Composite inhibitors have high inhibition efficiency, but the production cost is high, and the synthesis process is complicated. In practice, inhibitors are sprayed onto the coal or directly drill holes and inject inhibitor liquid into the coal wall that has begun to oxidize and heat. Wang et al. [35] examined the spraying process of the inhibitor. He sprayed nitrogen and atomized inhibitor liquid into the goaf for double fire prevention.2.2.4. Foam
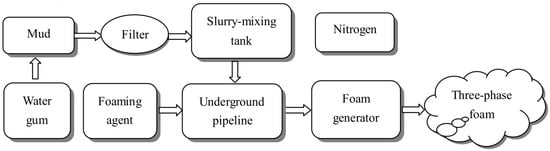
Foam is a kind of gas–liquid dispersed system with great importance. It is produced by physical foaming with a foaming device. The three-phase foam was first proposed to prevent the spontaneous combustion of coal in 2004 [36]. The three-phase foam gives full play to the fire protection advantages of yellow mud grouting and nitrogen injection technology. Meanwhile, it has been applied in many coal mines, such as the Longdong Coal Mine and the Geng Cun Coal Mine in Henan Province [37][38][37,38]. In addition, the preparation of three-phase foam in the coal mine site includes two processes (Figure 23): Firstly, the materials are mixed proportionally to form a slurry, and then the slurry is transported to the underground; secondly, a foaming device is used to fully mix and foam the slurry, foaming agent and nitrogen to form a three-phase foam, and the three-phase foam is transported to the area that needs to be prevented from extinguishing the fire [38]. Foam can cover the floating coal in the high place and effectively solve the defect that the grouting technology cannot be poured into the high place. At the same time, it can also be used as a carrier to transport solid phase materials and water to the coal body to improve the resistance effect [39][40][39,40].2.2.5. Gel
The gel is a special state between solid and liquid. According to the different chemical properties of the base material for preparing gel, it can be divided into inorganic gel, organic gel, and composite gel [41][42][43][41,42,43]. Gel fire prevention technology is to mix the base material, additives, and water in proportion and then press into the coal seam fire area with a slurry injection system. The solution in a liquid state before gel formation can penetrate the cracks and pores of the coal body. When the solution becomes a gel, it plugs these pores and fissures. The gel has a good water retention effect and absorbs heat through water evaporation to reduce the temperature of the coal. At the same time, the gel can cover the coal body and stop the reaction process between coal and oxygen. However, the flow rate of the gel is small, the fluidity is poor, and the colloid will crack and form fissures after a long period [44].3. Structure and Principle of Gel Foam
3.1. Structural Features of Gel Foam
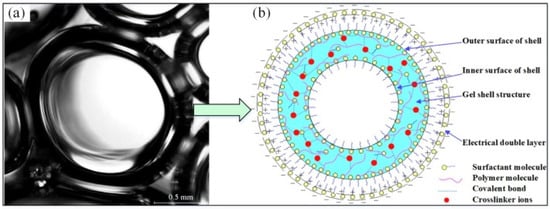
Gel foam is a non-equilibrium structure with gas uniformly dispersed in the gel. It is prepared by mechanical stirring of a gelling agent, cross-linking agent, and blowing agent in a gas [45][46][47][45,46,47]. In China, gel foam was first used to extract oil to enhance oil recovery. And it was gradually applied to coal mine fire prevention in the 20th century [48][49][48,49]. Tian [50] built the constitutive model of gel foam with relevant factors. He carried out a systematic study on the theory and technology of gel foam in coal mines. Qin et al. [51] proposed a multi-phase gel foam combining the gel and three-phase foam. It used fly ash as the base material and explored its weak cross-linking characteristics. Differing from gel and foam, gel foam is a mixed system with gel as the continuous phase and gas as the dispersed phase [52]. The foam transports the gel-forming material into the coal body, while the foam film contains a large amount of gas and water inside its membrane. After some time, the gel-forming material forms a colloid with a three-dimensional network structure and wraps the foam [53]. As shown in Figure 34, (a) is the structure of the gel foam, and its structural characteristics are shown in (b). The unique formation process and structure of gel foam are applied in coal mine spontaneous combustion control by combining the advantages of both gel and foam.3.2. Principle of Gel Foam Action
Gel foam is a new material based on gel and foam. The principle of spontaneous coal combustion is controlled by preventing or delaying the oxidation process of coal. It is essential to explore the role of gel foam in spontaneous coal combustion and explain its mechanism of action. The action mechanism of gel foam has been divided into three categories based on extensive experimental characterization and analysis [54][55][56][54,55,56]:- (a)
-
The cooling effect [57]
- (b)
-
The plugging effect
- (c)
-
The inhibition effect