Nanoparticulated manganese cyanoferrate (K2Mn[Fe(CN)6]) and its analogs are non-toxic complexes and prime candidates for the next generation non-gadolinium magnetic resonance imaging (MRI) agents. L-edge X-ray absorption spectroscopy (L-XAS) and 57Fe specific nuclear resonant vibrational spectroscopy (NRVS) can be combined as a modern spectroscopic method to evaluate the element specific and isotope specific information about the oxidation states, electronic spin states and the coordination environments for the metals inside these complexes.
- Non-gadolinium magnetic resonant imaging agents
- nanoparticulate manganese cyanoferrates
- L-edge X-ray absorption spectroscopy
- L XAS
- nuclear resonant vibrational spectroscopy
- NRVS
1. Introduction to MRI agents
Magnetic resonance imaging (MRI) has emerged as a prominent non-invasive and nonradioactive tool for diagnosing various diseases and/or testing human organ functions [1, 2][1][2]. To aid the diagnoses, it is often necessary to administer an imaging agent to improve the contrast [1, 2][2][1]. This is especially true for imaging the brain or spine where small details are often pursued. For example, Figure 1(a) illustrates the use of a contrast agent for the MRI of the brain: the right picture shows a much clearer and more detailed image with the use of a contrast agent in comparison with the left one where no contrast agent is used. The current clinical MRI agents, e.g., Gd-DTPA, all contain a single paramagnetic metal. These Gd based agents are used in about 1 in 3 of MRI scans to improve the clarity of the images for the internal structures of the human body [3, 4][3][4]. Often, use of an MRI contrast agent improves the visibility of inflammation, tumours, and blood vessels, or, in some cases, even blood flow or the real time function of blood vessels. In turn, a better MRI image provides a better medical diagnostic accuracy [3, 5][3][5].
Although Gd contrast agents have proven to be invaluable, they are not without drawbacks. In extreme cases, these agents can lead to a serious disease called nephrogenic systemic fibrosis (NSF) [3, 5][3][5]. An example of NFS patients’ skin appearance as well as the detailed area looking are shown as in Figure 1(b) and its right insert. In 2010, synchrotron radiation (SR)-based X-ray fluorescence microscopy (SXRF) and extended X-ray absorption fine structure spectroscopy (EXAFS) were used to chemically and spectroscopically characterize the Gd deposits in the skin from an NSF patient [5][5]. It has been concluded that the Gd deposits in that NSF case consist of a Gd-phosphate material with almost no Gd remaining coordinated to the original organic chelator [5][5]. One reason is that Gd3+ ion has a very similar radius with that of Ca2+ (i.e., 1.07 Å for Gd3+ and 1.02 Å for Ca2+) and can replace the Ca2+ in human body and skin [5][5]. Therefore, Gd-DTPA or other Gd-based agents are still considered harmful in general [3, 5][3][5], and hence safer alternative for imagining contrast agents need to be explored.
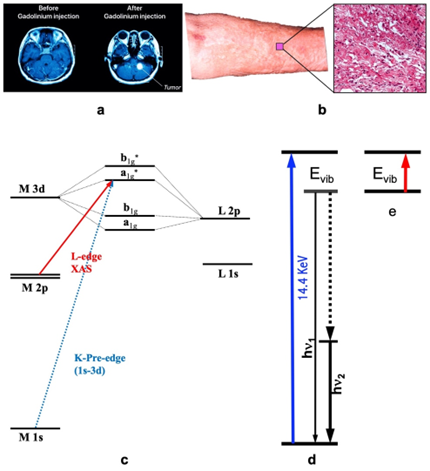
Figure 1. (a) The illustrative MRI images without (left) vs. with (right) contrast agent; (b) the skin surface and its microscopic profile of NFS; (c) illustrative K- and L-edge X-ray absorption spectroscopy (XAS) transitions (and the associated energy levels); (d) the transitions of nuclear resonant vibrational spectroscopy (NRVS) which measures the vibrations indirectly but with many advantages; (e) Infrared absorption spectroscopy (IR) which measures vibrations directly.
From the entire periodic table, there are only a few elements that are considered suitable as MRI contrast agents. The suitable candidates include, for example, Gd(III) (S=7/2), Eu(II) (S=7/2), Fe(III) (S=5/2), and Mn(II) (S=5/2). Among them, Mn(II) ion has a high electronic spin (S=5/2), a fast rate in water exchange, a long electronic relaxation time, stable oxidation states, and is more compatible with biological cells than other metals [e.g. vs. Gd(III)]. It therefore possesses almost ideal physical, chemical, and biological properties for MRI applications[6] [6] and led to two U.S. Food and Drug Administration (FDA)-approved contrast agents for clinical use, i.e., MnDPDP (DPDP = dipyridoxyl diphosphate; Teslascan®) and MnCl2 (LumenHance®). However, all Mn complexes including MnDPDP are kinetically labile and susceptible to the interaction with other metals such as Zn, Cu and Fe, etc. [7][7]. MnCl2 in aqueous solution can exist as [Mn(H2O)6]2+ and Cl- ions in the stomach. Although Mn(II) has much less toxicity than Gd(III), absorption of excessive amount of Mn can still cause neurotoxicity. Other single metal centred coordination complexes also show various disadvantages and various potential toxic concerns.
2. Nanoparticulated Prussian blue complexes
These concerns may be addressed by incorporating Mn (or other proper metals including Gd) into the Prussian blue (PB) structure in the form of nanomaterials [8-10][8][10]. PB has an extremely high formation constants (e.g., Kf = 1.6x1031 for PB vs. 1023 for Gd-DTPA) and therefore is much safer for medical use. For example, it is one of the most important medications on the World Health Organization's List of Essential Medicines. In general, it has a chemical formula of FeIII4[FeII (CN)6]3·xH2O. A quarter of the [FeII(CN)6]4- ions have to be absent from the crystal lattice in order to maintain a ratio of Fe(III):[FeII(CN)6]4- = 4:3 to realize the electrical neutrality of PB [11]. This arrangement creates void inside the structure that is often filled by a varying number of H2O molecules (i.e., x = 1 to 12). For PB–like analogs such as the manganese cyanidoferrates K2Mn[Fe(CN)6], Mn:Fe=1 is maintained instead, while a counterion of K+ can be used to maintain the electrical neutrality for the whole compound.
The K2Mn[Fe(CN)6] or similar PB analogs have attracted a great deal of attention as potential MRI contrast agents in the past ten years[8][10] [8-10] because: 1) the extremely high formation constant leads to an extremely stable complex [8, 9][8][9]. This in turn contributes to a much reduced risk of exposing the patients to the metal ions; 2) these complexes have a long blood circulation time, which is desirable for detailed imaging studies [12]; and 3) a given nanoparticle can form a superparamagnetic domain. Such material can thus possess a much higher magnetic susceptibility as compared with the paramagnetic materials composed of single molecules. This property can produce a much higher MRI sensitivity. On the other hand, nanoparticles are still small enough to be freely transported inside the human bloodstream.
There are a few major scientific issues concerning K2Mn[Fe(CN)6], for example: 1) what are the oxidation states of the two metals inside the complex?, i.e. whether this complex has a Mn(II)/Fe(II), a Mn(II)/Fe(III), a Mn(III)/Fe(II) or a Mn(III)/Fe(III); 2) what are the electronic spin states (i.e., ls vs. hs, where ls = low spin and hs = high spin) of the two metal sites? 3) in PB, the ls-Fe(II) is taking the C-bound position in the [Fe(CN)6]4- ion and the hs-Fe(III) is taking the N bound position to the [Fe(CN)6]4- ion. In K2Mn[Fe(CN)6], whether the Mn takes the positions for the original N-bound hs-Fe(III) sites or those for the C-bound ls-Fe(II) sites in comparison with PB? SR-based modern spectroscopies have many advantages [13-16] and can be used to shine some light directly onto these issues, which are critical for evaluating its suitability as an MRI agent [8, 9][8][9].
3. Application of L-XAS
SR-based X-ray absorption spectroscopy [abbreviated as XAS hereafters, Figure 1(c)] measures the electronic transition from a core shell to a valence shell. As XAS is sensitive to valence electrons and it is related to core shell (and thus element-specific) at the same time, it is one of the best methods to investigate the oxidation state for a specific element in different chemical compounds or enzymes [13, 15, 17, 18][13][15]. For 3d transition metal ions (e.g., Mn, Co or Fe ion), K-edge XAS uses a hard X-ray beam of > 4000 eV to study the transitions of 1s → 3d, → 4p and → continuum [Figure 1(c), the transition designated by the dashed blue line]. Although the information obtained from the K-edge XAS seems comprehensive [13[13][15], 15], L-edge XAS (or L-XAS) [17, 18] [Figure 1(c), the transition designated by the solid red line] as well as L-edge RIXS or L-edge X-ray emission spectroscopy [19] have several advantages over the K-edge XAS for studying electronic structures such as oxidation states and electronic spin states. These advantages include a direct probe of the ligand-metal bonding orbital (3d); a dipole-allowed 2p → 3d transition (vs. 1s → 3d in K-edge XAS); a better energy resolution (e.g. 0.1 eV vs. 1 eV); and a rich spectral multiplet which is specific to particular electronic structures and coordination environments [16, 17, 20-22].
Among these advantages, the spectral multiplets are more essential to identify metal’s oxidation states and electronic spin states and then to find its coordination symmetries based on electronic information [16, 18, 21]. The match of their spectral multiplets in two spectra indicates that the two samples have the same metal sites (or at least extremely similar metal sites). Due to its fingerprint-like multiplets, L-XAS is sensitive to and has been widely used to identify the oxidation states, spin states and other information for metal centers in various 3d transition metal complexes and metalloenzymes [17, 21]. For example, it has been used to identify Ni(I, II, III) and even Ni(II) with different electronic spin state (hs vs. ls) successfully [16, 18, 21, 22].
4. Application of NRVS
Nuclear resonant vibrational spectroscopy (or NRVS for short) is another SR-based modern spectroscopic technique that was widely used by physicists, chemists, biochemists, and materials scientists. It measures the phonons, or, in other words, vibrational modes associated with the nuclear transition for a specific isotope, as illustrated in Figure 1(d). The most frequently used NRVS to date is the 57Fe NRVS which has a nuclear resonant transition at 14.4 keV [14, 23-26]. In short, it is a scattering spectroscopy in general and a nuclear resonant scattering spectroscopy in particular. In comparison with the conventional infrared absorption spectroscopy (IR) [Figure 1(e)], it measures vibrations indirectly but has several distinct advantages. The most prominent advantages include but not limited to being isotope (e.g., 57Fe) specific for studying complicated systems [25-27], having an almost zero background [14, 25], and being able to obtain a theoretically calculable partial vibrational density of state (PVDOS) [14, 28]. These advantages make it a better method in comparison with the laboratory-based IR, Raman and laser induced fluorescence (LIF) spectroscopies [14, 29] as well as SR-based inelastic X-ray scattering (IXS) [14]. This modern spectroscopy became available in mid 1990s due to the development of the third-generation SR sources which provides the strong beam pulses with specific timing structure, advanced X-ray optics which lead to a monochromator with an 1 meV energy resolution to measure vibrations, and modern detectors which extract weak nuclear scattering signal from the huge electronic scattering counts in the time domain, and in turn, it has pushed a great advancement in physics, chemistry, biochemistry and materials science, etc. for the past 28 years [23, 25-27]. In chemistry and bioinorganic chemistry, for example, this technique has uncovered Fe-S/P/Cl, Fe-CO/CN/NO and Fe-H/D vibrational modes inside various inorganic complexes as well as dilute iron enzymes [14, 30, 31], and thus become an excellent pinpointing tool to study iron-specific electronic and structural properties, including iron oxidation state(s) and coordination environment. For PB–like compounds, it can pinpoint to the features attributable to the N-bound hs-Fe(III) or those to the C-bound ls-Fe(II) [14, 32].
5. Concluding Remarks on K2Mn[Fe(CN)6]
In the associated publication, wresearchers have conducted detailed measurements of L-edge XAS on Mn and Fe for the nanoparticulate PB analog K2Mn[Fe(CN)6] that has been synthesized and evaluated as a potential non-gadolinium MRI agent [8, 10][8][10]. The results obtained from such L XAS experiments allow uresearchers to unambiguously conclude that K2Mn[Fe(CN)6] has a hs-Mn(II) bound to N and a ls-Fe(II) bound to C in the PB structure. For comparison, Co and Fe L XAS on KFeCo[(CN)6] shows that KFe[Co(CN)6] has a hs-Fe(II) surrounded by N and a ls-Co(III) surrounded by C in the PB structure. Accordingly, K+ ions have to be incorporated into the structures to maintain electroneutrality of their overcall formula and a ratio of Mn:Fe=1:1 or Fe:Co=1:1.
To rule out the possible issues of the surface effect, the bulk sensitive 57Fe NRVS measurement is also performed on K2Mn[Fe(CN)6] and its reference samples KEu[Fe(CN)6] and KGd[Fe(CN)6]. The NRVS results are consistent with the above results obtained from the surface sensitive L XAS.
In addition to the evaluation on the electronic properties of K2Mn[Fe(CN)6] to assess its suitability as a prime candidate for the next-generation MRI agents, the current studies well illustrate the many significant advantages of using L XAS and NRVS in combination on the same sample in revealing the site-specific information for nanoparticulate PB analog complexes as well as other complexes in general.
References
- Kandanapitiye, M.S., et al., Gallium Analogue of Soluble Prussian Blue KGa Fe(CN)(6) center dot nH(2)O: Synthesis, Characterization, and Potential Biomedical Applications. Inorganic Chemistry, 2013. 52(6): p. 2790-2792.
- Buser, H.J., et al., The crystal structure of Prussian Blue: Fe4[Fe(CN)6]3.xH2O. Inorganic Chemistry, 1977. 16(11): p. 2704-2710.
- Shin, J., et al., Hollow Manganese Oxide Nanoparticles as Multifunctional Agents for Magnetic Resonance Imaging and Drug Delivery. Angewandte Chemie International Edition, 2009. 48(2): p. 321-324.
- Gu, W.W., et al., Refinement of the nickel site structure in Desulfovibrio gigas hydrogenase using range-extended EXAFS spectroscopy. Journal of Inorganic Biochemistry, 2003. 93(1-2): p. 41-51.
- Wang, H., et al., Nuclear Resonance Vibrational Spectroscopy: A Modern Tool to Pinpoint Site-Specific Cooperative Processes. Crystals, 2021. 11(8): p. 909.
- Penner-Hahn, J.E., X-ray Absorption Spectroscopy, in eLS. 2005, Wiley Online Library.
- Stöhr, J., NEXAFS Spectroscopy. 2003, New York: Springer-Verlag.
- Jang, J.-W., et al., Enabling unassisted solar water splitting by iron oxide and silicon. Nature Communications, 2015. 6(1): p. 7447.
- Geraldes, C.F.G.C. and S. Laurent, Classification and basic properties of contrast agents for magnetic resonance imaging. Contrast Media & Molecular Imaging, 2009. 4(1): p. 1-23.
- Parizel, P.M., et al., Magnetic Resonance Imaging of the Brain, in Clinical MR Imaging, P. Reimer, et al., Editors. 2010, Springer Berlin Heidelberg: Berlin, Heidelberg. p. 107-195.
- Wahsner, J., et al., Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers. Chemical Reviews, 2019. 119(2): p. 957-1057.
- Llamas, M. Gadolinium-Based Contrast Agents. 2022; Available from: https://www.drugwatch.com/gadolinium/.
- Winter, M.B., et al., Porphyrin-Substituted H-NOX Proteins as High-Relaxivity MRI Contrast Agents. Inorganic Chemistry, 2013. 52(5): p. 2277-2279.
- Tan, M., et al., Synthesis and Evaluation of Nanoglobular Macrocyclic Mn(II) Chelate Conjugates as Non-Gadolinium(III) MRI Contrast Agents. Bioconjugate Chemistry, 2011. 22(5): p. 931-937.
- Shokouhimehr, M., et al., Dual purpose Prussian blue nanoparticles for cellular imaging and drug delivery: a new generation of T-1-weighted MRI contrast and small molecule delivery agents. Journal of Materials Chemistry, 2010. 20(25): p. 5251-5259.
