Climate variations directly impacts on the primary productive sectors, such as agriculture, forestry, livestock, and commercial fishing, which present the subsequent economic relevance. As a result, the effect of climate change has been widely discussed for years on flora and fauna. However, the aftermath evaluation over the worldwide microbiota is a challenging task poorly considered, up to date. In fact, most of these effects have yet to be quantified, but the proliferation and geographycally spread of pathogens of plants, animals, or humans are providing clues of the possible results. These microbial issues should be kept in mind and are the core of this entry.
- climate change
- microorganisms
1. Microorganism-Mediated Effect on Productive Sectors
Climate variations (e.g., temperature increase, rainfall instability, glacial retreat, extreme weather events, etc.) have foreseeable impacts on certain industries. These impacts encompass technical concerns (e.g., power supply) or extend to more severe problems in sectors such as agriculture, forestry, livestock and commercial fishing, which are primary sectors of great economic relevance, whose global value grew by 73% between 2000 and 2019, reaching USD 3.5 trillion in 2018 [1]. Nowadays, farmers and scientists are already both turning their eyes to the use of biofertilizers and eco-friendly tillage practices in order to protect soil health, reduce GHG emissions and increase carbon sequestration [2]. However, the increase in outbreaks of several diseases, as well as the geographical expansion of different pathogens, is compromising the balance between eco-friendly activities and the way to face these new climate-change‑derived risks [3] (Figure 1). Thus, the microorganisms involved in these processes play a crucial role as detailed below.
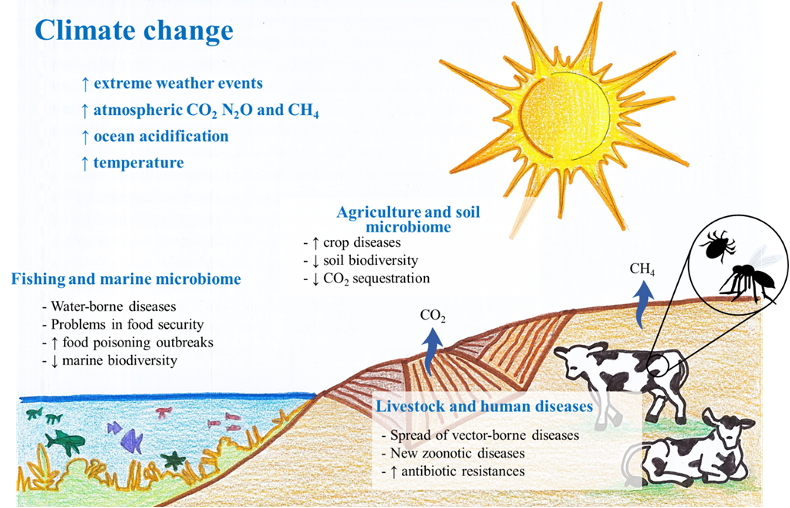
Figure 1. Main effects of climate change on microorganisms: (i) expansion and increase in waterborne diseases affecting fish and shellfish and causing an increase in food poisoning outbreaks and loss of marine biodiversity; (ii) expansion and intensification of pathogenic fungal infections in crops all around the world; and (iii) outbreaks of livestock diseases, emergence of new zoonotic diseases and increase in antibiotic resistance.
2. Agriculture and Soil Microbiome: Eternal Feedback
One of the most important worldwide primary sectors is agriculture, which relies on food production. However, the variation in soil microbial communities influenced by climatic change affects the physiology, temperature sensitivity or plant growth rate, which jeopardizes the final yields and compromises the future of this sector [4]. Thus, increased temperatures accelerate microbial decomposition activities, leading to faster CO2 emissions. As a result, soils will become a carbon dioxide source rather than a sink [4]. This CO2 enrichment enhances the development of rhizobial populations and the increase in N-fixing microorganisms in controlled environments, although multiple resource limitations dampen rhizobial responses in natural systems. In contrast, high temperatures lead to drought, which reduces colonization by arbuscular mycorrhizal fungi [4][5].
In addition, temperature and soil humidity play a critical role in microbial-soil abundance, diversity and metabolic functionality, since climatic factors greatly modify the type and quantity of some plant species that predominate in a landscape [6][7]. Furthermore, the consequences of climate change on soil communities and nutritional balances depend on the specific soil type, vegetation cover and management techniques. As a result, the total effects of climate change on soil communities and nutritional balances seem logical but unclear [4][5][8].
|
Disease |
Pathogen |
Host |
Origin |
Spread and Development |
Ref. |
|
Ash dieback |
Hymenoscyphus fraxineus |
Ash trees |
Asia |
Asia, Europe and Africa |
|
|
Bacterial blight or Bacterial leaf blight |
Xanthomonas oryzae |
Rice |
Japan |
Worldwide (especially Asia and Africa) |
|
|
Bacterial canker |
Pseudomonas syringae |
Fruit trees |
Depends on pathovar |
Worldwide |
|
|
Brown rust or Leaf rust |
Puccinia recondita |
Cereals (wheat, rye and barley) |
Eastern Australia |
Worldwide |
|
|
Brown spot |
Bipolaris oryzae |
Rice |
USA |
Asia, Europe and South America |
|
|
Bunch rot or Gray mold |
Botrytis cinerea |
Wide range |
Unknown |
Worldwide |
[31] |
|
Chestnut canker |
Cryphonectria parasitic |
Chestnut tree |
Asia |
North America |
[32] |
|
Disease dependent on the Diaporthe species |
Diaporthe spp. |
Wide range |
Germany |
Europe, Australia and Asia |
|
|
Disease dependent on the X. fastidious subspecies |
Xylella fastidiosa |
Wide range |
USA |
South and North America and Europe |
|
|
Downy mildew |
Plasmopara viticola |
Grape |
North America |
Worldwide |
|
|
Dry root rot |
Rhizoctonia bataticola (also Macrophomina phaseolina) |
Chickpea |
India |
North America, Asia and Africa |
[38] |
|
Dutch Elm disease |
Ceratocystis ulmi (also Ophistoma ulmi) |
Elm |
Asia |
Worldwide |
[39] |
|
Fire blight |
Erwinia amylovora |
Apple, Pearl and some Rosaceae |
North America |
Europe and Asia |
[40] |
|
Rice blast |
Magnaporthe oryzae (anamorph Pyricularia oryzae) |
Rice |
Brazil |
South America, Asia, Africa and Europe |
|
|
Stewart’s wilt |
Pantoea stewartii (formerly Erwinia stewartii) |
Corn |
USA |
Italy, Malaysia |
|
|
Yellow rust or Stripe rust |
Puccinia striiformis |
Wheat |
Transcaucasia (Armenia, Georgia and Azerbaijan) |
Worldwide |
|
|
Wheat blast |
Pyricularia graminis-tritici |
Wheat |
Brazil |
North and South America and Asia |
[45] |
-
Brown rust (mainly caused by Puccinia recondita), in the case of wheat, is forecast to increase its pressure on the crop by 20–100%, and yellow rust (caused by Puccinia striiformis) will increase by 5–20% in cold regions.
- An increase in cases of diseases in leafy vegetable and cereal crops has already been reported in Italy, as a result of several pathogens’ effect, such as Plectosphaerella cucumerina, Alternaria sp., Fusarium equiseti, Myrothecium verrucaria, Myrothecium roridum, Phoma valerianellae, Pleospora betae, Peronospora belbahrii and Pythium ultimum, as well as the appearance of new pathogens like different species of Pythium (Pythium aphanidermatum, Pythium irregulare, Pythium dissotocum, Pythium coloratum, Pythium diclinum or Pythium lutarium) and new species causing yellow rust or stem rust [17][Rice pathogens such as Pyricularia oryzae (the main cause of blast) and Bipolaris oryzae (or brown spot) are favored in all European rice districts, with the most critical situation in northern Italy (with an increase of close to 100%).
-
Among the new infections that have been reported in recent years in southern Europe and the Mediterranean coast, different species of the fungus Diaporthe have been found infecting several citruses, and the bacterium Xylella fastidiosa has also been discovered in olive trees [20].In the case of grape, Plasmopara viticola (also called downy mildew) will increase by 5–20% across Europe, while Botrytis cinerea (or bunch rot) will have diverse impacts, ranging from a 20% decrease to a 100% increase in infection events [17].
3. Livestock and Climate Change: An Arthropoda Matter
- ]
- . Other cases come from the
- Vibrio genus, as in the case of Vibrio parahaemolyticus and Vibrio vulnificus. Both species are related to typical human infections associated with seafood consumption, and the risk area for both vibrio infections has greatly increased during warmer water temperature episodes in the last years [78][91].
-
Last, habitat expansion is likely to cause novel contact among populations, pathogens and vectors, potentially increasing interspecies infections. For example, novel species of Brucella named Brucella ceti and Brucella pinnipedialis have been reported to cause infection in marine mammals like cetaceans and seals, respectively [87].
|
Disease |
Pathogen |
Vector |
Host |
Origin |
Spread and Development |
Ref. |
|
Anaplasmosis |
Anaplasma phagocytophilum |
Ixodes scapularis, Ixodes pacificus |
Sheep and cattle |
Scotland |
Worldwide |
|
|
Babesiosis |
Babesia microti, Babesia venatorum and Babesia divergens |
Ixodes ricinus, I. scapularis |
Mammals |
Romania |
Europe and North America |
[52] |
|
Babesia bovis and Babesia bigemina |
Rhipicephalus microplus |
Mammals |
Asia, Africa, South and Central America |
Europe and North America |
[53] |
|
|
Bluetongue Virus |
Orbivirus |
Culicoides imicola |
Ruminants |
South Africa |
USA, Canada, Australia, South and Central Europe |
[54] |
|
Canine Babesiosis |
Babesia spp. |
Dermacentor reticulatus |
Mammals (especially cattle) and birds |
Romania |
Worldwide |
[55] |
|
Colorado tick fever |
Coltivirus |
Dermacentor andersoni |
Mammals |
Western US |
Europe and North America |
|
|
Ehrlichiosis |
Ehrlichia chaffeensis and Ehrlichia ewingi |
Amblyomma americanum |
Mammals |
Canada |
North America and Europe |
|
|
Leptospirosis |
Leptospira spp. |
Environmental transmission |
Mammals |
Japan and Europe |
Asia, Australia, America and South Europe |
|
|
Lyme disease |
Borrelia burgdorferi |
Ixodes scapularis |
Rodents |
USA |
North America and Eurasia |
|
|
Powassan virus disease |
Powassan virus |
Several tick species |
Mammals |
Unknown |
North America |
[65] |
|
Q fever |
Coxiella burnetii |
D. reticulatus |
Ruminants (cattle, goat and sheep) |
Australia |
Europe and North America |
[66] |
|
Rocky Mountain spotted fever |
Rickettsia rickettsii |
D. andersoni |
Mammals |
South and Central America |
North America |
[67] |
|
Rickettsia parkeri |
A. maculatum |
Central and North America |
||||
|
Disease |
Pathogen |
Vector |
Host |
Origin |
Spread and Development |
Ref. |
|
Anaplasmosis |
Anaplasma phagocytophilum |
Ixodes scapularis, Ixodes pacificus |
Sheep and cattle |
Scotland |
Worldwide |
|
|
Babesiosis |
Babesia microti, Babesia venatorum and Babesia divergens |
Ixodes ricinus, I. scapularis |
Mammals |
Romania |
Europe and North America |
[52] |
|
Babesia bovis and Babesia bigemina |
Rhipicephalus microplus |
Mammals |
Asia, Africa, South and Central America |
Europe and North America |
[53] |
|
|
Bluetongue Virus |
Orbivirus |
Culicoides imicola |
Ruminants |
South Africa |
USA, Canada, Australia, South and Central Europe |
[54] |
|
Canine Babesiosis |
Babesia spp. |
Dermacentor reticulatus |
Mammals (especially cattle) and birds |
Romania |
Worldwide |
[55] |
|
Colorado tick fever |
Coltivirus |
Dermacentor andersoni |
Mammals |
Western US |
Europe and North America |
|
|
Ehrlichiosis |
Ehrlichia chaffeensis and Ehrlichia ewingi |
Amblyomma americanum |
Mammals |
Canada |
North America and Europe |
|
|
Leptospirosis |
Leptospira spp. |
Environmental transmission |
Mammals |
Japan and Europe |
Asia, Australia, America and South Europe |
|
|
Lyme disease |
Borrelia burgdorferi |
Ixodes scapularis |
Rodents |
USA |
North America and Eurasia |
|
|
Powassan virus disease |
Powassan virus |
Several tick species |
Mammals |
Unknown |
North America |
[65] |
|
Q fever |
Coxiella burnetii |
D. reticulatus |
Ruminants (cattle, goat and sheep) |
Australia |
Europe and North America |
[66] |
|
Rocky Mountain spotted fever |
Rickettsia rickettsii |
D. andersoni |
Mammals |
South and Central America |
North America |
[67] |
|
Rickettsia parkeri |
A. maculatum |
Central and North America |
4. Fishing and Marine Microbiome
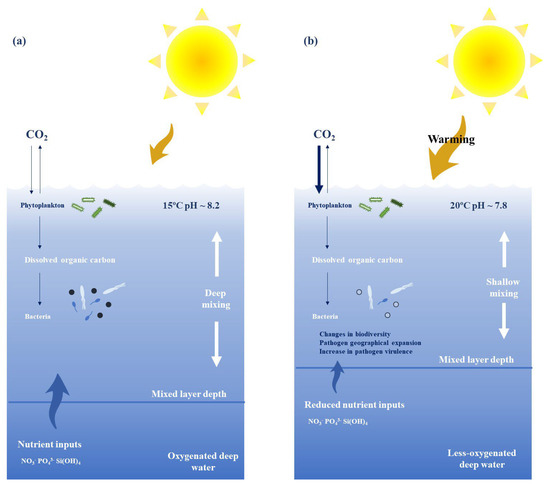
-
First, environmental changes like those caused by climate change may lead to stress in both fish and shellfish species, leading to lower immune responses against various pathogens and diseases [3]. Urchins have shown a strong correlation between mass mortality events and long-term elevated temperatures [87].
-
Second, a rise in temperatures leads to an increase in several marine pathogens’ virulence by increasing their metabolism and inducing higher rates of transmission [87]. Such as the case of the host–pathogen interaction between Pocillopora damicornis and Vibrio coralliilyticus. At temperatures above 27 °C, pathogen virulence increases and cause lysis and mortality in P. damicornis [88].
References
- FAO. Statistical Yearbook 2021: World Food and Agriculture; FAO (Food and Agriculture Organization): Rome, Italy, 2021.
- Mangalassery, S.; Sjögersten, S.; Sparkes, D.L.; Sturrock, C.J.; Craigon, J.; Mooney, S.J. To what extent can zero tillage lead to a reduction in greenhouse gas emissions from temperate soils?. Sci. Rep. 2015, 4, 4586.
- Cavicchioli, R.; Ripple, W.J.; Timmis, K.N.; Azam, F.; Bakken, L.R.; Baylis, M.; Behrenfeld, M.J.; Boetius, A.; Boyd, P.W.; Classen, A.T.; et al.et al. Scientists’ warning to humanity: Microorganisms and climate change.. Nat. Rev. Microbiol. 2019, 17, 569–586.
- Kannojia, P.; Sharma, P.K.; Sharma, K.. Climate Change and Soil Dynamics: Effects on Soil Microbes and Fertility of Soil. In Climate Change and Agricultural Ecosystems; Elsevier: Amsterdam, The Netherlands, 2019; pp. 43–64.
- Mekala, S.; Polepongu, S.. Impact of Climate Change on Soil Microbial Community. In Plant Biotic Interactions; Springer International Publishing: Cham, Switzerland, 2019; pp. 31–41.
- Pugnaire, F.I.; Morillo, J.A.; Peñuelas, J.; Reich, P.B.; Bardgett, R.D.; Gaxiola, A.; Wardle, D.A.; van der Putten, W.H. Climate change effects on plant-soil feedbacks and consequences for biodiversity and functioning of terrestrial ecosystems.. Sci. Adv. 2019, 5, 1834.
- Sheik, C.S.; Beasley, W.H.; Elshahed, M.S.; Zhou, X.; Luo, Y.; Krumholz, L.R. Effect of warming and drought on grassland microbial communities.. ISME J. 2011, 5, 1692–1700..
- Classen, A.T.; Sundqvist, M.K.; Henning, J.A.; Newman, G.S.; Moore, J.A.M.; Cregger, M.A.; Moorhead, L.C.; Patterson, C.M. Direct and indirect effects of climate change on soil microbial and soil microbial-plant interactions: What lies ahead?. Ecosphere 2015, 6, art130.
- Lobell, D.B.; Asseng, S. Comparing estimates of climate change impacts from process-based and statistical crop models.. Environ. Res. Lett 2017, 12, 015001.
- Liu, B.; Asseng, S.; Müller, C.; Ewert, F.; Elliott, J.; Lobell, D.B.; Martre, P.; Ruane, A.C.; Wallach, D.; Jones, J.W.; et al.et al. Similar estimates of temperature impacts on global wheat yield by three independent methods.. Nat. Clim. Change 2016, 6, 1130–1136.
- Zampieri, M.; Ceglar, A.; Dentener, F.; Toreti, A. Wheat yield loss attributable to heat waves, drought and water excess at the global, national and subnational scales.. Environ. Res. Lett. 2017, 12, 064008.
- Matiu, M.; Ankerst, D.P.; Menzel, A. Interactions between temperature and drought in global and regional crop yield variability during 1961–2014.. PLoS ONE 2017, 12, e0178339.
- Zhang, P.; Zhang, J.; Chen, M. J. Economic impacts of climate change on agriculture: The importance of additional climatic variables other than temperature and precipitation.. Environ. Econ. Manag. 2017, 83, 8-31.
- Almeida, F.; Rodrigues, M.L.; Coelho, C. The Still Underestimated Problem of Fungal Diseases Worldwide.. Front. Microbiol. 2019, 10, 214.
- Miedaner, T.; Juroszek, P. Climate change will influence disease resistance breeding in wheat in Northwestern Europe.. Theor. Appl. Genet. 2021, 134, 1771–1785.
- FAO. Plant Pests and Diseases in the Context of Climate Change and Climate Variability: Food Security and Biodiversity Risks; FAO (Food and Agriculture Organization): Budapest, Hungary, 2019; Volume 41.
- Bregaglio, S.; Donatelli, M.; Confalonieri, R. Fungal infections of rice, wheat, and grape in Europe in 2030–2050.. Agron. Sustain. Dev. 2013, 33, 767–776.
- Bhattacharya, S. Deadly new wheat disease threatens Europe’s crops. . Nature 2017, 542, 145–146.
- Gilardi, G.; Gullino, M.L.; Garibaldi, A. Emerging foliar and soil-borne pathogens of leafy vegetable crops: A possible threat to Europe. . EPPO Bull 2018, 48, 116–127.
- Guarnaccia, V.; Crous, P.W. Emerging citrus diseases in Europe caused by species of Diaporthe.. IMA Fungus 2017, 8, 317–334.
- Ryabov, E.V.; Childers, A.K.; Chen, Y.; Madella, S.; Nessa, A.; VanEngelsdorp, D.; Evans, J.D. Recent spread of Varroa destructor virus-1, a honey bee pathogen, in the United States.. Sci. Rep. 2017, 7, 17447.
- Wallen-Russell, C.; Wallen-Russell, S. Topical Probiotics Do Not Satisfy New Criteria for Effective Use Due to Insufficient Skin Microbiome Knowledge.. Cosmetics 2021, 8, 90.
- Kirby, K.J.; Watkins, C.. Europe’s Changing Woods and Forests: From Wildwood to Managed Landscapes; Kirby, K.J.; Watkins, C., Eds.; CABI: Wallingford, UK, 2015; pp. ISBN 9781780643373.
- Yasmin, S.; Hafeez, F.Y.; Mirza, M.S.; Rasul, M.; Arshad, H.M.I.; Zubair, M.; Iqbal, M. Biocontrol of Bacterial Leaf Blight of Rice and Profiling of Secondary Metabolites Produced by Rhizospheric Pseudomonas aeruginosa BRp3.. Front. Microbiol. 2017, 8, 1895.
- Banerjee, A.; Roy, S.; Bag, M.K.; Bhagat, S.; Kar, M.K.; Mandal, N.P.; Mukherjee, A.K.; Maiti, D. A survey of bacterial blight (Xanthomonas oryzae pv. oryzae) resistance in rice germplasm from eastern and northeastern India using molecular markers. Crop Prot. 2018, 112, 168–176.
- Wang, R.; Li, Q.; He, S.; Liu, Y.; Wang, M.; Jiang, G. Modeling and mapping the current and future distribution of Pseudomonas syringae pv. actinidiae under climate change in China.. PLoS ONE 2018, 13, e0192153.
- Krawczyk, K.; Łochyńska, M. Identification and characterization of Pseudomonas syringae pv. mori affecting white mulberry (Morus alba) in Poland.. Eur. J. Plant Pathol. 2020, 158, 281–291.
- Park, R.F.; Burdon, J.J.; McIntosh, R.A. Studies on the origin, spread, and evolution of an important group of Puccinia recondita f. sp. tritici pathotypes in Australasia.. Eur. J. Plant Pathol. 1995, 101, 613–622.
- Peksa, K.; Bankina, B. Characterization of Puccinia recondita, the causal agent of brown rust: A review.. Res. Rural Dev. 2019, 2, 70-76.
- Schwanck, A.A.; Meneses, P.R.; Farias, C.R.J.; Funck, G.R.D.; Maia, A.H.N.; Del Ponte, E.M. Bipolaris oryzae seed borne inoculum and brown spot epidemics in the subtropical lowland rice-growing region of Brazil.. Eur. J. Plant Pathol. 2015, 142, 875–885.
- Rosslenbroich, H.-J.; Stuebler, D. Botrytis cinerea—History of chemical control and novel fungicides for its management.. Crop Prot. 2000, 19, 557–561.
- FAO. The Impact of Disasters and Crises on Agriculture and Food Security; FAO (Food and Agriculture Organization): Rome, Italy, 2021.
- Thompson, S.M.; Tan, Y.P.; Shivas, R.G.; Neate, S.M.; Morin, L.; Bissett, A.; Aitken, E.A.B. Green and brown bridges between weeds and crops reveal novel Diaporthe species in Australia. . Persoonia Mol. Phylogeny Evol. Fungi 2015, 35, 39-49.
- Rapicavoli, J.; Ingel, B.; Blanco-Ulate, B.; Cantu, D.; Roper, C. Xylella fastidiosa: An examination of a re-emerging plant pathogen.. Mol. Plant Pathol. 2018, 19, 786-800.
- Kim, B.; Lee, J.S.; Choi, Y.-J. Plasmopara viticola Causing Downy Mildew on Vitis davidii in Korea.. Plant Dis. 2021, 105, 3309.
- Williams, M.G.; Magarey, P.A.; Sivasithamparam, K. Effect of temperature and light intensity on early infection behaviour of a Western Australian isolate of Plasmopara viticola, the downy mildew pathogen of grapevine.. Australas. Plant Pathol. 2007, 36, 325.
- Thines, M. Recent outbreaks of downy mildew on grape ivy (Parthenocissus tricuspidata, Vitaceae) in Germany are caused by a new species of Plasmopara.. Mycol. Prog. 2011, 10, 415-422.
- Sharma, M.; Ghosh, R.; Pande, S. Dry root rot (Rhizoctonia bataticola (Taub.) Butler): An emerging disease of chickpea—Where do we stand?. Arch. Phytopathol. Plant Prot. 2015, 48, 797–812.
- Hantula, J.; Vainio, E.J.. Viruses as components of forest microbiome.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 371–382.
- Gaganidze, D.; Sadunishvili, T.; Aznarashvili, M.; Abashidze, E.; Gurielidze, M.; Carnal, S.; Rezzonico, F.; Zubadalashvili, M. Fire blight distribution in Georgia and characterization of selected Erwinia amylovora isolates.. J. Plant Pathol. 2021, 103, 121-129.
- Singh, P.K.; Gahtyari, N.C.; Roy, C.; Roy, K.K.; He, X.; Tembo, B.; Xu, K.; Juliana, P.; Sonder, K.; Kabir, M.R.; et al.et al. Wheat Blast: A Disease Spreading by Intercontinental Jumps and Its Management Strategies.. Front. Plant Sci. 2021, 12, 1467.
- Bragard, C.; Dehnen-Schmutz, K.; Di Serio, F.; Gonthier, P.; Jacques, M.; Jaques Miret, J.A.; Justesen, A.F.; MacLeod, A.; Magnusson, C.S.; Milonas, P.; et al.et al. Risk assessment of the entry of Pantoea stewartii subsp. stewartii on maize seed imported by the EU from the USA.. EFSA J. 2019, 17, e05851.
- Abidin, N.; Ismail, S.I.; Vadamalai, G.; Yusof, M.T.; Hakiman, M.; Karam, D.S.; Ismail-Suhaimy, N.W.; Ibrahim, R.; Zulperi, D. Genetic diversity of Pantoea stewartii subspecies stewartii causing jackfruit-bronzing disease in Malaysia. . PLoS ONE 2020, 15, e0234350.
- Ali, S.; Gladieux, P.; Leconte, M.; Gautier, A.; Justesen, A.F.; Hovmøller, M.S.; Enjalbert, J.; de Vallavieille-Pope, C. Origin, Migration Routes and Worldwide Population Genetic Structure of the Wheat Yellow Rust Pathogen Puccinia striiformis f.sp. tritici. . PLoS Pathog. 2014, 10, e1003903.
- McDonald, B.A.; Stukenbrock, E.H. Rapid emergence of pathogens in agro-ecosystems: Global threats to agricultural sustainability and food security.. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20160026.
- Ali, M.Z.; Carlile, G.; Giasuddin, M. Impact of global climate change on livestock health: Bangladesh perspective.. Open Vet. J. 2020, 10, 178-188.
- FAO. Meat Market Review; FAO (Food and Agriculture Organization): Rome, Italy, 2019.
- Bett, B.; Kiunga, P.; Gachohi, J.; Sindato, C.; Mbotha, D.; Robinson, T.; Lindahl, J.; Grace, D. Effects of climate change on the occurrence and distribution of livestock diseases.. Prev. Vet. Med. 2017, 137, 119-129.
- Gale, P.; Drew, T.; Phipps, L.P.; David, G.; Wooldridge, M. The effect of climate change on the occurrence and prevalence of livestock diseases in Great Britain: A review. . J. Appl. Microbiol. 2009, 106, 1409–1423.
- Woldehiwet, Z. The natural history of Anaplasma phagocytophilum.. Vet. Parasitol. 2010, 167, 108-122.
- Uminski, K.; Kadkhoda, K.; Houston, B.L.; Lopez, A.; MacKenzie, L.J.; Lindsay, R.; Walkty, A.; Embil, J.; Zarychanski, R. Anaplasmosis: An emerging tick-borne disease of importance in Canada.. IDCases 2018, 14, e00472.
- Gray, J.S.; Ogden, N.H. Ticks, Human Babesiosis and Climate Change.. Pathogens 2021, 10, 1430.
- Marques, R.; Krüger, R.F.; Peterson, A.T.; de Melo, L.F.; Vicenzi, N.; Jiménez-García, D. Climate change implications for the distribution of the babesiosis and anaplasmosis tick vector, Rhipicephalus (Boophilus) microplus.. Vet. Res. 2020, 51, 81.
- Wilson, A.J.; Mellor, P.S. Bluetongue in Europe: Past, present and future.. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2669–2681.
- Solano-Gallego, L.; Sainz, Á.; Roura, X.; Estrada-Peña, A.; Miró, G. A review of canine babesiosis: The European perspective.. Parasites Vectors 2016, 9, 336.
- Hughes, H.R.; Velez, J.O.; Fitzpatrick, K.; Davis, E.H.; Russell, B.J.; Lambert, A.J.; Staples, J.E.; Brault, A.C. Genomic Evaluation of the Genus Coltivirus Indicates Genetic Diversity among Colorado Tick Fever Virus Strains and Demarcation of a New Species.. Diseases 2021, 9, 92.
- Viral CNS Infections. In Hunter’s Tropical Medicine and Emerging Infectious Disease; Elsevier: Amsterdam, The Netherlands, 2013; pp. 344–379.
- Paddock, C.D.; Childs, J.E. Ehrlichia chaffeensis: A Prototypical Emerging Pathogen.. Clin. Microbiol. Rev. 2003, 16, 37-64.
- Marcdante, K.J.; Kliegman, R.M.. Zoonoses and Vector Borne Infections. In Nelson Essentials of Pediatrics; Marcdante, K.J., Kliegman, R.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 439–447.
- Adler, B. History of leptospirosis and leptospira.. Curr. Top. Microbiol. Immunol. 2015, 387, 1-9.
- Lau, C.L.; Smythe, L.D.; Craig, S.B.; Weinstein, P. Climate change, flooding, urbanisation and leptospirosis: Fuelling the fire?. Trans. R. Soc. Trop. Med. Hyg. 2010, 104, 631-638.
- Howard, S.; Ginsberg, P.; Jannelle Couret, P.M.; Jason Garrett, B.M.; Thomas, N.; Mather, P.; Roger, A.; Lebrun, P. Potential effects of climate change on tick-borne diseases in Rhode Island.. Rhode Isl. Med. J. 2021, 104, 29-33.
- Steinbrink, A.; Brugger, K.; Margos, G.; Kraiczy, P.; Klimpel, S. The evolving story of Borrelia burgdorferi sensu lato transmission in Europe.. Parasitol. Res. 2022, 121, 781-803.
- Brownstein, J.S.; Holford, T.R.; Fish, D. Effect of Climate Change on Lyme Disease Risk in North America.. Ecohealth 2005, 2, 38-46.
- Bouchard, C.; Dibernardo, A.; Koffi, J.; Wood, H.; Leighton, P.; Lindsay, L. Increased risk of tick-borne diseases with climate and environmental changes.. Can. Commun. Dis. Rep. 2019, 45, 83-89.
- Schimmer, B. Dutch Q Fever Epidemic in a ‘One Health’ Context: Outbreaks, Seroprevalence and Occupational Risks. Ph.D. Thesis, University of Utrecht, Utrecht, The Netherlands, 2018.
- Alkishe, A.; Peterson, A.T. Climate change influences on the geographic distributional potential of the spotted fever vectors Amblyomma maculatum and Dermacentor andersoni.. PeerJ 2022, 10, e13279.
- Guo, X.; Feng, J.; Shi, Z.; Zhou, X.; Yuan, M.; Tao, X.; Hale, L.; Yuan, T.; Wang, J.; Qin, Y.; et al.et al. Climate warming leads to divergent succession of grassland microbial communities.. Nat. Clim. Change 2018, 8, 813-818.
- Flahault, A.; de Castaneda, R.R.; Bolon, I. Climate change and infectious diseases.. Public Health Rev. 2016, 37, 21.
- Le Conte, Y.; Navajas, M. Climate change: Impact on honey bee populations and diseases.. Rev. Sci. Tech. 2008, 27, 499–510.
- Etxegarai-Legarreta, O.; Sanchez-Famoso, V. The Role of Beekeeping in the Generation of Goods and Services: The Interrelation between Environmental, Socioeconomic, and Sociocultural Utilities.. Agriculture 2022, 12, 551.
- Giliba, R.A.; Mpinga, I.H.; Ndimuligo, S.A.; Mpanda, M.M. Changing climate patterns risk the spread of Varroa destructor infestation of African honey bees in Tanzania.. Ecol. Process. 2020, 9, 48.
- Rowland, B.W.; Rushton, S.P.; Shirley, M.D.F.; Brown, M.A.; Budge, G.E. Identifying the climatic drivers of honey bee disease in England and Wales.. Sci. Rep. 2021, 11, 21953.
- Veen, N.; Yadav, A.S. Seasonal Incidence of European Foul Brood and Sac Brood Disease in Apis mellifera L. and their Correlation with Colony and Weather Parameters.. Int. J. Curr. Microbiol. Appl. Sci. 2021, 10, 13-24.
- Budge, G.E.; Simcock, N.K.; Holder, P.J.; Shirley, M.D.F.; Brown, M.A.; Van Weymers, P.S.M.; Evans, D.J.; Rushton, S.P. Chronic bee paralysis as a serious emerging threat to honey bees.. Nat. Commun. 2020, 11, 2164.
- Stephan, J.G.; de Miranda, J.R.; Forsgren, E. American foulbrood in a honeybee colony: Spore-symptom relationship and feedbacks between disease and colony development.. BMC Ecol. 2020, 20, 16.
- FAO. The State of World Fisheries and Aquaculture 2020: Sustainability in Action; FAO (Food and Agriculture Organization): Budapest, Hungary, 2020.
- Vezzulli, L.; Grande, C.; Reid, P.C.; Hélaouët, P.; Edwards, M.; Höfle, M.G.; Brettar, I.; Colwell, R.R.; Pruzzo, C. Climate influence on Vibrio and associated human diseases during the past half-century in the coastal North Atlantic.. Proc. Natl. Acad. Sci. USA 2016, 113, E5062–E5071.
- Lafferty, K.D. Marine Infectious Disease Ecology.. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 473-496.
- Hillebrand, H.; Brey, T.; Gutt, J.; Hagen, W.; Metfies, K.; Meyer, B.; Lewandowska, A.. Climate Change: Warming Impacts on Marine Biodiversity. In Handbook on Marine Environment Protection; Springer International Publishing: Cham, Swizterland, 2018; pp. 353–373.
- Canonico, G.; Buttigieg, P.L.; Montes, E.; Muller-Karger, F.E.; Stepien, C.; Wright, D.; Benson, A.; Helmuth, B.; Costello, M.; Sousa-Pinto, I.; et al.et al. Global Observational Needs and Resources for Marine Biodiversity.. Front. Mar. Sci. 2019, 6, 367.
- Bunse, C.; Lundin, D.; Karlsson, C.M.G.; Akram, N.; Vila-Costa, M.; Palovaara, J.; Svensson, L.; Holmfeldt, K.; González, J.M.; Calvo, E.; et al.et al. Response of marine bacterioplankton pH homeostasis gene expression to elevated CO2. . Nat. Clim. Change 2016, 6, 483–487.
- Kling, J.D.; Lee, M.D.; Fu, F.; Phan, M.D.; Wang, X.; Qu, P.; Hutchins, D.A. Transient exposure to novel high temperatures reshapes coastal phytoplankton communities.. ISME J. 2020, 14, 413-424.
- Wang, Z.; Tsementzi, D.; Williams, T.C.; Juarez, D.L.; Blinebry, S.K.; Garcia, N.S.; Sienkiewicz, B.K.; Konstantinidis, K.T.; Johnson, Z.I.; Hunt, D.E.; et al. Environmental stability impacts the differential sensitivity of marine microbiomes to increases in temperature and acidity.. ISME J. 2021, 15, 19-28.
- Hutchins, D.A.; Fu, F. Microorganisms and ocean global change.. Nat. Microbiol. 2017, 2, 17058.
- Abirami, B.; Radhakrishnan, M.; Kumaran, S.; Wilson, A. Impacts of global warming on marine microbial communities.. Sci. Total Environ. 2021, 791, 147905.
- Cohen, R.; James, C.; Lee, A.; Martinelli, M.; Muraoka, W.; Ortega, M.; Sadowski, R.; Starkey, L.; Szesciorka, A.; Timko, S.; et al.et al. Marine Host-Pathogen Dynamics: Influences of Global Climate Change.. Oceanography 2018, 31, 182–193.
- Ben-Haim, Y.; Zicherman-Keren, M.; Rosenberg, E. Temperature-Regulated Bleaching and Lysis of the Coral Pocillopora damicornis by the Novel Pathogen Vibrio coralliilyticus. . Appl. Environ. Microbiol. 2003, 69, 4236–4242.
- Malek, J.C.; Byers, J.E. Responses of an oyster host (Crassostrea virginica) and its protozoan parasite (Perkinsus marinus) to increasing air temperature.. PeerJ 2018, 6, e5046.
- Marcos-López, M.; Gale, P.; Oidtmann, B.C.; Peeler, E.J. Assessing the Impact of Climate Change on Disease Emergence in Freshwater Fish in the United Kingdom.. Transbound. Emerg. Dis. 2010, 57, 293–304.
- De Souza Valente, C.; Wan, A.H.L. Vibrio and major commercially important vibriosis diseases in decapod crustaceans. . J. Invertebr. Pathol. 2021, 181, 107527.
- Li, X.; Yang, B.; Shi, C.; Wang, H.; Yu, R.; Li, Q.; Liu, S. Synergistic Interaction of Low Salinity Stress With Vibrio Infection Causes Mass Mortalities in the Oyster by Inducing Host Microflora Imbalance and Immune Dysregulation.. Front. Immunol. 2022, 13, 859975.
- Dorfmeier, E.M. Ocean Acidification and Disease: How Will a Changing Climate Impact Vibrio tubiashii Growth and Pathogenicity to Pacific Oyster Larvae? Master’s Thesis, University of Washington, Seattle, WA, USA, 2012.
