Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Federico Roggio and Version 2 by Lindsay Dong.
Adolescent idiopathic scoliosis (AIS) is the predominant orthopedic disorder in children, affecting 1–3% of the global population. Research in this field has tried to delineate the genetic factors behind scoliosis and its association with heredity since AIS is considered a polygenic disease and has different genetic and epigenetic factors.
- adolescent idiopathic scoliosis
- biomarkers
- protein
1. Introduction
Spinal deformities, particularly scoliosis, represent the most common orthopedic deformities in the pediatric population, with idiopathic scoliosis being the most prevalent form [1]. The spine is characterized by structural changes in the vertebrae that result in rotation and translation relative to the normal body axis [2]. Scoliosis is defined as idiopathic because, until now, no identifiable cause of it has clearly been recognized. Theories include a broad idea of genetic and hormonal factors or more specific causes related to the asymmetric growth of the musculoskeletal system [3]. According to the age of diagnosis, it can be classified as infantile when it occurs between the ages of 0 and 3 years, juvenile when occurring in the age range of 4–10, adolescent at ages 10–18, and adult when diagnosed after the age of 18 [4]. It is important to properly consider the age of onset, since different age ranges have unique physiological aspects to consider, such as bone, muscle, and hormonal maturation (Figure 1). The diagnostic approach comprises clinical analysis using the forward bending test and a scoliometer, followed by X-rays to identify the degree of curvature of the scoliosis. In addition to diagnosis, these methods allow us to observe the condition’s progression and choose the most suitable approach for treatment. According to the Scoliosis Research Society, scoliosis is diagnosed as such only when there is an axial rotation of the vertebrae and the curve is greater than the Cobb angle [5].
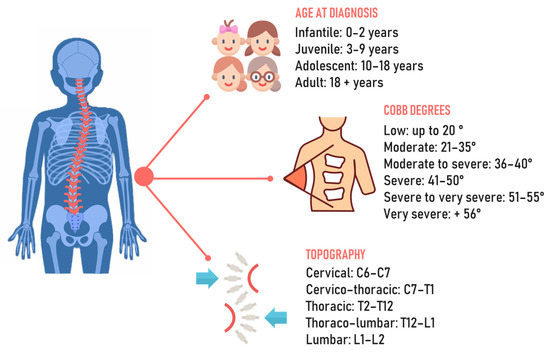
Figure 1.
Chronological, angular, and topographic classification of scoliosis.
Scoliosis screening programs are important because early detection of scoliosis can slow its progression and prevent additional musculoskeletal disorders [6]. As observed in a previous investigation, the presence of clinical signs of scoliosis among Italian high school adolescents was 36% in the sample examined [7], highlighting the lack of adequate screening programs.
Scoliosis diagnosis involves the forward bending or Adam’s test, scoliometer use for trunk rotation measurement, and radiography, the gold standard for identifying and quantifying spinal alterations [8][9][10][8,9,10]. To avoid exposure to repeated X-rays in a short period of time, different non-invasive methods have been proposed to help clinicians to select the most appropriate treatment to prevent scoliosis progression, such as Moiré topography [11], infrared thermography [12], rasterstereography [13], 3D scanner [14], and 3D ultrasound imaging [15]. These methods cannot diagnose AIS, but their ease of use can highlight possible improvements or worsening at follow-up, helping to better tailor treatment. Monitoring AIS is necessary as progression may be aggressive, resulting in the need for surgery if scoliosis is not adequately treated.
These different methods are a valid source of support; however, research has been trying to classify the genetic factors behind scoliosis [16] and its association with heredity, first described in 1934 by Garland [17]. He observed that the frequency of scoliosis in first-degree relatives is much higher than in the general population. Epidemiological and genetic studies consider AIS a polygenic disease, describing an increased risk of onset of scoliosis due to different genetic and epigenetic factors [18][19][18,19]. Different loci have been identified as strongly associated with susceptibility to AIS, but this clinical approach is still limited to supporting the prediction of scoliosis or its progression [20], rather than diagnosis before its onset. Since genetic markers represent valid prognostic tools, it is important to promote studies on the classification of scoliosis and its progression to help clinicians manage personalized treatments.
The exploration of molecular biomarkers in AIS has yielded significant insights into its pathogenesis and opened potential opportunities for early diagnosis and treatment. For instance, a study by Seki et al. [21] revealed an association of ligamentum flavum hypertrophy with AIS progression, identifying the ERC2 and MAFB genes as significant contributors to this phenomenon. These genes were linked to increased expression of collagen by ligamentum flavum cells, highlighting a molecular pathway that could be targeted for therapeutic intervention. Another pivotal study, conducted by Sun et al. [22], explored potential metabonomic biomarkers for AIS, identifying seven differential metabolites, including PC(20:4), 2-hexenoylcarnitine, and beta-D-glucopyranuronic acid. These metabolites indicated a disrupted lipid metabolism in AIS, suggesting that lipid metabolism plays a significant role in the pathogenesis of AIS. This discovery sheds light on the development of diagnostic biomarkers and also provides a deeper understanding of metabolic alterations in AIS.
2. Role of Muscle Biomarkers in Adolescent Idiopathic Scoliosis
Research on AIS is important in orthopedics due to its impact during musculoskeletal development. This condition can cause not just physical deformities [23][37] but also muscle imbalances [24][38] and diminished lung capacity, stemming from changes to the spine and ribcage [25][26][39,40] (Figure 13).
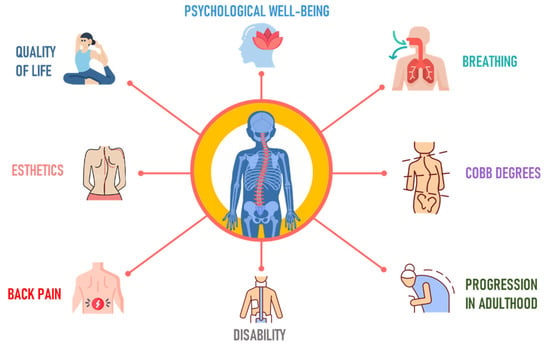
Figure 13.
Goals in the treatment of adolescent idiopathic scoliosis.
The term ‘idiopathic’ refers to the inability to identify a specific cause and is why research has been trying to map all possible genetic or epigenetic factors associated with AIS in recent decades. Other studies summarized the main characteristics of the relationship between scoliosis and some specific biomarkers. Zhu et al. [27][41] found that vitamin D deficiency may be involved in AIS pathogenesis because it affects the regulation of calcium phosphate metabolism in bones. Recent studies explore how leptin and ghrelin signaling might contribute to the changes observed in body mass index in AIS patients [28][42]. Leptin is mainly produced in white adipose tissue, while ghrelin is a polypeptide hormone regulating growth hormone secretion and bone formation. Recent research indicates a correlation between ghrelin and the abnormal cartilage development seen in AIS patients [29][43].
The alpha subunits of the Gi protein mainly inhibit cAMP-dependent pathways by inhibiting the activity of adenylyl cyclase and reducing the production of cAMP from ATP [30][50]. Akoume et al. [31][24] analyzed the contribution of Gi-protein-coupled receptor signaling in the pathogenesis of AIS. They classified patients with AIS into three endophenotypes (FG1, FG2, and FG3) and observed that the FG2 group was the most likely to develop severe scoliosis (Cobb angle > 45°) in both osteoblasts and myoblasts. Gαi2 is related to skeletal muscle hypertrophy and myoblast differentiation [32][33][51,52], so the differences found by Akoume et al. [31][24] in the three groups of Gαi proteins may be related to muscular imbalance due to postural asymmetry in scoliosis. Buchan et al. [34][25] postulated the same concept of muscle imbalance in their investigation of fibrillin-1 and fibrillin-2, a topic first broached by Miller et al. in 1997 [35][53]. While the FBN1 gene mutation is tied to Marfan syndrome [36][54], its association with skeletal muscle microfibrils suggests that rare variants in AIS patients could lead to vertebral and muscular structural changes due to altered elasticity. FBN2, which is known to regulate TGF-β (transforming growth factor-β) signaling [37][55], plays a role in the inflammatory response of skeletal muscle. It inhibits muscle regeneration and governs the remodeling of the extracellular matrix [38][56]. Probably, muscle alterations related to FBN1 and FBN2 and their activity on TGF-β may explain muscle and bone impairments in patients with AIS. Also, a study by Dai et al. [39][26] discussed muscle changes in AIS. They analyzed the concentration of dipeptidyl peptidase-4, a transmembrane protein that modulates insulin metabolism. The authors found that AIS muscles demonstrated reduced sensitivity to glucose and insulin, which could be linked to cell viability during myogenesis.
Regarding the analysis of SNPs, the studies are mainly based on the GWAS protocol. LBX1 is an important regulator of muscle precursor migration [40][57] and was the most investigated gene in the present study. Two SNPs, rs11190870 and rs678741 [41][27], were found to have a higher OR for individuals with AIS compared to the control group, while the results of Man et al. [42][29] and Xu et al. [43][34], although significant, had a lower OR. LBX1 mRNA expression was also found to be lower in the muscles of the concave side compared to the convex one [44][35]. This gene was also the most investigated among the selected studies.
PAX3 is another gene providing conflicting results for the SNP rs13398147. Man et al. [42][29] found a lower OR of 0.88 than Xu et al. [43][34], who found an OR of 1.46, while in another study, the authors provided an OR = 1.48 [19], similar to that of Xu et al. [43][34]. Man et al. [42][29] and Xu et al. [43][34] were also in conflict regarding the SNP rs241215 (OR = 1.11 vs. OR = 0.75) of the gene AJAP1. Both authors also evaluated the genes SOX9/KCNJ2, GPR126, BCL2, BNC2, MEIS1, MAGI1, and TNIK, but they identified different SNPs, making them non-comparable. Wu et al. [45][31] found significant differences in two SNPs in the genes ABO and CDH13, while Li et al. [46][36] found a lower expression of the SNP rs1978060 in TBX1 in patients with AIS compared to patients with congenital scoliosis. Xia et al. [47][32] analyzed two SNPs in IRX, with a higher OR for rs12517904 but not for rs117273909. Liu et al. [48][61] evaluated an SNP of the IL-6 gene, reporting no significant association between this SNP and AIS. These results are in contrast to those of Nikolova et al. [49][62], who found the IL-6 gene as a modifier factor for idiopathic scoliosis.
Molecular differences could inspire new treatment strategies. Therapies targeting the Gαi protein, for instance, could potentially be developed to address the muscle imbalances caused by scoliosis’s postural asymmetry, thereby reducing the severity of the condition. Understanding the role of genes such as FBN1 and FBN2 in skeletal muscle microfibrils could pave the way for innovative treatment approaches. Targeted gene therapy could also potentially be used to correct these mutations and prevent the muscular and vertebral alterations associated with the disease. Furthermore, identifying specific SNP variations could guide genetic counseling and foster a personalized medicine approach, optimizing interventions based on a patient’s genetic risk. A blood test for these biomarkers could facilitate earlier detection and treatment of AIS, potentially mitigating disease progression and improving patient outcomes.
Results show a complex picture of genetic variability in individuals with AIS, with conflicting results and multiple genes and SNPs that may be associated with the disease. More research is needed to clarify these associations and their potential implications for the diagnosis, treatment, and management of AIS.
Adolescent idiopathic scoliosis is an orthopedic deformity prevalent in the pediatric population. Despite its frequency, the exact cause remains elusive, with hypotheses largely revolving around genetic and hormonal influences. Protein biomarkers, including Gi-protein alpha subunits, fibrillin-1 and -2, and differentially expressed proteins, might contribute to muscle alterations in AIS. Concerning single-nucleotide polymorphism studies, LBX1 was the most analyzed gene, and two SNPs (rs11190870 and rs678741) exhibited a higher odds ratio for AIS relative to the control group. The genes PAX3, AJAP1, BNC2, and PAX1 were also studied, yielding mixed results for some SNPs. Persistent efforts in molecular research are crucial to the treatment of AIS before the attainment of full skeletal maturity, thereby reducing severe complications associated with postural alterations and compromised quality of life.
