One of the most prevalent primary malignant brain tumors is glioblastoma (GB). About 6 incidents per 100,000 people are reported annually. Most frequently, these tumors are linked to a poor prognosis and poor quality of life. There has been little advancement in the treatment of GB. In recent years, some innovative medicines have been tested for the treatment of newly diagnosed cases of GB and recurrent cases of GB. Surgery, radiotherapy, and alkylating chemotherapy are all common treatments for GB. A few of the potential alternatives include immunotherapy, tumor-treating fields (TTFs), and medications that target specific cellular receptors. To provide new multimodal therapies that focus on the molecular pathways implicated in tumor initiation and progression in GB, novel medications, delivery technologies, and immunotherapy approaches are being researched. Of these, oncolytic viruses (OVs) are among the most recent. Coupling OVs with certain modern treatment approaches may have significant benefits for GB patients.
- glioblastoma
- malignant brain tumor
- neurosurgery
- treatment of glioblastoma
1. Introduction
2. Overview Glioblastoma
2.1. Introduction of Glioblastoma
Initially, it was believed that GB only came from glial cells, but research now reveals that they may also come from other cell types that have characteristics of neural stem cells. These cells are in various phases of development from stem cells to neurons to glia, with molecular changes in signaling pathways acting largely as a determinant of phenotypic diversity rather than diverse cell types of origin [22].2.2. Molecular Description
More than 600 genes were sequenced from more than 200 human tumor samples as a result of genomic profiling and the Cancer Genome Atlas project by Parsons et al., 2008, which revealed the complex genetic profile of GB and established a set of three core signaling pathways that are frequently activated (namely, the tumor protein p53 (p53) pathway, the receptor tyrosine kinase/Ras/phosphoinositide 3-kinase signaling pathway, and the retinoblastoma pathway) [23]. Epidermal growth factor receptor (EGFR) overexpression, mutations in the PTEN gene, and deletion of chromosome 10q are among the genetic changes common to primary GBM. Chromosome 19q deletion, p53 mutations, and isocitrate dehydrogenase 1 (IDH1) mutations are typically observed in secondary GB [24][25][26][24,25,26]. Transcriptome studies have become significant methods for grouping cancers into molecular subgroups that differ in terms of their clinical behavior and reaction to treatment [27][28]. However, when taking into account the extremely aggressive isocitrate dehydrogenase (IDH) wild-type group, the transcriptome categorization has not been able to predict prognosis and pharmacologic vulnerability for specific cancers, such as GBM [28][29][29,30]. Specifically, the absence of correlation between survival and physiologically defined subgroups of IDH wild-type GBM has impeded the quest to identify the distinct mechanisms that maintain tumor development in patient subgroups. The transcriptome subgroups utilized to define GBM are preferentially concentrated in tumor cells displaying unique lineage-specific biological states, according to recent results in single cells. The possibility of using the basic biological processes of individual GBM cells to create a clinically meaningful categorization of bulk tumors is still unproven.2.3. Risk Factors
One of the few recognized risk factors that has been proven to increase the likelihood of developing gliomas is exposure to ionizing radiation [30][35]. Radiation-induced GB is generally diagnosed years after receiving therapeutic radiation that was prescribed for another tumor or illness. [31][36]. Other environmental risk factors for the growth of gliomas include exposure to vinyl chloride, pesticides, smoking, petroleum refining, and the production of synthetic rubber. It has not been demonstrated that exposure to electromagnetic fields, formaldehyde, or nonionizing radiation from cell phones causes GB [32][37]. Less than 1% of people with glioma have a recognized hereditary disease; however, some specific genetic diseases, such as neurofibromatosis 1 and 2, tuberous sclerosis, Li–Fraumeni syndrome, retinoblastoma, and Turcot syndrome, are associated with an elevated risk of glioma development [33][38].2.4. Clinical Presentation and Imaging
The anatomical components of the affected brain and the size and location of the tumor can all have a significant impact on how a patient with newly diagnosed GB presents [34][35][39,40]. Patients frequently have headaches and localized or progressive neurologic impairments as signs of elevated intracranial pressure. Up to 25% of patients have a seizure as their first symptom, and up to 50% of patients can have one later on in the course of the disease [36][37][41,42]. Antiepileptic medicines (AEDs) are already part of the standard of care for patients who present with seizures, although routine use of AEDs in individuals without seizures is not advised [38][39][43,44]. At the time of diagnosis, corticosteroids are frequently prescribed to patients to assist in reducing vasogenic edema and relieve related signs and symptoms.2.5. Current Treatment Options
The current standard of care is for concomitant radiotherapy with temozolomide and the greatest amount of safe surgical resection possible [40][46]. Because these tumors are commonly invasive and typically located in expressive regions of the brain, such as regions that regulate speech, motor function, and the senses, extensive and full surgical resection of GBM is challenging. Radical removal of the initial tumor mass is not curative due to the high degree of invasiveness, and infiltrating tumor cells typically stay in the nearby brain, causing the disease to develop or return in the future [41][47].2.6. Role of Immunosuppressive Mechanism in Glioblastoma and Resistance to Immunotherapy
One typical feature of GBM that limits a favorable prognosis is recurrence. Not all patients had access to second-line therapy at this time (about 50% did not receive any treatment while their condition progressed) [42][43][56,57]. Numerous studies demonstrate that GBM is associated with an immunosuppressive microenvironment as a result of an increase in factors generated by tumor cells, including FASL, PD-1, indolamine 2, 3dioxygenase (IDO), and STAT3. Additionally, microglia cells have the ability to create IL-1 and TGF-B, which in turn regulate local myeloid and lymphatic immune cells and support systemic immunosuppression [44][58]. By modifying the expression of several extracellular and intracellular mediators, myeloid cells promote the tumor by ensuring an immunosuppressive microenvironment [45][59]. These variables all alter the cytotoxic T lymphocyte (CTL) phenotype, which raises the quantities of immunosuppressive markers like PD-1. Several research aims to boost anticancer immune responses by utilizing these approaches. For example, vaccination therapy or anti-PD-1 and anti-CTLA-4 treatments are used to kill tumor cells that have GBM-associated antigens like EGFRvIII [46][60]. On the other hand, a treatment called viral oncolytic therapy applies a virus that can stimulate the immune system against the tumor. Attenuated oncolytic viruses propagate into tumor cells by taking advantage of the absence of a viral defense system [47][61]. In recent times, the Hippo pathway has been extensively researched as a molecular mechanism to regulate angiogenesis, invasion, migration, and proliferation of tumors. Numerous investigations demonstrate that YAP may establish contact between immune cells and tumors, especially with TAMs [48][66].3. Novel Oncolytic Viral Therapy for Treatment of Glioblastoma
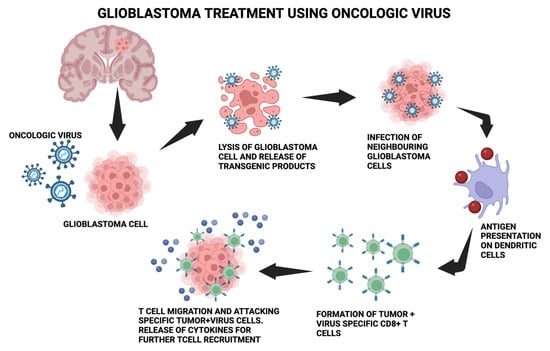
OVs are useful in treating GB because of their ability to replicate virally quickly in rapidly proliferating cells, their absence of distant metastases, and their alignment with the brain environment [49][50][67,68]. The anticancer immune response begins by converting “cold tumors” that are immunosuppressed by the microenvironment into “hot tumors” that are sensitive to the immune system [51][52][53][54][69,70,71,72]. Inducing an immunological response to inadvertently kill cancer cells through several mechanisms, including apoptosis, necrosis, and autophagy, is known as immunogenic cell death (ICD) [55][56][57][73,74,75]. Releases of damage-associated molecular patterns (DAMPs), viral pathogen-associated molecular patterns (PAMPs), tumor-associated antigens (TAAs), and several other cytokines are indicative of this [58][59][76,77]. Oncolytic viruses enhance the function of antigen-presenting cells (APCs), which reach lymph nodes to recruit cytotoxic CD8+ T lymphocytes (CTLs) and attract them to the infection site, where they destroy tumor-inducing cells [60][61][62][78,79,80]. Figure 1 depicts the molecular process mentioned.