You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Masahito Inagaki and Version 2 by Catherine Yang.
Messenger RNA (mRNA) is produced in living organisms by transcription from genomic DNA, and proteins are produced based on the sequence information from mRNA. The COVID-19 pandemic generated interest in the medicinal applications of mRNA. It is expected that mRNA will be applied, not only to vaccines, but also to regenerative medicine. The purity of mRNA is important for its medicinal applications.
- messenger RNA
- cell regeneration
- pluripotent cells
- cellular differentiation
1. mRNA for Cell Reprogramming
ES cells are created by collecting cells from a fertilized egg that is in the process of becoming an embryo, and there are ethical issues involved [1][59]. On the other hand, iPSCs are cells with ES cell-like pluripotency and proliferation ability, established by introducing several types of factors into somatic cells and culturing them [2][60]. Unlike ES cells, which require early embryos, iPSCs can be created from any somatic cells, so there are no ethical issues or concerns about immune rejection. iPSCs can be differentiated into cells of almost any tissue or organ, so they are expected to have a wide range of applications [3][61]. The creation of models of damaged tissue/organ-like disease using iPSCs that can be used to examine the activity and safety of drug candidates is a good example. To generate iPSCs, reprogramming technology is required to initialize the epigenetic modification of the differentiated cells. iPSCs were first developed by Yamanaka et al. [4][40]. They discovered four genes, called Yamanaka factors (Oct4, Sox2, Klf4, and c-Myc), and injected them into fibroblasts using retroviral vectors to induce cell reprogramming. However, with retrovirus-based techniques, there is the possibility that the transgene will integrate into nuclear genomic DNA. Regarding the Yamanaka factors, there is concern that if Klf4 and c-Myc, which are involved in carcinogenesis, are accidentally inserted into genomic DNA, there is a risk of disease onset and tumor formation [5][6][62,63].
Since then, various techniques for creating iPSCs have been developed (Table 1). For example, several methods for introducing plasmid vectors and proteins are known, and although these are safer than the viral vectors, their cell introduction and reprogramming efficiency are insufficient [7][64]. Other reprogramming techniques using small molecules have been reported, such as ACTH 1-24 peptide (fragment of adrenocorticotropic hormone) [8][65], A83-01 (selective inhibitor of activin receptor-like kinase) [9][66], CHIR99021 (inhibitor of lycogen synthase kinase 3β) [10][67], SU5402 (FGF receptor inhibitor) [11][68], DAPT (inhibitor of γ-secretase) [12][69], LDN193189 (inhibitor of bone morphogenetic protein) [13][70], PD0325901 (selective inhibitor of MEK/MAPKK) [14][15][71,72], SB431542 (activin receptor-like kinase inhibitor) [16][17][73,74], SU5402 (tyrosine kinase inhibitor specific to fibroblast growth factor receptor) [18][75], and thiazovivin (improves the survival rate of human ES cells against trypsin treatment) [19][76]. Although reprogramming methods using such small molecules are extremely simple and innovative, it is necessary to confirm the required dosage and the presence or absence of cytotoxicity [20][21][22][77,78,79]. Furthermore, it is difficult to determine whether existing small-molecule reprogramming can cover all applications. It is necessary to expand the types of proteins and receptors that can be targeted and to search for further compounds. MicroRNA-induced reprogramming from somatic cells by injecting members of the mir-302 family (mir-302a, 302b, 302c, 302d, pre-microRNA cluster) is also reported in animal models [23][80]. The mir-302 family is highly expressed in slowly proliferating human ES cells, and rapidly decreases as the cells differentiate and proliferate. Reprogramming using microRNA is an effective method, but the only microRNAs that have been found to be involved in reprogramming are mir-302 [23][80], mir-372 [24][81], the miR-17-92 cluster [25][82], mir-19 [26][83], mir-524 [27][84], mir-371 [28][85], and mir-31 [29][86]; thus, in the future, it is necessary to explore the applicability of various microRNAs [30][87].
Table 1.
Advantages and disadvantages of cell reprogramming strategies.
| Reprogramming Method | Advantages | Disadvantages |
|---|---|---|
| Retroviral vectors |
|
|
|
|
|
| Plasmid vectors |
|
|
| Small molecules |
|
|
|
|
|
|
|
|
| microRNA |
|
|
|
|
|
| mRNA |
|
|
|
|
|
|
A reprogramming method using mRNA has been developed in recent years (
A reprogramming method using mRNA has been developed in recent years (
Figure 1a) [31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50]. Similar to mRNA vaccines, mRNA reprogramming creates iPSCs by introducing mRNA containing genetic information to create cell reprogramming factors in cells and expressed target proteins. Compared to the retrovirus method of delivering DNA encoding reprogramming factors, mRNA is unstable within the cells and degrades gradually, so it does not remain in the iPSCs [51]. As a result, mRNA does not cause mutations in genomic DNA, and there is no risk of tumor development. It is also known that the reprogramming efficiency is higher compared to those of existing methods using viral vectors [35]. In reprogramming using mRNA, as in using mRNA as a vaccine, the introduction of chemical modifications is recommended [52][53][54]. Following the awarding of this year’s Nobel Prize in Physiology or Medicine, the use of chemically modified mRNA in COVID-19 vaccines has been attracting increased attention [55][56], but the application of chemically modified mRNA to cell reprogramming has also been considered since around 2010 [35].
4a) [88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107]. Similar to mRNA vaccines, mRNA reprogramming creates iPSCs by introducing mRNA containing genetic information to create cell reprogramming factors in cells and expressed target proteins. Compared to the retrovirus method of delivering DNA encoding reprogramming factors, mRNA is unstable within the cells and degrades gradually, so it does not remain in the iPSCs [108]. As a result, mRNA does not cause mutations in genomic DNA, and there is no risk of tumor development. It is also known that the reprogramming efficiency is higher compared to those of existing methods using viral vectors [92]. In reprogramming using mRNA, as in using mRNA as a vaccine, the introduction of chemical modifications is recommended [109,110,111]. Following the awarding of this year’s Nobel Prize in Physiology or Medicine, the use of chemically modified mRNA in COVID-19 vaccines has been attracting increased attention [15,34], but the application of chemically modified mRNA to cell reprogramming has also been considered since around 2010 [92].
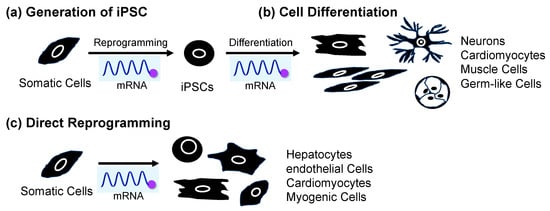
Figure 14. Application of mRNA for (a) cell reprogramming to produce iPSCs from somatic cells, (b) differentiation from iPSCs, and (c) direct reprogramming from somatic cells.
LNP formulations similar to mRNA vaccines, electroporation, and liposomes have also been reported as cellular introduction methods. The mRNA used for cell reprogramming is loaded with 5-methylcytosine, pseudouridine, and 5′-cap structure [52][109]. In addition to OCT4, SOX2, KLF4, and c-MYC, LIN28 is commonly added as a reprogramming factor encoded by mRNA. Examples of mRNA-mediated reprogramming that have been reported to date include somatic cells such as fibroblasts [33][35][57][58][59][60][90,92,112,113,114,115], adipose-derived stem cells (ADSCs) [61][116], bone marrow-derived mesenchymal stem cells (BMSCs) [62][117], and amniotic fluid stem cells [63][118]. On the other hand, there are challenges in creating iPSCs using mRNA from blood cells, which are generally used to create iPSCs because they are easy to culture. mRNA needs to be injected every day due to its biological instability. Blood cells are resistant to cationic lipids [64][119], so lipofection cannot be used, and electroporation is the method of choice, but multiple courses of electroporation increase the risk of cell death [65][120]. Therefore, it is necessary to improve the intracellular stability of mRNA to ensure sustainable protein expression and reduce the number of administrations. Increased mRNA stability is important, but multiple turnovers of mRNA are found in cancer cells. Therefore, the signaling pathway change in cancer development induced by carcinogenic properties due to mRNA stabilization should be carefully investigated. Although there have been some successful cases of reprogramming using mRNA, there are limitations to its applicability, and further research and development are required.
A recent successful example of mRNA-induced reprogramming is the establishment of iPSCs derived from Alzheimer’s disease patients. In 2022, Supakul et al. established iPSCs for patients with mild Alzheimer’s disease using an iPSC establishment kit sold by ReproCELL, a biotech company [66][121]. They succeeded in establishing iPSCs from cells collected from a patient’s urine by administering an mRNA cocktail by lipofection. To date, most iPSCs have been produced from fibroblasts found in the skin or blood. Since urine is easier to collect than skin or blood, it is expected that it will become easier to generate iPSCs from patients with diseases, as well as from children, from whom it was previously difficult to collect samples [67][122]. Research using the generated iPSCs is thought to provide clues to solving social problems associated with aging, such as the increasing number of patients with dementia. In the future, when more examples of reprogramming using mRNA accumulate, it is expected that this will lead to the elucidation of the mechanisms of the development of various diseases and their application to therapeutic research. In addition to cell reprogramming, cell differentiation is also an important technique in regenerative medicine. The application of mRNA for cell differentiation is discussed in the following section.
2. mRNA-Induced Cell Differentiation from iPSCs
Applying iPSCs to regenerative medicine also requires technology to induce differentiation of reprogrammed iPSCs. There are three general methods for inducing differentiation of iPSCs into target tissue cells. The first is to prepare a cell culture medium containing a combination of various cell growth factors, cell differentiation factors, and small-molecule drugs and culture pluripotent stem cells in this medium [68][123]. In many cases, cells are differentiated by exposing them to different culture solutions sequentially. The second method is to create clusters or aggregates of pluripotent stem cells, which allows the cells to change and interact with each other within the clusters (self-organization) and differentiate into various types of cells [69][70][124,125]. These methods require multiple steps, so it takes time for the cells to differentiate into the desired cells, and it is necessary to confirm whether the cells are the same as the original cells existing in the body. The third method takes advantage of the fact that genes involved in transcriptional regulation determine the differentiation state of cells, and it induces differentiation by activating these genes in pluripotent stem cells [71][72][73][126,127,128]. This method directly manipulates transcriptional regulatory factors that determine the differentiation state of the cells, resulting in rapid differentiation. However, since it requires genome editing technology, such as the clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (CRISPR/Cas9) system, there is a risk of cancer or malfunction due to the introduction of off-target mutations that cleave and edit sequences other than the target sequence [74][75][129,130]. On the other hand, this problem can be overcome by introducing mRNA encoding transcriptional regulatory factors. For this reason, research on differentiation induction using mRNA is currently attracting attention (Figure 14b).
As an example of the usefulness of differentiation induction using mRNA, a 2017 report showed that neurons could be rapidly generated from iPSCs derived from patients with Gaucher’s disease [76][77][131,132]. Glucocerebrosidase (GBA) is an enzyme that decomposes the glycolipid glycosylceramide, and Gaucher’s disease is caused by mutations in the GBA gene [78][133]. Glycolipids cannot be broken down, and the main symptoms include enlargement of the liver and spleen, anemia, and thrombocytopenia, but neurological symptoms can also appear, and the disease is classified into three types based, on the presence and severity of these symptoms (type I–III) [79][134]. Although type I Gaucher’s disease is relatively mild and does not cause neurological symptoms, it is known that the risk of developing Parkinson’s disease is extremely high (9% to 12%) in these patients as they get older [80][135]. It has been suggested that excessive accumulation of glycolipids in the brain influences the onset of Parkinson’s disease, but the mechanism is unknown. The relationship between glycolipid accumulation and α-synuclein was investigated using nerve cells generated from iPSCs derived from patients with type I Gaucher’s disease [76][131]. When the researchers synthesized mRNA encoding a transcription factor that promotes neural differentiation and administered it to patients, they were able to confirm glycolipid accumulation just 10 days after the start of differentiation. Although α-synuclein aggregation was not been detected at that point, it was found that the phosphorylation modification of α-synuclein involved in the process was enhanced, making it susceptible to neurodegeneration. In addition, by forcing the normal GBA gene to promote glycolipid degradation, α-synuclein phosphorylation could be suppressed, suggesting that glycolipid accumulation is directly involved in the onset of Parkinson’s disease. On the other hand, it was reported that with conventional neural differentiation techniques, glycolipids accumulated 60 days after the start of differentiation. With this method, it takes more than a month for neurons to form; therefore, it takes even longer to detect the phenotype. Thus, it was shown that the synthetic mRNA differentiation method not only enables short-term differentiation, but is also effective at rapidly reproducing disease-related phenotypes.
A recent research result is the successful creation of sperm stem cell precursors from iPSCs of the marmoset, an experimental primate [81][136]. The researchers induced marmoset iPSCs to become primordial germ-like cells (PGCLCs) by transfecting them with mRNA encoding the SOX17 gene, a master regulator of primordial germ cells. They transplanted the created marmoset PGCLCs under the kidney capsule of an immunodeficient mouse, and succeeded in developing pre-spermatogonia (sperm stem cell precursors). Gene expression and DNA methylation analysis revealed that this process nearly faithfully reproduced the in vivo germ cell development process (up to the newborn stage), and the newly developed method is useful for research on early germ cell development in primates. Sperm production from iPSCs has not yet been achieved in primates, including humans, and the process has only progressed to the production of pre-spermatogonia. The author hopes to advance the development toward sperm production, which will lead to the investigation of the causes of infertility and applications in reproductive medicine in the future. By combining mRNA-induced cell reprogramming and differentiation methods, the next research objective is direct reprogramming using mRNA. The application of mRNA for both cell reprogramming and differentiation, as well as direct re-programming, is discussed in the following section.
3. mRNA for Cell Reprogramming and Differentiation Induction and Direct Reprogramming without Passage through Pluripotent Stem Cells
Examples of mRNA being used for reprogramming and differentiation induction were discussed above. It is also possible to generate functional tissues by administering mRNA as a differentiation-inducing factor to iPSCs that have been reprogrammed and established with mRNA. That is, mRNA can act as both a reprogramming and a differentiation-inducing factor. In 2010, Warren et al. reported the transformation of fibroblasts into embryonic stem cells, which then differentiated into contractile muscle tissue, using modified mRNAs [35][92]. They synthesized the mRNA encoding Yamanaka factors Oct4, Sox2, Klf4, and c-Myc. In this mRNA, cytidine was completely replaced with 5-methylcytidine, and uridine was completely replaced with pseudouridine. When the mRNA was administered to cells, immunostaining showed that the Yamanaka factors were expressed and localized in the nucleus. Furthermore, protein expression by this mRNA peaked 12 to 18 h after introduction and then rapidly decreased, indicating that it degraded within 10 h after administration and did not remain in the cells.
Researchers have also successfully reprogrammed somatic cells. A five-factor cocktail (KMOSL) containing four Yamanaka factors, plus mRNA encoding LIN28, was used in Detroit 551 (D551) cells, MRC-5 fetal fibroblasts, BJ neonatal fibroblasts, and primary cells from adult patients with cystic fibrosis. When the KMOSL–mRNA cocktail was introduced daily into four cultured skin-derived fibroblast-like cells (CF cells), many human ES cell-like colonies appeared, along with more than 10 iPSCs from each somatic cell line. Furthermore, the established iPSCs expressed OCT4, SOX2, NANOG, and hTERT, the Oct4 gene was demethylated, and pluripotency-related genes, including SOX2, REX1, NANOG, OCT4, LIN28, and DNMT3B, were observed. Transcripts were elevated to levels comparable to those of human ES cells, indicating that mRNA-reprogrammed iPSCs are more similar to human ES cells than to virus-generated iPSCs. In addition, the conversion of BJ fibroblasts introduced to the iPSCs using the 5-factor mRNA cocktail exhibited an efficiency of 2%, regardless of the presence or absence of the Rho-associated kinase (ROCK) inhibitor Y-27,632. It was found to be two orders of magnitude more efficient than conventional virus-based methods. Next, the researchers introduced KMOS-mRNA or KMOS retrovirus into dH1f fibroblasts in parallel, and found that ES cell-like colonies began to appear after 2 weeks in those into which mRNA had been introduced, and on day 16, transfection occurred. By the last day of transfection, there was an outgrowth of ES cell-like colonies, whereas when using the KMOS retrovirus, no ES cell-like colonies appeared by this time point, but only from day 24 after gene transfer. The efficiency of iPSC establishment, determined by counting the beginning of colony appearance and TRA-1-60 positive colonies, was 1.4% and 0.04% for KMOS mRNA and KMOS retrovirus, respectively, with KMOS mRNA being 36 times more efficient. Fibroblast growth factor (FGF) was removed from the medium of the iPSC line established using mRNA, serum was added, the medium was spread on a gelatin coat, and mRNA encoding the muscle differentiation-inducing MyOD gene was introduced. After an additional 3 days of culture under low serum conditions, myogenin and MyHC double-positive myotubes appeared, with high efficiency. These results indicate that mRNA directly differentiates pluripotent stem cells into terminally differentiated cells.
It is expected that induction into tissue cells via ES cells and iPSCs will be applied in regenerative medicine. However, there are concerns about the risk of tumor formation due to undifferentiated cells and the low engraftment efficiency of treatments using pluripotent stem cells [49][106]. Direct reprogramming is attracting attention as a reuse method to solve the problems of stem-cell-derived cell transplantation [82][137]. This is a method for directly producing desired cells from fibroblasts, etc., without using iPSCs, and it is possible to produce tissue in vivo by introducing genes into target sites. The concept of direct reprogramming was proposed in 1987. The first report identified MyoD as a master factor for skeletal muscle, and by forcing the expression of the MyoD gene in fibroblasts, the researchers succeeded in producing fibroblasts, which are the precursors of skeletal muscle [83][138]. In 2010, Ikeda et al. were able to coax fibroblasts into becoming beating heart muscle. Using retroviral vectors, they revealed that Gata4, Mef2c, and Tbx5 (GMT) genes are essential for direct myocardial reprogramming. When these three factors were introduced into fibroblasts, a cardiac muscle-specific gene expression pattern was observed, as well as the expression of cardiac muscle-specific structural proteins, such as α-actinin and cardiac troponin (cTnT), with a sarcomere structure [84][139].
Since then, efforts have been made to identify factors that promote direct reprogramming. In 2014, Muraoka et al. reported the use of microRNA as a factor to promote direct reprogramming of the heart muscle [85][140]. They revealed that adding miR-133 to GMT efficiently induced myocardium in a short period. The enhancement of direct reprogramming using lower-cost small molecules is also being investigated. In 2015, Zhao et al. hypothesized that fibroblast plasma maintenance mechanisms inhibit reprogramming into myocardium. By using small molecules that suppress the TGF-β and ROCK pathways, which promote fibrosis, they succeeded in improving the efficiency of guiding mouse fetal fibroblasts to myocardium [86][141]. Furthermore, in 2019, Muraoka et al. showed that diclofenac, a nonsteroidal anti-inflammatory drug, suppressed age-related inflammation, thereby improving the efficiency of direct reprogramming from adult mouse fibroblasts to myocardium, which was difficult to induce [87][142]. Cardiomyocyte induction, using only small molecules and without using any genes, has also been reported. In 2016, Cao et al. reported that by introducing nine small molecules, they could induce human fibroblasts to become functional heart muscle [88][143]. The advantage of this method is that it is safer and provides relative ease of control regarding the cell culture conditions because it does not use genes or viral vectors. On the other hand, direct reprogramming using mRNA, which offers less risk of gene insertion, is also attracting attention (Figure 14c).
In 2014, Simeonov et al. reported the direct reprogramming of human fibroblasts to hepatocyte-like cells using synthetic mRNA [89][144]. They confirmed the generation of hepatocyte-like cells by the lipofection of three types of mRNAs, consisting of HNF1A and two genes from FOXA1, FOXA3, and HNF4A, into human fibroblast cells in an optimized haptic growth medium. In 2017, Pham et al. achieved the direct reprogramming of endothelial progenitor cells from skin fibroblasts using the mRNA encoding ETV2 gene [90][145]. Endothelial progenitor cells are important for angiogenesis, but their abundance in the human body is limited. With the development of this technology, it is expected that it will be applied to autologous transplantation by administering mRNA to skin fibroblasts.
Only recently has research been conducted on direct reprogramming using mRNA. Several applied studies using model animals in regenerative medicine have been reported. In 2021, Kaur et al. demonstrated direct reprogramming from non-cardiomyocytes to cardiomyocytes by using mRNA encoding four cardiac reprogramming genes (Gated, Mef2c, Tbx5, and Hand2) and three reprogramming-helper genes (dominant-negative TGFb, dominant-negative Wnt8a, and acid ceramidase). Using a lineage-tracking model of acute myocardial infarction in mice, they administered an mRNA cocktail at the time of myocardial infarction and found that 25% of cardiomyocyte-like cells in the scarred area were reprogrammed. As a result, significant improvements in cardiac function, scar size, long-term survival rate, and capillary density were observed. Through this research, the author expects the development of safe and highly efficient regenerative drugs for ischemic diseases using mRNA [91][146]. In August 2023, Qabrat et al. demonstrated direct reprogramming of mouse fibroblasts to myogenic progenitor cells (iMPCs) by administering MyoD-expressing mRNA and small molecules promoting myoD expression (forskolin, a cyclic AMP agonist; RepSox, a TGF-β receptor inhibitor; and CHIR99210, a GSK3 inhibitor). The generated iMPCs were shown to express a series of myogenic stem cell markers and to differentiate into contractile myotubes. Furthermore, in a mouse model of Duchenne muscular dystrophy, iMPCs strongly engrafted into skeletal muscle and restored dystrophin expression in hundreds of myofibers [92][147].
To improve the efficiency of direct reprogramming using mRNA, it is important to innovate the technology for introducing mRNA into cells. In 2015, Lee et al. were able to induce cardiomyocyte cells from cardiac fibroblast cells in mice by adding polyarginine-fused heart-targeting peptide (CRPPR-R9) to lipofectamine, a common lipofection reagent. They administered mRNA encoding Gata4, Mef2c, and Tbx5 (GMT) genes for 2 weeks. They showed that by adding CRPPR-R9, the efficiency of intracellular introduction was approximately two times higher compared to the rates for conventional lipofection, and the resulting translational efficiency was confirmed to be approximately three times higher. In this way, the development of highly efficient delivery technology is expected to lead to the ability to promote direct reprogramming within the human body [40][97]. In the above sections, the application of mRNA for cell reprogramming, differentiation and direct reprogramming was introduced. The next topic is the application of mRNA, based on the analytical aspect, for the development of purification methods of iPSCs and iPSC-derived cells, which can contribute to the research and development of regenerative medicine.
