Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Matteo Nadile and Version 4 by Jessie Wu.
Cervical cancer is the fourth most commonly diagnosed cancer among women worldwide. While treatments exist to stop growth of cervical cancer in humans, they are often associated with major side effects and the development of resistance to therapy. Traditionally, plant-derived compounds have been used to treat many ailments, including cancer. The search for novel plant-derived chemicals is important, as they can potentially provide effective treatment with less severe side effects and importantly overcome drug resistance. Genistein and its analogues have been shown to decrease survival and proliferation as well as induce cell death in cell culture models of cervical cancer and reduce tumor volume in a mouse model.
- cervical cancer
- genistein
- proliferation
- survival
- apoptosis
- signaling cascades
1. Genistein against Cervical Cancer: In Vitro Studies
Cervical cancer cell lines have been used to examine the effects of genistein using in vitro methods. These studies are summarized here, and the key findings are highlighted below in Table 1 and Figure 12.
HeLa and ME-180 cervical cancer cells treated with genistein had reduced cell growth and invasion, and increased cell cycle arrest and apoptosis [1][30]. The half-maximal inhibitory concentration (IC50) value for HeLa cells was 35 µM, with the majority of cells arrested in the S phase while for ME-180 cells, the IC50 value was 60 µM, with cells arrested in the G2/M phase (Table 1) [1][30].
Genistein treatment decreased colony formation in ME-180 (lethal dose (LD50): 11 µM) and CaSki (LD50: 24µM) cervical cancer cell lines. Cytochrome c was increased in both cell lines following treatment with 2.5 µM and 10 µM genistein, suggesting activation of apoptotic pathways [2][31].
Cervical cancer cells (HeLa) treated with genistein showed a decrease in cell survival (a 50% decrease was seen with 18.47 µM) and increased cell cycle arrest (increased the percentage of cells in S and G2/M phase) and apoptosis [3][32]. Western blot analysis revealed increased levels of cleaved poly (ADP-ribose) polymerase (PARP), a marker of apoptosis, after treatment with genistein in HeLa cells, while no cleaved PARP was seen in L929 cells (normal mouse fibroblasts). Treatment with genistein led to decreased protein expression of cyclin B1 and cyclin-dependent kinase 1 (CDK1), and decreased tyrosine phosphorylation of cell division control protein 2 (cdc2) (Table 1) [3][32].
Treatment of HeLa and CaSki cervical cancer cells with 5–80 µM of genistein for 24–48 h resulted in a significant inhibition of growth [4][33]. Treatment with genistein resulted in a time-dependent decrease in phosphorylated Akt and extracellular signal-regulated kinase (ERK), while phosphorylated p38 and c-Jun N-terminal kinase (JNK) were increased [4][33]. The authors used small molecule inhibitors in combination with genistein to view the effects on cell viability. Cell viability was significantly decreased (compared to cells treated with genistein alone) when both cervical cancer cell lines were treated with genistein and PD98059 (ERK inhibitor), while cell viability was increased (compared to cells treated with genistein alone) when SP600125 (p38 inhibitor) and genistein were used. These results suggest that genistein inhibits cell growth and decreases cell viability through decreasing the phosphorylation/activation of ERK, while increasing phosphorylation/activation of p38 and JNK [4][33].
Treatment of HeLa, CaSki, and C33A cervical cancer cells with 5–60 µM of genistein for 48 h resulted in inhibition of cell growth and decreased cell viability in all three cell lines, with the greatest response seen in HeLa cells [5][34]. Following treatment with genistein, the percentage of apoptotic cells (measured by flow cytometry) was increased in a dose-dependent manner, while the expression of pro-caspase-3, -8, and -9 was decreased. In line with these findings, the levels of cleaved PARP and the proapoptotic protein Bid (total) decreased while Bax levels were increased. Levels of Bcl2 remained unchanged (Table 1) [5][34].
Squamous cervical cancer cells (SiHa) treated with genistein showed reduced viability (IC50 value of 80 µM) [6][35]. Ethidium bromide staining revealed the formation of apoptotic bodies in these cells following genistein treatment, and internucleosomal DNA fragmentation was observed, consistent with apoptosis induction. Tumor-suppressor genes can be silenced by methylation; genistein (20 µM) was able to reverse RARβ2 tumor suppressor gene methylation after 72 h and continued demethylation for six days. Genistein treatment also increased mRNA expression of RARβ2 in SiHa cells [6][35]. This researchtudy provides evidence for genistein to not only act as an anti-cancer agent through the induction of apoptosis but also through its ability to de-methylate the tumor suppressor RARβ2 leading to its re-activation. This is an important finding, as re-activation of tumor suppressors is a possible target for cancer therapies.
Genistein treatment decreased viability of HeLa cells and caused nuclear morphological changes characteristic of apoptosis: nuclear condensation, fragmentation, blebbing, and the appearance of apoptotic bodies [7][36]. Flow cytometry showed an increased number of cells in the G2/M phase following genistein treatment, suggesting cell cycle arrest at this stage. Treatment with genistein inhibited HeLa cell migration (wound closure assay). These results were correlated with a decrease in mRNA expression of MMP-9 and an increase in TIMP-1 expression [7][36].
HeLa cells treated with genistein resulted in decreased cell viability an effect that was associated with decreased expression of nuclear factor kappa B (NF-kB) p65 subunit [8][37]. Furthermore, phosphorylated levels of Akt, mammalian target of rapamycin (mTOR), p70S6K1, and Eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1) were significantly reduced with genistein treatment. These data suggest that genistein is able to decrease the rate of cell growth and proliferation, while potentially inducing apoptosis (Table 1) [8][37]. While this researchtudy indicates genistein’s ability to downregulate cell growth, and proliferation through inhibition of Akt- mTOR signaling, it fails to evaluate its ability to induce apoptosis or autophagy by examining caspases, PARP cleavage, and LC-3.
Cervical cancer cells (HeLa) treated with varying doses of genistein (0–100 µM) for 48 h were seen to have reduced cell viability and induction of apoptosis [9][38] as evidenced by the increased levels of cleaved caspase-3 and cleaved PARP. Furthermore, endoplasmic reticulum (ER) stress was shown by the increase in protein levels of GRP78, a molecular marker for ER stress, and C/EBP homologous protein (CHOP), a transcription factor [9][38]. These data suggest that genistein has the ability to induce apoptosis by increasing ER stress in HeLa cervical cancer cells.
Genistein (5 µM) decreased the viability of HeLa cervical cancer cells, an effect that was associated with increased expression of cleaved caspases -9 and -3, reduced mitochondrial membrane potential, and DNA fragmentation [10][39].
Treatment of HeLa cells with genistein resulted in inhibition of proliferation and migration that was associated with reduced levels of phosphorylated protein focal adhesion kinase (FAK) and paxillin, which are both involved in migration and invasion [11][40]. Moreover, β-catenin and vimentin levels, proteins involved in migration and metastasis, were reduced. Phosphorylation/activation of p38 and p42/44 mitogen activated protein kinase (MAPK) were also reduced with genistein treatment. mRNA expressions of FAK, paxillin, Snail, and twist were reduced with genistein treatment, suggesting suppression of the epithelial to mesenchymal transition (EMT) [11][40]. In another study, HeLa and CaSki cervical cancer cells treated with genistein had significantly reduced cell viability [12][41].
Sundaram et al. (2020) treated HeLa cervical cancer cells with 50 µM of genistein for 24 and 48 h and found increased cell cycle arrest, nuclear condensation, fragmentation, and the formation of apoptotic bodies [13][42]. Flow cytometry revealed an accumulation of cells in the G0 phase and cell cycle arrest in the G2/M phase in a dose-dependent manner. Nitric oxide (NO) levels were seen to increase significantly (2.37 µM) in cells treated with genistein. Genistein treatment upregulated many pro-oxidants while downregulating antioxidants at the transcript level (Table 1) [13][42].
HeLa cells treated with genistein (12.5, 25, 50, and 100 µM) had reduced proliferation and colony formation [14][43]. In addition, treatment with genistein caused a significant decrease in cell adhesion and migration/invasion (assessed with a wound-healing and a trans-well assay). RNA sequencing was performed to elucidate the mechanisms involved in these anti-cancer effects of genistein. Differentially expressed genes (DEGs) that were downregulated were associated with ribosomal subunits, metabolic processes, and mitochondrial translation. Western blot analysis showed a significant decrease in phosphorylated FAK and paxillin and total β-catenin, and vimentin protein levels with genistein treatment. A decrease in mRNA levels of FAK, PAXILIN, Snail, and TWIST was also seen with genistein treatment [14][43]. Based on these data, the authors concluded that genistein has anti-proliferative and anti-metastatic properties against cervical cancer through inhibition of the FAK/paxillin pathway.
While all the above studies provide evidence that genistein inhibits proliferation and survival of cervical cancer cells in vitro, one study suggests it may promote the growth of cervical cancer cells.
Chen et al. (2018) found that HeLa cervical cancer cells treated with low concentrations of genistein (0.001–1 µM) showed an increase in cell proliferation [15][44]. Genistein altered the cell cycle by decreasing the portion of cells in G1 phase while increasing the portion of cells in the S phase. The apoptotic rate was significantly lower in genistein-treated cells. Western blot analysis revealed an increase in estrogen receptor alpha ERα, phosphorylated Akt, and nuclear NF-kB p65 protein levels with 0.1 µM genistein treatment [15][44]. The effects of genistein were attenuated in the presence of an inhibitor of ERα (MPP), a phosphoinositide 3 kinase (PI3K)-Akt inhibitor (LY294002), or a nuclear NF-kB p65 inhibitor (PDTC), indicating that the increased cell viability seen with genistein is due to the activation of ERα-PI3K/Akt -NF-kB p65 signaling cascade.
Table 1.
Effects of genistein against cervical cancer: in vitro studies summarized.
| Cell Line | Genistein Concentration/Duration | Effect | Reference |
|---|---|---|---|
| HeLa ME-180 |
IC50 35 and 60 µM | ↑ Cell cycle arrest S phase (HeLa) ↑ Cell cycle arrest G2/M phase (ME-180) ↓ Invasion ↑ Apoptosis |
[1][30] |
| ME-180 CaSki |
10–40 µM 6 days (colony formation) 48 h |
↓ Colony formation ↑ Cytochrome c |
[2][31] |
| HeLa | 25–150 µM 48 h |
↓ Cell survival ↑ Cell cycle arrest ↑ PARP cleavage ↓ Cyclin B1 ↓ cdc2 ↓ P-Tyr cdc2 |
[3][32] |
| HeLa CaSki |
5–80 µM 24–48 h |
↓ Cell growth ↓ Cell viability ↓ p-Akt ↓ p-ERK ↑ p-p38 ↑ JNK |
[4][33] |
| HeLa CaSki C33A |
5–60 µM 24–48 h |
↓ Cell viability ↑ Apoptosis ↓ Procaspase-3, -8, -9 ↑ Cleaved PARP ↓ Bid ↑ Bax |
[5][34] |
| SiHa | 80 µM 48–72 h |
↓ Cell viability ↑ Apoptosis ↓ RARβ2 methylation |
[6][35] |
| HeLa | 75, 100, and 150 µM 24 and 48 h |
↓ Cell viability ↑ Apoptosis G2/M Cell cycle arrest ↓ Migration ↓ MMP-9 mRNA expression ↑ TIMP-1 mRNA expression |
[7][36] |
| HeLa | 25 µM 24 h |
↓ Cell viability ↓ NF-kB p65B ↓ p-mTOR ↓ p-p70S6K1 ↓ p-4E-BP1 ↓ p-Akt |
[8][37] |
| HeLa | 0–100 µM 48 h |
↓ Cell viability ↑ Apoptosis ↑ Cleaved caspase-3 ↑ Cleaved PARP ↑ GRP78 ↑ CHOP |
[9][38] |
| HeLa | 5 µM 24–72 h |
↓ Cell viability ↑ Cleaved caspase -9 ↑ Cleaved caspase -3 ↑ DNA fragmentation |
[10][39] |
| HeLa | 0–150 µM 24–48 h |
↓ Proliferation ↓ Migration ↓ Invasion ↓ p-FAK ↓ p-paxillin ↓ p-p38 ↓ p-p42/44 ↓ β-catenin ↓ Vimentin ↓ p-paxillin mRNA levels ↓ p-p38 mRNA levels ↓ Snail mRNA levels ↓ twist mRNA levels |
[11][40] |
| HeLa CaSki |
120–180 µM 24 h |
↓ Cell Viability | [12][41] |
| HeLa | 50 µM 48 h |
↑ Cell cycle arrest G2/M phase ↑ Nuclear condensation ↑ Nuclear fragmentation ↑ NO levels |
[13][42] |
| HeLa | 0.01–1 µM 48–72 h |
↑ Cell proliferation ↑ S-phase arrest ↓ Apoptosis ↑ p-Akt ↑ Erα ↑ Nuclear NF-kB |
[15][44] |
Table legend: ↑ increased, ↓ reduced, p- phosphorylated.
Collectively, these in vitro studies provide evidence of inhibition of cervical cancer cell proliferation and survival and induction of apoptosis with genistein treatment. These effects were associated with inhibition of FAK, ERK, Akt, mTOR, and BID while the apoptotic markers Bax and cleaved caspases and PARP were enhanced (Figure 12). p38 was found to be activated by genistein in one study [4][33] and inhibited in another [11][40]. The reasons for these differences are not clear, as both studies examined the effects in the same cervical cancer cells (HeLa). Furthermore, genistein inhibited cervical cancer cell migration, an effect that was correlated with enhanced MMP-9 protein levels (Figure 12).
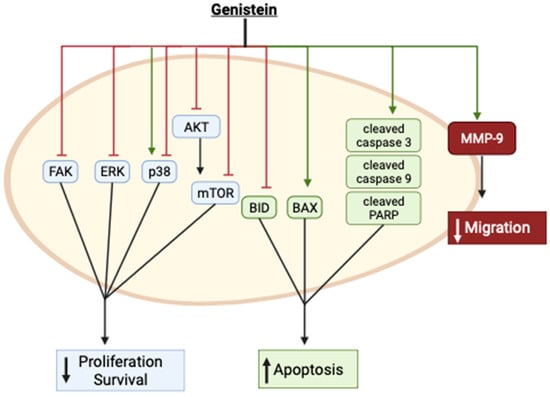
Figure 12.
Summary of the effects of genistein in cervical cancer cells in vitro. The image was created using
2. Genistein Analogues and Nanoparticles against Cervical Cancer: In Vitro Studies
Genistein has low solubility and bioavailability. In an effort to increase bioavailability and drug uptake, which will increase genistein’s effectiveness as an anti-cancer drug, some researchers have examined genistein analogs or nanoparticle delivery options.
Nanoparticle technology has been employed for the encapsulation and delivery of compounds with low absorption and bioavailability. Genistein-loaded nanoparticles using inulin-stearic acid have shown potent and specific toxicity against human colorectal cancer cells [16][45]. In addition, genistein encapsulated in zein/chicory polysaccharide nanoparticles had a greater inhibition of hepatic (HepG2) cell proliferation when compared to parent genistein [17][46]. Studies which examined genistein analogues and conjugates against cervical cancer are presented and summarized below and in Table 2.
Xiong et al. (2015) synthesized seven analogues of genistein and examined their effects in human cervical cancer (HeLa) cells. Each of the analogues had a much lower inhibitory effect on HeLa cell proliferation than the parent compound. The cell growth inhibition ranged from 3.4 to 13.1% when exposed to 1 µM of the analogues and 8.5 to 26.8% with 10 µM, as compared to the parent compound genistein which showed a cell growth inhibition of 9.9% (1 µM) and 54.0% (10 µM) (Table 2) [18][47]. Genistein’s effect on proliferation had IC50 value of 10.0 ± 1.5 µM. The IC50 values of the different analogues were in the range of 51.3 µM ± 5.0 to 1000 µM. These high IC50 values clearly suggest the analogues are much less effective. The data of this researchtudy indicate that the parent compound, which contains hydroxyl groups located at carbon-5 and carbon-7, is more effective, indicating an important role of these hydroxyl groups on its antiproliferative activity [18][47].
Genistein was encapsulated in folic-acid-conjugated chitosan nanoparticles (FGCN) with a hydrodynamic diameter less than 200 nm, suggesting they are small enough to penetrate tumor tissues and resist rapid clearance. Cervical cancer cells, HeLa, had significantly decreased cell viability when treated for 24 h with FGCN compared to cells treated with free genistein (IC50 14.6 µg/mL and 33.8 µg/mL, respectively) [19][48]. Cell number was decreased following treatment with FGCN, and visual examination revealed rounded cells suggesting damage and/or cell stress. Flow cytometry analysis showed a significant increase in the percentage of apoptotic cells following treatment with FGCN compared to free genistein. Fluorescence analysis showed a significantly higher cellular uptake of conjugated genistein over four hours [19][48]. Based on these findings, the authors concluded the folic acid-conjugated chitosan nanoparticles increased the toxicity of genistein to the cervical cancer cells. Folic acid receptors are present in high numbers on cervical cancer cells, so conjugating the nanoparticles to folic acid increases the affinity to folic acid receptors, thereby increasing drug uptake to the cells and increasing the biological effects. This paper provides evidence for the benefits of conjugating natural compounds to molecules with known cell membrane receptors to increase their effectiveness as cancer treatments.
Table 2.
Effects of genistein analogues and nanoparticles against HeLa cervical cancer studies summarized.
| Cell Line | Nanoparticle/Conjugate | Effect | Reference |
|---|---|---|---|
| HeLa | 1 & 10 µM 3 days |
↓ Proliferation ↑ Inhibitory rate |
[18][47] |
| HeLa | Folic acid chitosan conjugate | ↓ Cell viability ↑ Apoptosis |
[19][48] |
Table legend: ↑ increased, ↓ reduced.
