Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Takuro Kanekura and Version 2 by Mona Zou.
CD147/Basigin, a transmembrane glycoprotein belonging to the immunoglobulin superfamily, is a multifunctional molecule with various binding partners. CD147 binds to monocarboxylate transporters (MCTs) and supports their expression on plasma membranes. MTC-1 and MCT-4 export the lactic acid that is converted from pyruvate in glycolysis to maintain the intracellular pH level and a stable metabolic state. Under physiological conditions, cellular energy production is induced by mitochondrial oxidative phosphorylation. Glycolysis usually occurs under anaerobic conditions, whereas cancer cells depend on glycolysis under aerobic conditions. T cells also require glycolysis for differentiation, proliferation, and activation. Human malignant melanoma cells expressed higher levels of MCT-1 and MCT-4, co-localized with CD147 on the plasma membrane, and showed an increased glycolysis rate compared to normal human melanocytes. CD147 silencing by siRNA abrogated MCT-1 and MCT-4 membrane expression and disrupted glycolysis, inhibiting cancer cell activity. Furthermore, CD147 is involved in psoriasis. MCT-1 was absent on CD4+ T cells in CD147-deficient mice. The naïve CD4+ T cells from CD147-deficient mice exhibited a low capacity to differentiate into Th17 cells. Imiquimod-induced skin inflammation was significantly milder in the CD147-deficient mice than in the wild-type mice. Overall, CD147/Basigin is involved in the development of malignant tumors and T-cell-mediated immunological disorders via glycolysis regulation.
- CD147
- glycolysis
- malignant tumor
- psoriasis
- immune disorder
1. CD147/Basigin
1.1. Discovery of CD147/Basigin
Cell surface glycoproteins regulate cellular activities such as proliferation, differentiation, adhesion, migration, and transmembrane transportation [1][2][12,13]. CD147/Basigin was cloned as a carrier of Lewis X, a cell surface carbohydrate antigen expressed in embryonal carcinoma (EC) cells using the λgt11 expression library of F9 EC cells in our study of developmentally regulated cell surface markers [3][1]. CD147/Basigin is a transmembrane glycoprotein comprising two immunoglobulin (Ig)-like extracellular domains, a single transmembrane domain, and a short C-terminal cytoplasmic tail [3][1]. Extracellular Ig-like domains have strong homology with the Ig variable domain and the major histocompatibility complex class II β-chain. Based on the structural analysis, CD147/Basigin was identified as a new member of the Ig superfamily [3][1]. CD147/Basigin is expressed at similar levels in various adult organs, mouse embryos, and EC cells. Because of its broad distribution, the researchers considered that this molecule has basic or fundamental functions and termed “Basigin (Bsg)” as an abbreviation of basic immunoglobulin [3][1]. It is located on chromosome 19 at p13.3 and consists of 10 exons spanning approximately 12 kb [4][14]. The molecular weight of this protein portion is 28 kDa. CD147 has three asparagine (ASN) glycosylation sites on its Ig-like domains and is highly glycosylated. The molecular weights of the glycosylated forms ranged from 43 to 66 kDa. Their diverse molecular weights are due to the different modes of the glycosylation of molecules from various organs [5][2].
1.2. Discovery of Related Molecules
Identical molecules have been found independently from various perspectives in several laboratories and are given different names: M6, extracellular matrix metalloproteinase inducer (EMMPRIN), HT7, neurothelin, 5A11, gp42, OX-47, and CE9 [6][7][8][9][10][11][12][13][15,16,17,18,19,20,21,22]. Among these, M6 was cloned using peripheral granulocytes from patients with rheumatoid arthritis (RA) and was identified as a human leukocyte activation antigen [6][15]. EMMPRIN, previously known as tumor cell collagenase stimulatory factor (TCSF) [14][23], is implicated in the induction of matrix metalloproteinases (MMPs). Its expression is enhanced in various human carcinomas, including MM, and correlates with tumor progression and invasion by inducing the production of MMPs by stromal fibroblasts [15][16][17][18][19][20][24,25,26,27,28,29]. HT7 is a highly glycosylated protein localized in brain endothelial cells that is a receptor involved in cell surface recognition at the blood–brain barrier (BBB) [8][17]. Neurothelin is an inducible cell surface glycoprotein of BBB-specific endothelial cells and distinct neurons and regulates the interaction between vascularization and neuronal differentiation [9][18]. Fadool and Linser reported that 5A11 is a functional molecule involved in neural–glial mutual recognition in the avian neural retina [10][19]. A murine fibroblast membrane glycoprotein, gp42, is a fibronectin-receptor-associated antigen involved in neural cell adhesion [11][20]. OX-47 is a lymphocyte activation antigen. It presents in lymphocytes whose expression is markedly induced on activation with mitogens [12][21]. CE9 is a posterior-tail domain-specific integral plasma membrane glycoprotein cloned from a rat spermatozoon and plays an important role in spermatogenesis [13][22]. These studies suggest that CD147/Basigin is a multifunctional molecule involved in various physiological and pathological phenomena [21][30].
The gene and protein names given to these molecules are Basigin both in humans (Locus Link) and mice (Mouse Genome Informatics) and the symbol provided by the Human Genome Organization is BSG in humans [4][14] and Bsg in mice [22][31]. Because Basigin is expressed in leukocytes, the Cluster of Differentiation Nomenclature “CD147” was given at the 5th International Workshop in 1993 [23][32].
2. Chaperone-Like Function of CD147/Basigin
Previous studies have revealed that CD147 has various binding partners such as cyclophilin A (CyPA) [24][33], integrins [25][26][34,35], P-glycoprotein [1][21][27][12,30,36], and MCTs [28][4]. The pleiotropic functions of CD147 are attributable to its binding partners. CD147 acts as a chaperone for their proper plasma membrane expression and catalytic activity and participates in many pathophysiological processes through these molecules [1][21][12,30].
CyPA, the major target of the immunosuppressive drug cyclosporin A, is a ubiquitously distributed intracellular protein. CyPA is secreted by cells in response to inflammatory stimuli and is a potent neutrophil and eosinophil chemoattractant. In the inflammation process, CD147 acts as a cell surface receptor for CyPA and initiates signaling cascades leading to ERK activation [24][33]. CD147 binds to β1-integrin, which, in turn, induces the aggregation of promonocyte line U937 cells via protein tyrosine phosphorylation. The antibody against CD147 inhibits the aggregation and the tyrosine phosphorylation by blocking the binding between β1-integrin and CD147 [25][34]. The multidrug resistance (MDR) of cancer cells is often associated with the overexpression of P-glycoprotein (P-gp), a transmembrane ATP-dependent transporter. The expression of P-gp and CD147 is upregulated in the adriamycin (ADR)-resistant human mammary carcinoma cell line MCF7 (MCF7/Adr) compared to its non-ADR-resistant counterpart MCF7. The silencing of CD147 in MCF7/Adr by siRNA targeting CD147 resulted in the downregulation of P-gp expression and a reduction in drug resistance [27][36].
The association between CD147 and MCTs yielded important clues for understanding the role of CD147. Cross-linking studies showed that CD147 forms a homo-oligomer [29][37]. Homodimerized CD147 binds to two MCT monomers and facilitates its proper folding and expression on the cell membrane (Figure 1). In MCT-transfected COS cells, expressed MCT proteins accumulate in the perinuclear compartment, whereas co-transfection with CD147 cDNA enables the expression of functional MCT-1 or MCT-4 on the plasma membrane [28][4]. As mentioned previously, CD147 consists of two Ig-like extracellular domains: a single transmembrane domain and a short cytoplasmic tail at the C-terminus [3][1]. MCT-1 attaches itself directly to the transmembrane domain of CD147 [21][30][30,38]. The cryo-EM structure of the CD147/MCT complex has been determined [31][32][39,40] and Glu218 in the CD147 transmembrane domain is the binding site for MCT-1 [31][33][39,41]. Philp et al. demonstrated the role of CD147 in the retina, in association with MCTs [34][42]. CD147-deficient mice exhibit visual impairment. Their morphology of the retina at the light microscopic level and the fundus and the fluorescein fundus angiography appeared to be normal until 8 weeks of age, whereas the amplitude of all components of both the photopic and scotopic electroretinograms was decreased, indicating that both rod and cone functions were severely affected [35][43]. In these mice, the cell membrane expression of MCT-1 and MCT-4 was greatly reduced in retinal photoreceptor cells and adjacent Müller cells. Müller cells are retinal glial cells, whose major role is to maintain the functional and structural stability of photoreceptor cells. Müller cells provide photoreceptor cells with lactate as fuel for normal functions [34][42]. Because of the absence of MCTs on the membrane of facing cells, the flux of lactate from Müller cells to photoreceptor cells is disrupted, and photoreceptor cell activity is lost owing to energy depletion [34][42]. The absence of CD147 results in the impaired expression of MCTs on the cell membrane, leading to loss of vision.
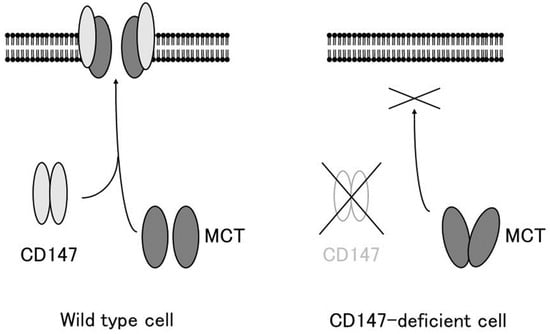
Figure 1. CD147 associates with MCT-1 and MCT-4 and facilitates their expression in the plasma membrane. In the absence of CD147, MCTs accumulate in the cytosol and are not expressed on the plasma membrane (modified from [36]).
CD147 associates with MCT-1 and MCT-4 and facilitates their expression in the plasma membrane. In the absence of CD147, MCTs accumulate in the cytosol and are not expressed on the plasma membrane (modified from [7]).
Other binding partners of CD147 include glucose transporter-1 (GLUT1), CD44, the major hyaluronan receptor, CD43, CD98, γ-secretase, NOD2, γ-catenin, platelet glycoprotein VI (GPVI), and apolipoprotein D. Molecular interactions between CD147 and these binding partners have been previously documented [21][30].
3. CD147 and Glycolysis
The elucidation of the mechanism of lactate flux by CD147 and MCTs in the retina prompted us to investigate the role of CD147 in glycolysis. Glycolysis is the enzymatic conversion of glucose to pyruvate to generate energy, which is stored in the form of ATP. Pyruvate is further converted to lactic acid, which is exported through the plasma membrane and is required for metabolism and intracellular pH regulation [30][38]. Lactic acid is transported by proton-linked/lactate co-transporters, MCTs, on the plasma membrane. The detailed metabolic pathway of glycolysis was initially studied in cancer cells. Under physiological conditions, cellular energy is provided by mitochondrial oxidative phosphorylation, and glycolysis results from anaerobic enzymatic conversion. Warburg first reported that cancer cells depend on glycolysis for energy even in the presence of oxygen, i.e., under aerobic conditions [37][3]. They take up excess glucose through GLUT-1 or GLUT-3, which is then enzymatically metabolized to ATP to drive the pathological processes involved in cell division and growth [38][44]. In cancer cells, pyruvate is transformed into lactic acid during aerobic glycolysis and then released from the cytoplasm into the extracellular milieu by means of MCTs. Altered metabolism requires tumor cells to rapidly efflux lactate into the surrounding microenvironment to prevent self-poisoning. MCTs facilitate proton-linked monocarboxylate transport, leading to a decrease in the extracellular pH of tumors. The acidity of the tumor microenvironment produces more aggressive phenotypes in cancer cells that exhibit increased proliferation, invasiveness, metastasis, and VEGF production [39][40][41][42][45,46,47,48].
4. CD147 in Cancer Cell Glycolysis
Based on the observation in the retina, the researchers investigated the involvement of CD147 in cancer cell glycolysis using MM cells. Human MM cells (A375) expressed higher levels of MCT-1, MCT-4, and CD147 and showed an increased glycolysis rate compared to normal human melanocytes. CD147 co-localized with MCT-1 and MCT-4 on the A375 cell membrane. CD147 silencing by siRNA abrogated MCT1 and MCT-4 expression and their co-localization with CD147. The glycolysis rate and ATP production were dramatically decreased and the extracellular pH increased. Subsequently, cell proliferation, invasiveness, and VEGF production were significantly inhibited [43][5]. Gallagher et al. documented similar findings in the highly metastatic breast cancer cell line MDA-MB-231. In accordance with the findings in MM, lactate efflux was mediated by MCTs and the accessory subunit, CD147. CD147 was highly expressed in MDA-MB-231 cells and its expression was linked to MCT expression. MCT-4 mRNA and protein expression were increased in MDA-MB-231 cells compared to cells derived from normal mammary tissue. MCT-4 co-localized with CD147 in the plasma membrane. CD147 silencing resulted in the loss of MCT4 in the plasma membrane and the accumulation of the transporter in endo-lysosomes. On the other hand, the silencing of MCT-4 impaired the maturation and trafficking of CD147 to the cell surface, resulting in the accumulation of CD147 in the endoplasmic reticulum [44][49]. Recent studies revealed the involvement of CD147 in cancer cell glycolysis in various malignant tumors including non-small-cell lung cancer, hepatocellular carcinoma, colorectal cancer, prostate cancer, and anaplastic large-cell lymphoma [45][46][47][48][49][50][50,51,52,53,54,55]. These findings strongly suggest that CD147 interacts with MCT-1 and MCT-4 to promote glycolysis in tumor cells, resulting in tumor progression (Figure 2).
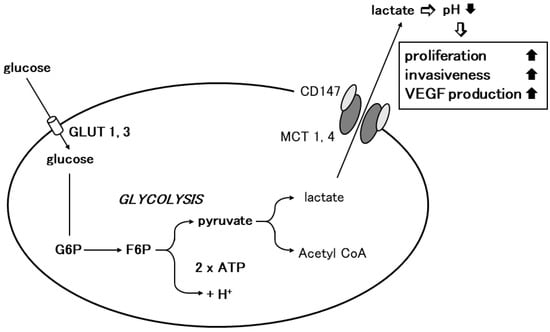
Figure 2. Involvement of CD147 in tumor cell glycolysis. Glycolysis is the enzymatic conversion of glucose to pyruvate to produce ATP. Cancer cells depend on glycolysis for energy aerobic conditions. Cancer cells take up excess glucose through GLUT-1 or GLUT-3, which is enzymatically converted to ATP to provide energy for pathophysiological processes such as cellular growth and proliferation. Through MCT-1 or MCT-4, pyruvate is further transformed into lactic acid during aerobic glycolysis in cancer cells, which is then released from the cytoplasm into the surrounding extracellular milieu. Homodimerized CD147 associates with two monomers of MCT1 or 4 and regulates lactate transport (modified from [36][7]).
5. T Cell Differentiation/Proliferation and Glycolysis
Glycolysis is also important for the differentiation, proliferation, and activation of lymphocytes, including T cells, B cells, and natural killer cells. Activated lymphocytes engage in robust growth and rapid proliferation. For these processes, lymphocytes adopt glycolysis [51][8]. Stimulated CD4+ T cells differentiate into effector T cells or inducible regulatory T cells. The differentiation of CD4+ T cells into distinct subsets, Th1, Th2, and Th17 cells, requires aerobic glycolysis. Th1, Th2, and Th17 cells express high surface levels of GLUT-1 and are highly glycolytic [52][56]. Halestrap and Wilson demonstrated the importance of MCT-1 in T cell activation and proliferation. In T cells, energy metabolism is largely glycolytic even under aerobic conditions, and lactic acid efflux from T cells is mediated by MCT-1. MCT-1 is important during the activation and proliferation of resting T cells, which is accompanied by a switch from aerobic to glycolytic metabolism and a remarkable increase in lactate production and export [53][57]. In line with this, Murray et al. showed that a strong and specific inhibitor targeting MCT-1 functioned as an immunosuppressive drug and prevented T cell proliferation [54][58]. T cell glycolysis is regulated by CD147 because MCT-1 requires CD147 as an ancillary protein in T cells [53][57]. Studies on the role of CD147 in T cell biology by Hahn et al. documented that energy metabolism depends on CD147, which is also involved in T cell development, activation, proliferation, migration, invasion, and adhesion. The rapid proliferation and activation of T cells require glycolysis instead of oxidative phosphorylation to respond to their energy demands. Although glycolysis generates energy faster than oxidative phosphorylation, energy production is less efficient and leads to the intracellular accumulation of lactic acid. CD147 plays a role in alleviating lactate efflux for cell stability [55][59]. In patients with RA, CD147 mRNA expression was elevated in peripheral blood mononuclear cells. Th17 cell differentiation from CD4+ T cells is facilitated by CD147 and induces the production of Th17-secreting cytokine, interleukin (IL)-17, and Th17-differentiation-regulating cytokines, IL-6 and IL-1β [56][60]. These results strongly imply a role for CD147 in the pathophysiology of immune disorders mediated by T cells.
