Professional divers exposed to pressures greater than 11 ATA (1.1 MPa) may suffer from high-pressure neurological syndrome (HPNS). Divers who use closed-circuit breathing apparatus and patients and medical attendants undergoing hyperbaric oxygen therapy (HBOT) face the risk of CNS hyperbaric oxygen toxicity (HBOTx) at oxygen pressure above 2 ATA (0.2 MPa). Both syndromes are characterized by reversible central nervous system (CNS) hyperexcitability, accompanied by cognitive and motor deficits, and N-methyl-D-aspartate receptor (NMDAR) plays a crucial role in provoking them. Various NMDAR subtypes respond differently under hyperbaric conditions. The augmented currents observed only in NMDAR containing GluN2A subunit increase glutamatergic synaptic activity and cause dendritic hyperexcitability and abnormal neuronal activity. Removal of the resting Zn2+ voltage-independent inhibition exerted by GluN2A present in the NMDAR is the major candidate for the mechanism underlying the increase in receptor conductance.
- NMDAR
- HPNS
- HBOTx
- high pressure
1. Introduction
- Introduction
Pressure, like temperature, is one of the fundamental physical factors affecting living organisms. Most humans are physiologically adapted to live at an ambient atmospheric pressure of 1 ATA (the average atmospheric pressure exerted at sea level, 0.1 MPa). However, some are exposed to pressures as low as 0.3 ATA (0.03 MPa) on the summit of Mt. Everest at an altitude of 8848 m or as high as 70 ATA (7.09 MPa) at a depth of 690 m under the ocean surface [1]. Although only relatively few highly trained personnel are exposed to these extreme environments, many others frequently encounter levels of hyperbaric pressures (HP, i.e., more than 1 ATA) on a more regular basis. Recreational divers using an underwater breathing apparatus (SCUBA), professional divers (for the oil and salvage industry), and combat divers; patients and medical attendants undergoing hyperbaric oxygen therapy (HBOT); and working in the compressed atmosphere of a subterranean environment such as tunnelling [2].
Organisms exposed to HP develop behavioral and physiological changes such as hyperexcitability, tremors, myoclonus, and complete seizures. The compression rate governs the type of adaptation mechanisms available in response to HP, which only cope with relatively slow rates. It has been shown that, for humans, a compression of 50–60 ATA would require between 5 and 6 days to return to a fully functioning state [3]. Moreover, the adaptation process is not linear—the higher the pressure, the longer adaptation takes at a steady state. For any given species, there is a pressure beyond which adaptation cannot compensate for pressure effects. Approximately 1000 ATA (10 km sea water) appears to be the ultimate limit for the feasibility of any complex life form since higher pressures would dictate protein coagulation and total inhibition of enzymatic activity. The less complex a life form with a less complex nervous system, the more easily it adapts to HP.
On the other hand, over 20 species of marine mammals routinely dive to extreme depths, ranging between 600 and over 2000 m (60–200 ATA) for as long as 20 up to 120 min [4][5][6][7][4,5,6,7]. These species have evolved modifications to critical physiological processes that allow their body, specifically their nervous system, to withstand these great pressures.
In contrast, professional deep divers exposed to pressures greater than 11 ATA exhibit reversible central nervous system (CNS) hyperexcitability, tremor, myoclonic jerking [8][12], somnolence, EGG changes, visual disturbance, nausea, dizziness, and decreased cognitive performance [9][10][13,14]. This constellation of signs and symptoms is termed High Pressure Neurological Syndrome (HPNS) [11][12][13][14][8-11].
Another difficulty for human divers is that exposure to O2 partial pressure above 2 ATA may cause also CNS hyperexcitability, convulsions, and loss of conscience, which are usually reversible [15][16][17][18][26-29]. This response is is termed Hyperbaric Oxygen Toxicity (HBOTx).
2. NMDAR Subtypes and Their Response to Pressure
- NMDAR Subtypes and Their Response to Pressure
The NMDAR belongs to the family of ionotropic glutamate receptors that mediate the majority of excitatory neuronal transmission within the CNS [19][20][31,32]. Three families of NMDAR subunits have been identified [21][22][23][33,34,35]: GluN1 contains eight distinct isoforms (GluN1-1a to 4a and GluN1-1b to 4b) owing to RNA splicing [24][36]; the GluN2 family is composed of four members (GluN2A to D) encoded by four different genes; GluN3 subunits (GluN3A and GluN3B) arise from two separate genes. NMDARs function as heterotetrameric assemblies (see Figure 1), usually associating two GluN1 and two GluN2 subunits (GluN1⁄GluN2 complexes) or a mixture of GluN1, GluN2, and GluN3 subunits [20][25][26][32,37,38].
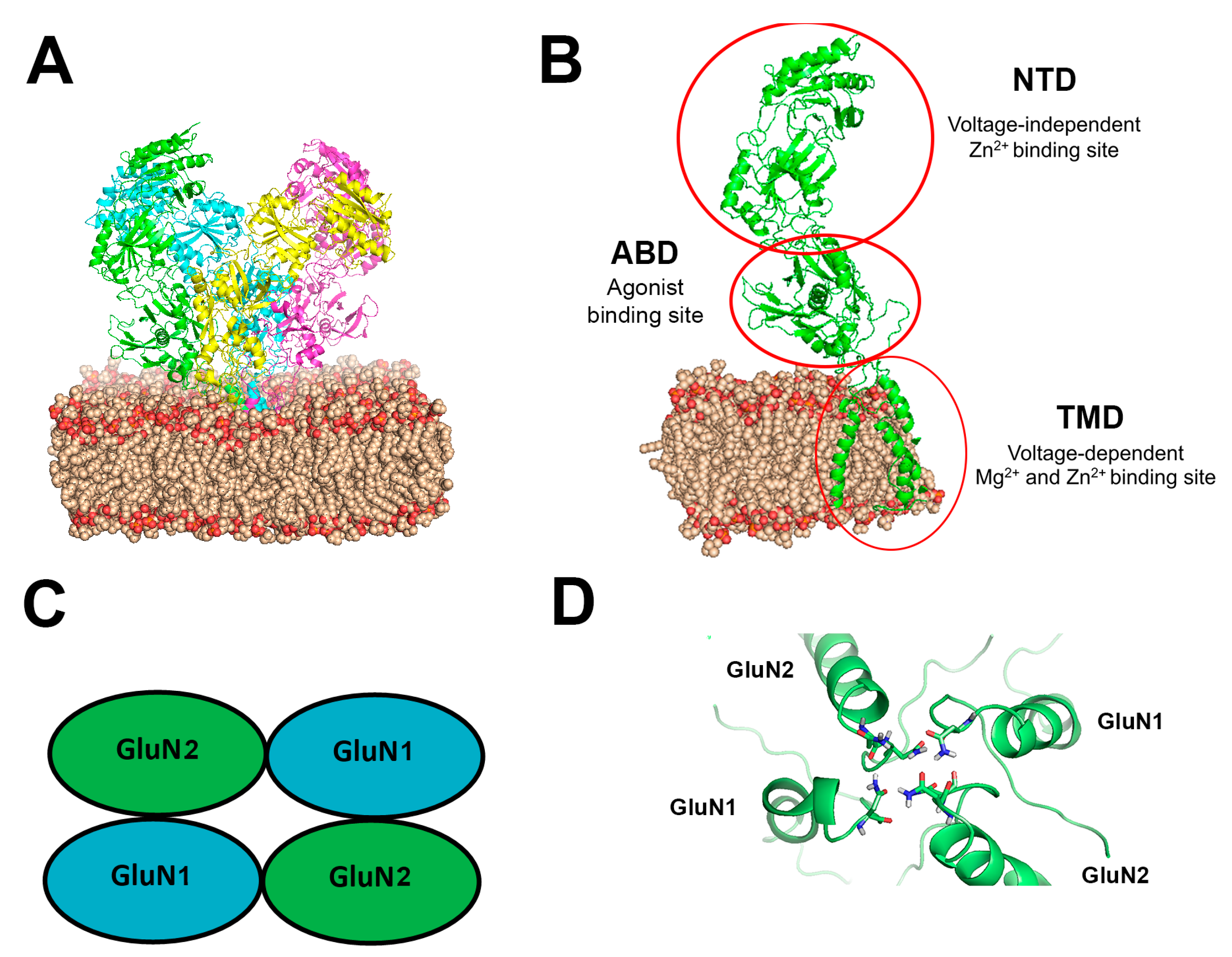
Figure 1. (A) Simulated NMDAR embedded in a DOPC lipid bilayer at 1 ATA pressure. The NMDAR is shown as a cartoon colored with chains of the four subunits (green, magenta, yellow, cyan), DOPC is presented as spheres in wheat and red color (colored by element), (B) One subunit of the NMDAR in the membrane, important sites indicated with a red circle. All GluN subunits share a modular architecture that is composed of four distinct domains: the N-terminal domain (NTD), the agonist-binding domain (ABD) that binds glycine or d-serine in GluN1 and GluN3 and glutamate in GluN2, the transmembrane domain (TMD) containing the ion channel, and an intracellular C-terminal domain (CTD) (not shown in the figure). The NTD and CTD are the most divergent regions. (C) Schematic topology of the NMDAR and its subunits (D) NMDAR asparagine (Asn) residues coordinations at the Mg2+ site. Asn residues are shown as sticks colored by element on the M2 region of the TMD of the four subunits.
To date, there are abundant but incomplete data on the NMDAR subtypes’ spatial distribution and function(s) in the mammalian brain [19][25][27][28][29][31,37,40,41,42]. GluN2A and GluN2B are the predominant subunits in the adult CNS, particularly in higher brain structures (such as the hippocampus and cortex) [28][30][31][41,43,44], indicating that they have central roles in synaptic function and plasticity. In addition, in the hippocampus and cortex, tri-heteromeric GluN1/GluN2A/GluN2B receptors also populate, with estimates of abundance ranging from 15% to >50% of the total receptor population [32][33][34][45,46,47]. Tri-heteromeric GluN1/GluN2A/GluN2C and GluN1/GluN2B/GluN2D receptors have also been described [25][26][37,38].
NMDAR has been implicated with CNS hyperexcitability as part of HPNS. Neuropharmacological studies at HP have suggested increased NMDAR responses in CA1 pyramidal cells [35][36][37][38][39][40][17,18,19,20,21,22]. In the same brain region, electrophysiological studies showed a significant increase in the synaptic NMDAR response followed by postsynaptic excitability changes [41][42][48,49]. The first attempt to measure the NMDAR currents at HP directly was made by Daniels and his colleagues (1998), showing that HP increased the receptors’ currents expressed in Xenopus laevis oocytes.
Electrophysiological studies from aour laboratory [43][50] have revealed differential current responses under HP He (obtaining HP pressure with He gas) conditions in NMDAR subtypes that contain either GluN1-1a or GluN1-1b splice variants co-expressed in Xenopus laevis oocytes, with all four GluN2A/B/C/D subunits. Further study from our lab in which six GluN1 splice variants (GluN1-3a/b) were co-expressed with the GluN2A subunit (which is most abundant in adult brains and CNS and plays a crucial role in long-term potentiation (LTP) and learning) showed a different amount of increase in the ionic currents at HP He [44][51] or "dichotomic" (either increased or decreased) responses in GluN1-4a/b splice variant co-expressed with GluN2A/B [45][52]. The control current amplitudes and the relative increase under HP He conditions differed and depended on the GluN1 variant in the subtype analyzed. The study on GluN1 variants and previous studies on GluN2 subunits [45][46][40][34][41][42][15,22,47–49,52] indicate a very complex picture of NMDAR response under HP He conditions. A recent intriguing study [47]from our lab [30] on the GluN1-1a co-expressed with GluN2A/B showed at HBO (obtaining HP pressure with 2-6 ATA oxygen gas) the same response as under HP He, namely, an increase in the GluN1-1a+Glu2A subtype response and no change in the GluN1-1a+GluN2B subtype response.
3. The Mechanism of the NMDAR Hyperexcitation
- The Mechanism of the NMDAR Hyperexcitation
3.1. Voltage-Dependent Mg2+ and Zn2+ Inhibition
Di-heteromeric receptors that contain GluN2A or GluN2B are well known to have high voltage-dependent sensitivity to mM range [Mg2+]o blockade, i.e., strong membrane depolarization (induced via an alternative pathway) can remove this inhibition. The Mg2+ binding site (containing Asn residues, N and N C 1 sites) is located at the channel pore in the trans-membrane domain (TMD, see Figure 1D) of the receptor [48][49][53,54]. Mg2+ voltage-dependent sensitivity is controlled by a single GluN2 residue in the M3 segment [50][55], significantly affecting the relative contribution of NMDAR subtypes to synaptic integration and plasticity. Similarly, [Zn2+]o in the range of [mM] can inhibit NMDARs through a voltage-dependent channel block that appears to occur within the ion channel pore and may involve residues (e.g., Asn616 in the M2 region of GluN1 and Asn614/5 on GluN2) that are known to be associated with other divalent ions blockade [23][51][52][53][54][55][35,56,57,58,59,60].
3.2. NMDAR Characteristics: Affinity, Stoichiometry, Surface Expression
A change in NMDAR affinity to glutamate under HP He conditions should also be considered [56][61]. The NMDAR current was markedly increased (128%), but EC50 was not reported. During LTP, additional NMDARs are inserted into the membrane [57][65]. In theour experiments, a similar process may occur during the 15–20 min time required for HP stabilization. The immunoprecipitation experiment indicated no increase in surface expression of NMDARs in the oocyte membrane under HP He conditions [58][63]. Taken together with additional investigations [44][45][47][59][58][60]in our laboratory [30,51,52,62,64], it is conceivable to conclude that there are no HP-induced modifications in NMDAR affinity to glutamate, stoichiometry, aggregate formation, its reversal potential, and surface expression that could explain the increase in the response.
3.3. Structural Differences of the GluN1 Variants
GluN2A co-expressed with different splice variants of GluN1 showed different currents at normobaric and HP He and depended on the GluN1 variant [44][51]. The measurements showed that GluN1-1a and GluN1-2a mean current amplitudes saw a greater increase due to HP He than their corresponding “b” splice variants (see [44][51] Figure 5A). In contrast, for GluN1-3a and GluN1-4a, the opposite behavior was observed. This may indicate that there might be a possible functional relevance to the existence of the exon 5-encoded 21 amino acids extracellular N1 loop [61][36] (in the N terminal domain (NTD)), which is present in “b” types of GluN1 variants and absent in “a” types. However, other sequence differences exist between the GluN1 variants, which are located in the intracellular C terminal domain (CTD) and result from differential splicing of exons 21 and 22 [62][66]. GluN1-1 splice variants have the longest CTD due to the expression of both exon 21 and the long portion of exon 22. GluN1-2 variants do not express exon 21 but have the same long exon 22 as GluN1-1. GluN1-3 and GluN1-4 variants have much shorter CTDs since only a small portion of exon 22 is present, and only GluN1-3 contains exon 21. From theour experiments, scholarswe may postulate that CTDs are also involved in current modulation. In general, the observed effect of HP He-induced increased/decreased inward ionic currents through the different NMDAR subtypes can be sufficiently explained by observing the increase/decrease in input conductance (the slope of the I/V curve of each variant) of the oocyte under HP conditions (see [45][52] Figure 2B). The good correlation between these two parameters may support the ‘single switch’ hypothesis for HP ‘channel’ opening.
3.4. Voltage-Independent Zn2+ Inhibition
The GluN2A is the only member of the GluN2 family, which has harbors for Zn2+ ions on its NTD that can act in a voltage-independent manner to reduce the frequency of channel opening. The concentration of Zn2+ needed for that is <5 nM [56-58,66]. NMDAR containing GluN2A unit is the only subtype of NMDAR that showed a significant increase of the current under HP He and HBO conditions. Although all experiments discussed above were carried out in nominally zero Zn2+, contamination of Zn2+ in the nM range was probably present, which means that the Zn2+ inhibition site of GluN2A could be occupied. Indeed, a study [47]from our lab [30] showed that the elimination of Zn2+ by TPEN (a selective powerful Zn2+ chelator) at normobaric pressure increased the NMDAR current by 50%, revealing the presence of strong resting voltage-independent Zn2+ inhibition of the GluN1-1a + GluN2A receptor. Most importantly, the addition of TPEN under both HP He and HBO conditions failed to alter the currents. The lack of any additional response to TPEN strongly supports the notion that voltage-independent inhibition by Zn2+ had already been removed under both conditions. The absence of any effect of HP He and HBO on the GluN1-1a + GluN2B NMDAR, which lacks the Zn2+ voltage-independent inhibition site, greatly strengthens this hypothesis. However, the increase in current at 5.4 ATA HBO was only 35%, whereas at 51 ATA HP He was 63%. It appears that the removal of Zn2+ inhibition via HBO is less effective than HP He, or there is some additional component in the response to the HP He increase in currents.
4. Clinical Aspects and Considerations
- Clinical Aspects and Considerations
HPNS symptoms are usually reversible upon decompression (see introduction). An intriguing Norwegian report [63][16] has suggested that repetitive exposure to HP over the years may cause chronic memory and motor impairments in professional divers. The HP He that they may have been exposed to was either sub-threshold to HPNS or “supra-threshold”, but HPNS symptoms were antagonized by the use of narcotic gas mixtures (such as Trimix containing O2, N2, and He). ScholarsWe hypothesize that even if clear HPNS symptoms were not observed, the glutamate NMDAR response was still potentiated, causing more Ca2+ flow into the neurons. Through several signal transduction pathways, an overload of Ca2+ can activate metabolic cascades that deteriorate the neuron and eventually may lead to cell death via apoptosis [64][67]. Therefore, the long-term health effects are not separate phenomena but rather an accumulation of minute deleterious changes inflicted by the potentiation of NMDARs during each deep dive. Thus, this represents a permanent consequence of at least part of the broad symptoms and signs of HPNS.
TheOur recent Molecular Dynamics simulation (MDS) study [65][64] suggested hydrostatic pressure and compression with He has different impacts on the cell membrane and the tertiary receptor structure. Professional divers who showed HPNS symptoms usually perform their dive with a gas mixture containing He. Taking this together with MDS results, it can be speculated that the presence of the He in diver breathing mixtures could partly contribute to HPNS symptoms. Another fact that may support this hypothesis is the ability of some marine mammals to perform very deep breath-hold dives when exposed only to hydrostatic pressure [59][62]. The MDS study shows that pressure per se has a less devastating influence on the membrane and the embedded protein, which may render their CNS less vulnerable to ambient HP.
