Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Andreia Garces.
Antibiotic resistance is a global concern that affects not only human health but also the health of wildlife and the environment. Wildlife can serve as reservoirs for antibiotic-resistant bacteria, and antibiotics in veterinary medicine and agriculture can contribute to the development of resistance in these populations.
- mammals
- wild
- carnivores
- bacteria
- antibiotics
- contamination
- resistance
1. Introduction
Antimicrobial resistance (AMR) is considered one of the leading public health problems of the 21st century [1]. Although AMR has always existed, the overuse and misuse of antibiotics have increased antibiotic-resistant strains [2]. In recent decades, selective pressure has been generated by the use of antibiotics in medicine, veterinary, and agricultural practices, which has been responsible for a significant increase in antibiotic resistance [3].
“One Health” is a concept wherein human, animal, and environmental health are interconnected [4]. One of the greatest problems with “One Health” is antimicrobial resistance. This problem affects these three groups simultaneously. Humans and domestic and wild animals can be hosts and spreaders of AMR bacteria. Moreover, bacteria are continuously exchanged between the different environmental niches [5,6][5][6].
Although most wildlife prefer to live far from humans, some species have adapted and can live in contact with domestic animals or humans in urban environments. Therefore, they can be recognized as potential indicators of AMR dissemination [7]. Wild animals usually do not receive antibiotics or veterinary care, except in cases of interventions in endangered animals, admissions to wildlife rehabilitation centers, or treatments during disease outbreaks [8]. Studies have shown that AMR in most wildlife is associated with environmental exposure to anthropogenic AMR contamination [8]. Air, water, land, and food are some of the sources of AMR [9]. Bodies of water, such as rivers, lakes, or seas, can be contaminated with industrial discharges, agricultural discharges (fecal sludge from farms), domestic sewage, discharges from hospitals (human and veterinary), and wastewater treatment plants, among others [8,10,11][8][10][11]. Fertilizers used in agriculture can be a source of AMR [8]. In addition to environmental pressures, there are intrinsic mechanisms in bacteria that may contribute to the development of antimicrobial resistance, such as bacterial permeability, efflux pumps, target receptor modification, or horizontal gene transfer between bacteria via mobile genetic elements (e.g., plasmids, transposons, integrons) [3,12][3][12]. The presence of AMR in wildlife is also associated with other factors, such as habitat use, foraging behavior, and species’ habitats [3,8][3][8]. Habitat destruction, the loss of biodiversity, climate change, the accumulation of toxic pollutants, and the invasion of exotic species and pathogens have also contributed to the spread of AMR [13].
Contact between anthropogenic source areas and wild animals has increased due to human expansion. Some animals—for example, foxes and hedgehogs—have adapted and now live and thrive in urban areas [1,14][1][14]. Animals in these areas can feed on human domestic waste [15]. These contacts can potentially contribute to the emergence of new pathogens and AMR in wildlife, which can promote higher mortality rates. When animals survive, they can become bacterial reservoirs and spread throughout the environment again [13,16][13][16]. A study performed in Botswana showed that the prevalence of AMR Escherichia coli was highest in carnivores (62.5%) and animals using urban habitats (25.6%) when compared to herbivores (9.1%) and animals using protected/rural habitats (9.0%) [8].
Despite the abundance of literature on AMR in the medical and veterinary fields, available studies focus mainly on some bacterial species, such as Escherichia coli or Salmonella spp., and some species of wild animals, mainly birds and mammals [6,7,8][6][7][8]. Carnivores are a very diverse group of species in Europe, with some populations living in remote areas and others in urban areas in close contact with humans [10,15][10][15].
2. European Wild Carnivorous
Carnivora is an order of mammals that eats meat, by predation or necrophagy. They have specialized teeth for their meat-based diet, with fang-like canines, which they use to kill their prey and cut the meat into pieces [17,18][17][18]. Some animals in this order can also consume vegetation, insects (omnivores), and meat [17]. Carnivores can be found in diverse habitats, including cold polar regions, desert regions, forests, open seas, and urban areas [19]. The order Carnivora includes 16 families and 9 terrestrial families: Canidae, Felidae, Ursidae, Procyonidae, Mustelidae, Herpestidae, Viverridae, and Hyaenidae. In Europe, there are approximately 63 species of carnivorous mammals, both terrestrial and marine. Some of these species are threatened according to the IUCN Red List of Threatened Species, such as the Iberian lynx (endangered) or the Balkan lynx (critically endangered) [17]. These include larger predators, such as wolves, bears, and lynxes, and smaller carnivores like foxes, weasels, and mustelids. Historically, throughout the continent, these species have all experienced a dramatic decline in their populations and distributions due to anthropogenic factors (hunting, habitat destruction, pollution) [18,20,21][18][20][21]. Table 1 presents some information regarding the distribution, conservation status, and diet of some of the carnivorous species included in this research, to understand better the source of the acquisition of AMR strains of bacteria.Table 1. Species, family, distribution, diet, habitat, behavior, and conservation status (LC—Least Concern, V—Vulnerable, NT—Near Threat) of wild carnivore species from Europe.
| Species | Family | Distribution | Diet | Habitat | Behavior | Conservation Status | Ref. |
|---|
| Species | SpeciesCountry | Year | Type of Sample | Isolated Bacteria | Antibiotic Resistance * | Resistance Genes | Ref. |
|---|---|---|---|---|---|---|---|
| Country | Year | Type of Sample | Isolated Bacteria | Antibiotic Resistance * | Resistance Genes | Ref. | |
| Beech marten (Martes foina, Erxleben, 1777) | Mustelidae | Europe, except most Mediterranean islands, the Balkan peninsula, the Scandinavian peninsula, and the United Kingdom | |||||
| Iberian Lynx (Lynx pardinus) | Portugal | 2008–2010 | Feces | E. casseliflavus | |||
| Eurasian otter ( | Plants, fruit, rats, mice, small mammals, birds | Urban areas, forest habitats, and rural areas | Crepuscular and nocturnal | LC | |||
| S. sciuri | |||||||
| group, | |||||||
| S. equorum | |||||||
| , | |||||||
| S. capitis | |||||||
| MET, CL, AMC, AMP, ENR, FD, DA, TET | |||||||
| mecA | |||||||
| [ | |||||||
| 8 | |||||||
| ] | |||||||
* AMC: amoxicillin/clavulanic acid; AMP: ampicillin; STR: streptomycin; E: erythromycin; ENR: enrofloxacin; E: erythromycin; BE: benzylpenicillin; C: chloramphenicol; CD: clindamycin; CEF: ceftiofur; CEP: cephalothin; CN: gentamicin; CPN: cephalexin; CRO: ceftriaxone; CTX: cefotaxime; DXT: doxycycline; F: nitrofurantoin; IMI: imipenem; INN: cefovecin; KF: cephalothin; MAR: marbofloxacin; NEO: neomycin; PRA: pradofloxacin; PX: cefpodoxime; SXT: trimethoprim/sulfamethoxazole; TE: tetracycline; N: nalidixic acid; CIP: Ciprofloxacin; KAN: kanamycin; VAN: vancomycin; Q–D: quinupristin–dalfopristin; CZA: ceftazidime; FEP: cefepime; FOX: cefoxitin: FA: fusidic acid; DA: clindamycin; MET: methicillin.
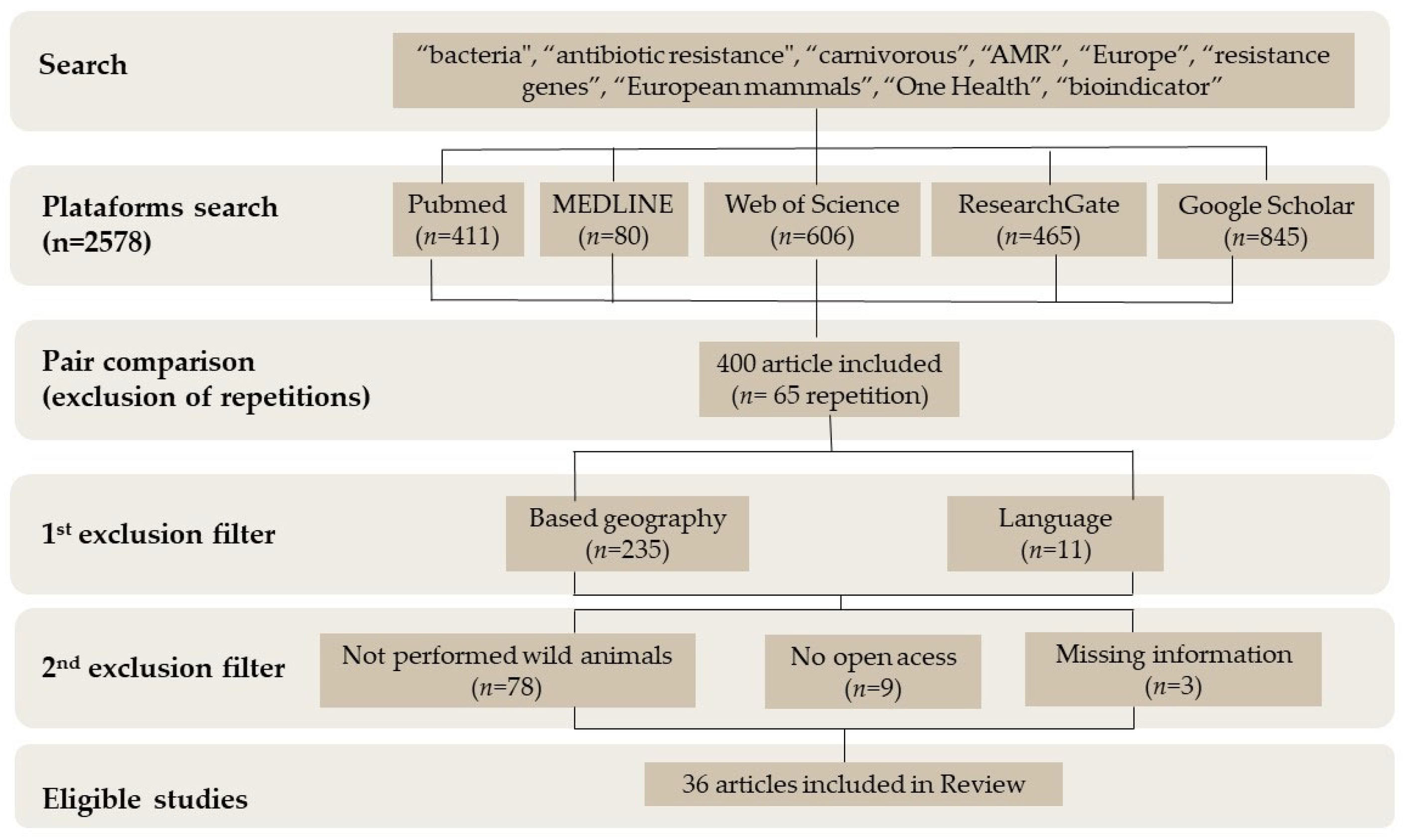
Table 3. Antibiotic resistance in animals from the family Ursidae regarding species, country, year, type of sample, bacteria isolated, antibiotic resistance, and resistance genes.
| Species | Country | Year | Type of Sample | Isolated Bacteria | Antibiotic Resistance * | Resistance Genes | Ref. | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polar bear (Ursus maritimus) | [ | Svalbard | 22 | ] | ||||||||||||||||
| 2014 | Fecal | Clostridiales | n/a | Lutra lutra) | bla | TEM | TE, Q–D, E, STR | vanC2, tetM, ermB, hyl, cylA, acm, ebpABCcylL, | , gelE, [37] | |||||||||||
| Portugal | 2006–2008 | Feces | E. faecalis | , E. faecium, E. durans | n/a | ace, cylA, tetM, pbp5, vanB, vanD | [49] | , aac(60)-Ie-aph | [53] | European polecat (Mustela putorius, Linnaeus, 1758) | Mustelidae | Western European Russia, Western Belarus, Western Ukraine, Central and Western Europe, and North Africa | Lagomorphs, small rodents, amphibians, birds, reptiles, and insects | PortugalRiparian and agricultural areas to meadows and forest areas | Nocturnal | LC | [ | |||
| Svalbard | 2004–2006 | Fecal | Clostridiales, Firmicutes, E. coli | 23 | ] | |||||||||||||||
| n/a | 2008–2010 | Feces | ||||||||||||||||||
| Portugal | 2015–2016 | Enterococcus | spp., E. coli | bla | TEM | TE, E, STR, N, SXT, | Feces | Enterococcus spp.cpd, cylB, and cylL, | AMC, AMP, C, CN, DA, ENR, P, TE, VANblaTEM, tetA, aadA, cmlA, [50] | n/a | dfrA1 + aadA1, dfrA12 + aadA2, fimA | [36] | Brown bear (Ursus arctos, Linnaeus, 1758) | Ursidae | Europe, Asia, Atlas Mountains, North America | Omnivore | Mountain woodlands, forest | Crepuscular | LC | [24] |
| Brown bears (Ursus arctos) | Slovenia | 2010–2012 | Fecal | E. coli | ||||||||||||||||
| [ | 54 | ] | n/a | fimH | , | ompT, kpsMT, ibeA, traT | [51 | Portugal] | 2008–201034] | |||||||||||
| Feces | Enterococcus | spp. | TE, E, KAN, N | Italy | ||||||||||||||||
| Portugal | 2022 | Forearm wound, exposed fracture | Streptococcus dysgalactiae spp. equisimilis, Leclercia adecarboxilara | AMP, C, CEF, CEP, CN, CPN, DX, ENR, INN, MAR, PRA, PX, SXT, TE | n/a | [34] | ||||||||||||||
| Italy | 2022 | Carpal wound, intraarticular swab | ||||||||||||||||||
| Slovakia | 2020 | Feces | Enterococcus spp. | TE, AMP, VAN, E | [46] | Streptococcus canis, E. coli, Pseudomonas aeruginosa | AMP, C, CPN, CEP, DXT, ENR, INN, MAR, NEO, PRA, SXT, TE, AMC, CEF, CN, CPN, IMI, F | n/a | [34] | |||||||||||
| Iberian wolves (Canis lupus signatus) | Portugal | 2008–2010 | Feces | E. coli | TE, AMP, STR, CEP, N, SXT, CIP | cdt, chuA, cvaC, eaeA, paa, bfpA, blaCTX-M-1, blaCTX-M-9 | [35] | |||||||||||||
| Portugal | 2008–2009 | Feces | Enterococcus faecium, E. hirae, E. faecalis, E. durans | AMP, TE, STR | tetM, tetL, ermB, blaTEM, tetA, tetB, aadA, strA-strB | [36] | ||||||||||||||
| Portugal | 2008–2010 | Feces | E. faecium, E. gallinarum | TET, VAN, AMP, E, KAN | vanC1, vanA, tetM, ermB; aph(3′)-IIIa, tet(L); Tn916, hyl | [37] | ||||||||||||||
| Golden jackal (Canis aureus) | Italy | 2023 | Lung, liver, spleen, kidney, and intestine | n/a | n/a | tetM, tetP, Southeastern Europe, Moldova, Asia Minor, and the Caucasus | Omnivorous diet, plants, fruit, rodents, rabbits | Valleys, beside rivers canals, lakes, seashores | Crepuscular | LC | [32] |
3. Antibiotic Resistance in Wild Carnivores
For this research, the inclusion criteria were as follows: studies only performed in free-range animals, species of European terrestrial carnivores, studies conducted in Europe, and studies that included bacteria, phenotypic resistance, and/or resistance genes. The initial search identified 2578 articles on the databases (ResearchGate, MEDLINE, PubMed, Web of Science, and Google Scholar) using the terms “bacteria”, “antibiotic resistance”, “carnivorous”, “AMR”, “Europe”, “resistance genes”, “European mammals”, “One Health”, and “bioindicator”. On the 2578 articles collected, a first screening was performed based on the information in the abstracts. A total of 2178 were excluded since they did not have the necessary information for the research. From the remaining 400, 65 were duplicates and therefore excluded. Another 235 were excluded due to geography (studies performed outside Europe). Eleven were removed due to language, since only English, Spanish, and Portuguese manuscripts were included in this research. With a secondary exclusion filter screening the full articles, 78 were excluded since they were not performed in wild animals or did not include all the information required, and 9 were not open-access full articles. Therefore, a total of 36 articles had all the information required and were included in this research (Figure 1). Table 2, Table 3, Table 4 and Table 5 is presented the information from the papers selected.
Figure 1.
Flow diagram of data collection.
Table 2. Antibiotic resistance in animals from the family Canidae regarding species, country, year, type of sample, bacteria isolated, antibiotic resistance, and resistance genes.
| Species | Country | Year | Type of Sample | Isolated Bacteria | Antibiotic Resistance * | Resistance Genes | Ref. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Apennine wolf (Canis lupus italicus) | Italy | 2015–2017 | Feces | Citrobacter spp., Escherichia coli, Hafnia alvei, Salmonella spp., Serratia spp. | AMC, AMP, STR | n/a | [33] | ||||||||
| Italy | 2017 | Feces | n/a | TE | tetA, tetP | [7] | |||||||||
| Italy | 2022 | Endocardial swab, lung, thoracic effusion | Staphylococcus pseudointermedius, Enterococcus faecalis, E. coli | 2006AMC, E, ENR, MAR, CXT, C, SXT, TE, P, DXT | Feces | Aeromonas hydrophila, A. hydrophila/caviae, A. sobriatetM, tetL, ermB, aac (6′)-Ie-aph(2″)-Ia, ant(6)-Ia, aph(3′)-IIIa | [52] | ||||||||
| Polar bear (Ursus maritimus, Phipps, 1774) | |||||||||||||||
| P, CLI, E, VAN, AMP | n/a | [ | 55 | ] | Ursidae | Greenland, Canada, Alaska, Russia, and the Svalbard Archipelago of Norway | Seals, walruses, sea birds, eggs, small mammals, fish, reindeer/caribou, seaweed/kelp, land plants | Portugal | 2008–2010Ice fields | Diurnal | V | Feces | E. coli | TE, STR, SXT, N, AMP, CIP | blaTEM, blaSHV, tetA[25] |
| n/a | [ | 34 | ] | ||||||||||||
| Italy | 2022 | Peritoneal effusion, lung, endocardial swab, liver parenchyma, pleural effusion | Klebsiella oxytoca | AMP | , tetB, aadA, strA-strB, aac(3)-II, aac (3)-IV, aadA1, dfrA1 + aadA1, estX + psp + aadA2, aer, cnf1, fimA, papC, papG-allele III | [52] | |||||||||
| Portugal | 2006–2008 | Feces | S. arizona, S. pullorum, S. choleraesuis arizona | AMC, C, P, AMP, CL, ENR, GN, NA, S, TE | n/a | [16] | Red fox (Vulpes vulpes, Linnaeus, 1758) | Canidae | Northern hemisphere | Plants, rodents, birds, leporids, porcupines, raccoons, opossums, reptiles, insects, invertebrates | Scrubland, forest, agricultural fields, urban areas | Nocturnal | Wild cat (Felis silvestrisLC | ) | Germany[26] |
| n/a | [ | 2014 | Nasal swab | ||||||||||||
| Portugal | 2009 | Feces | E. coli, | S. aureus | Enterococcusn/a | spp.gapA, katA, CoA, Spa, sbi, nuc1, sarA, saeS, | CTX, ENR, SvraS | n/a | , | [agrl, 13hid | [45] | European badger (Meles meles, Linnaeus, 1758) | |||
| ] | Mustelidae | Europe (except Scandinavia), Russia, and parts of Asia | Omnivores: plants, earthworms, large insects, small mammals, fruits | Lynx (Lynx lynx) | |||||||||||
| Spain | Sweden | 2006 | Liver tissue | 2018–2021 | FecesS. aureus | E. coli, Pseudomonas fluorescensDeciduous, mixed, and coniferous forests, agro-silver-pastoral landscapes, Mediterranean scrub forests, and open areas with patches of riparian vegetation | , Crepuscular and nocturnal | LC | Hafnia alvei, Serratia marcescensn/a | CIP, ENR, CN, SXT, TE, C | ermB, blaCTX-M-15, gapA, katA, CoA, Spa, sbi, nuc1, sarA, saeS, vraS, agrl, hid, agrlV, mecC[27] | ||||
| [ | 45 | ] | European otter (Lutra lutra, Linnaeus, 1758) | Mustelidae | Eurasia, North Africa, the Middle East, Sri Lanka, a part of India, and Indonesia | Fish, amphibians, insects | Rivers, streams, marshes, lagoons, and reservoirs | Nocturnal | NT | [28] |
* AMP: ampicillin; STR: streptomycin; E: erythromycin; SXT: trimethoprim/sulfamethoxazole; TE: tetracycline; N: nalidixic acid; CIP: ciprofloxacin; KAN: kanamycin; Q–D: quinupristin–dalfopristin.
Table 5. Antibiotic resistance in animals from the family Mustelidae regarding species, country, year, type of sample, bacteria isolated, antibiotic resistance, and resistance genes.
| tetM | |||||||||||||
| , | |||||||||||||
| bla | |||||||||||||
| CMY-2 | |||||||||||||
| , | |||||||||||||
| tetM | [ | 56 | ] | ||||||||||
| Germany | 2000–2012 | Nasal and perineal swabs | S. aureus | AMC, AMP, P, | mecC | [57] | |||||||
| Apennine wolf (Canis lupus italicus, Altobello, 1921) | Canidae | Italy, France, Spain, Switzerland | Roe deer, wild boar, red deer, livestock sheep, horses, Mouflon, Italian hare, birds, invertebrates, fruit, berries, grasses, herbs, and garbage | Temperate coniferous forests | Crepuscular, diurnal | ||||||||
| Portugal | 2018–2019 | Feces | E. coli, | AMP, SXT, TE, CTX, KAN, CN, PX, DXT, T | aac(3)-IV, aph(4)-Ia, aph(6)-Id, blaTEM-1B | V | , lnu(F), tet(B), aac(3)-Iva, aadA1, aac(2′)-Iia, qnrB19, adA5, aph(3″)-Ib, catA1, qnrB19, qnrB82, [29] | ||||||
| sulII | , | dfrA17 | [ | 58 | ] | Iberian wolves (Canis lupus signatus, Cabrera, 1907) | Canidae | Portugal, Spain | Wild boars, rabbits, roe deer, red deer, ibexes, small carnivores, and fish | Temperate forests | Crepuscular, diurnal | ||
| Slovakia | 2020 | Feces | EN | Enterococcus spp. | [ | 30 | ] | ||||||
| TE, E, AMP, VAN | n/a | [ | 46 | ] | Iberian Lynx (Lynx pardinus, Temminck, 1827) | Felidae | Portugal, Spain | Rabbits, small rodents | Mediterranean forests of woodland and shrubland interspersed with natural and artificial pastures | Crepuscular and nocturnal | EN | mcr-1, tetA, | |
| Spain | 2012–2015 | Nasal and rectal swabs | Staphylococcus spp. | N, P, FOX, FA | tetL | n/a | , | [49]tetM, tetO, sul3, [31] | |||||
| bla | TEM−1 | [ | 38 | ] | Golden jackal (Canis aureus, Linnaeus, 1758) | Canidae | |||||||
| Red Fox (Vulpes vulpes) | Portugal | 2008–2009 | Feces | E. coli | STR, TE, SXT, AMP | ||||||||
| Spain | adA, tetA, tetB, sul1, bla | TEM | 2015–2015 | [ | 39 | ] | |||||||
| Fecal | E. coli | AMP, TET, SXT | dfrA1 aadA1 qacE 1 | , | sul1, sul2, tetA | [59] | Portugal | 2008–2009 | Feces | E. faecium | TE | tetM, tetL, ermB, aph(30)-IIIa | [39] |
| Badger (Meles meles) | Ireland | 2018–2019 | Fecal, nasopharyngeal swabs | Salmonella spp., E. coli | AMP, CZA, CEP, CTX | n/a | [60] | Portugal | 2008–2009 | Feces | E. faecium, E. durans | ||
| Spain | TE, E | 2016–2017 | Swabs | E. coli | CIP, N, C, S, TermB, tetM, tetL, Tn916 | bla[40] | |||||||
| SHV-12 | [ | 61 | ] | Ireland | 2018–2019 | Fecal, nasopharyngeal swabs | E. coli | CZA, TE, SXT, CIP, AMP, FEP | |||||
| Poland | n/a | 2014–2018 | Rectal swabs | E. coli | AMP, S, KAN, C, CIP, S, N, TE | aph(3¢)-Ia, strA, aph(3¢)-Ia, sul2, tetA, tetB, floR, cat, sul3[41] | |||||||
| [ | 62 | ] | Norway | 2006 | Fecal | E. coli | SXT, TE, CIP, N | n/a | [1] | ||||
| Germany | 2011 | Pharyngeal swab | S. aureus | n/a | gapA, katA, CoA, Spa, sbi, nuc1, sarA, saeS, vraS, agrl, hid | [45] | Portugal | 2017–2019 | Fecal | E. coli, Enterococcus spp. | TE, C, CD, CN, AMC, AMP, BE, CEF, CEP, CZA, CPN, CRO | blaTEM, ermB, aadA, tetM, tetW, tetL, drfA1, drfA17 | [42 |
| Spain | ] | ||||||||||||
| 2015–2015 | Fecal | E. coli | AMP, TE | tetB | [ | 59] | Italy | 2016–2018 | Fecal | E. coli | |||
| Spain | n/a | 2012–2015 | eaeA | , | hlyA, stx1, and stx2, | Nasal and rectal swabs[43] | |||||||
| Staphylococcus | spp. | N, P, FOX, FA, CLI | n/a | [ | 63 | ] | Italy | 2002–2010 | Rectal swab | Salmonella enterica, S. typhimurium | AMC, TE, AMP, ENR | n/a | |
| Beech marten (Martes foina) | Poland | 2014–2018 | [ | 44 | ] | ||||||||
| Rectal swabs | E. coli | AMP, STR, KAN, C, CN, CIP, S, N, TE, CTX | strA | , | sul1 | , sul2, tetA, tetB, aph(3¢)-Ia, floR, cat, blaTEM-135 | [62] | Germany, Austria, Sweden | 2013, 2006, 2005, 2014 | Nasal swab | S. aureus | n/a | gapA, katA, CoA, |
| Spain | Spa | , | 2012–2015 | sbi | , nuc1, sarA, | Nasal and rectal swabs | Staphylococcus spp.saeS, | N, PEN, FOX, TEvraS, agrl, | n/ahid | [45] | |||
| [ | 49 | ] | Slovakia | 2020 | Feces | Enterococcus spp. | TE, AMP, VAN, E | n/a | [46] | ||||
| Spain | 2015–2015 | Fecal | E. coli | AMP, NAL, CIP | blaTEM-1b | [59] | Spain | 2012–2015 | Nasal and rectal swabs | Staphylococcus spp. | CD, F, AMP, BE, FOX, FA, NEO | n/a | [47] |
| Spain | 2016–2017 | Swabs | Citrobacter freundii | CIP, NAL, GEN, TET, SUL, TMP | black my-2, blaSHV-12 | [61] | Italy | 2017–2019 | Oral, skin, rectal, tracheal swab, feces | K. oxytoca | AMP, CD | ||
| European pine marten ( | n/a | Martes martes) | [ | 48 | ] | ||||||||
| Slovakia | 2020 | Feces | Enterococcus | spp. | TE, E, AMP, VAN | n/a | [46 | UK | 2007–2008 | Tissues |
* AMP: ampicillin; E: erythromycin; TE: tetracycline; VAN: vancomycin.
Table 4. Antibiotic resistance in animals from the family Felidae regarding species, country, year, type of sample, bacteria isolated, antibiotic resistance, and resistance genes.
| ] | |||||||
| Italy | |||||||
| 2002–2010 | |||||||
| Rectal swab | |||||||
| Salmonella | |||||||
| spp. | |||||||
| AM, AMC, TE | |||||||
| n/a | |||||||
| [ | |||||||
| 44 | |||||||
| ] | |||||||
| Italy | |||||||
| 2017–2019 | |||||||
| Oral, skin, rectal, tracheal swab, feces | E. coli | AMP, CD | n/a | [ | 48 | ] | |
| European polecat (Mustela putorius) | Poland | 2014–2018 | Rectal swabs | E. coli | AMP, STR, S, TET | strA, sul2, tetA | [62] |
* AMC: amoxicillin/clavulanic acid; AMP: ampicillin; STR: streptomycin; E: erythromycin; ENR: enrofloxacin; E: erythromycin; BE: benzylpenicillin; C: chloramphenicol; CD: clindamycin; CEF: ceftiofur; CEP: cephalothin; CN: gentamicin; CPN: cephalexin; CRO: ceftriaxone; CTX: cefotaxime; DXT: doxycycline; F: nitrofurantoin; IMI: imipenem; INN: cefovecin; KF: cephalothin; MAR: marbofloxacin; NEO: neomycin; PRA: pradofloxacin; PX: cefpodoxime; SXT: trimethoprim/sulfamethoxazole; TE: tetracycline; N: nalidixic acid; CIP: ciprofloxacin; KAN: kanamycin; VAN: vancomycin; Q–D: quinupristin–dalfopristin; CZA: ceftazidime; FEP: cefepime; FOX: cefoxitin: FA: fusidic acid; P: penicillin; T: tobramycin.
 Regarding phenotype resistance, Figure 4 considers the number of articles that describe, in particular, each type of antibiotic resistance reported in the various Carnivora families: Canidae, Ursidae, Felidae, and Mustelidae. The studies are also summarized in Table 2 and Table 3. Many papers report multi-resistant bacteria. The methodology used in these articles was very similar, using the disk diffusion method (DDM) as antibiotic sensitivity testing. All the terminology was also standardized to be included in this graphic.
Regarding phenotype resistance, Figure 4 considers the number of articles that describe, in particular, each type of antibiotic resistance reported in the various Carnivora families: Canidae, Ursidae, Felidae, and Mustelidae. The studies are also summarized in Table 2 and Table 3. Many papers report multi-resistant bacteria. The methodology used in these articles was very similar, using the disk diffusion method (DDM) as antibiotic sensitivity testing. All the terminology was also standardized to be included in this graphic.
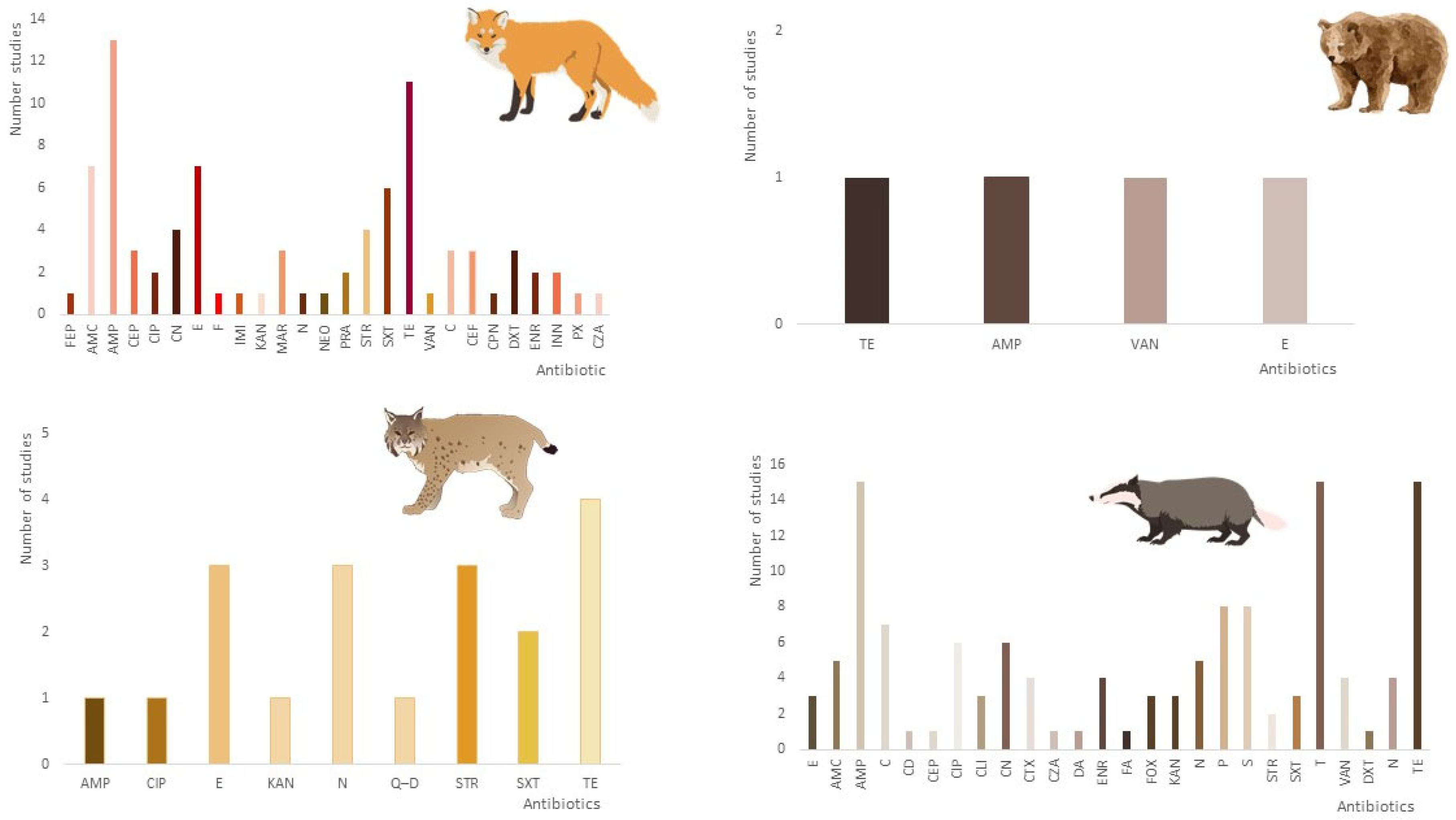 Based on the information collected in the different articles regarding the antibiotic resistance phenotype, it was possible to observe that three of the carnivore families—Canidae, Felidae, and Mustilidae—presented high levels of resistance to ampicillin and tetracyclines (Figure 4). In the case of the Ursidae family, it is impossible to extract any valid information due to the limited number of studies, and the resistance pattern is quite similar.
Based on the information collected in the different articles regarding the antibiotic resistance phenotype, it was possible to observe that three of the carnivore families—Canidae, Felidae, and Mustilidae—presented high levels of resistance to ampicillin and tetracyclines (Figure 4). In the case of the Ursidae family, it is impossible to extract any valid information due to the limited number of studies, and the resistance pattern is quite similar.
3.1. Species and Spatial Distribution
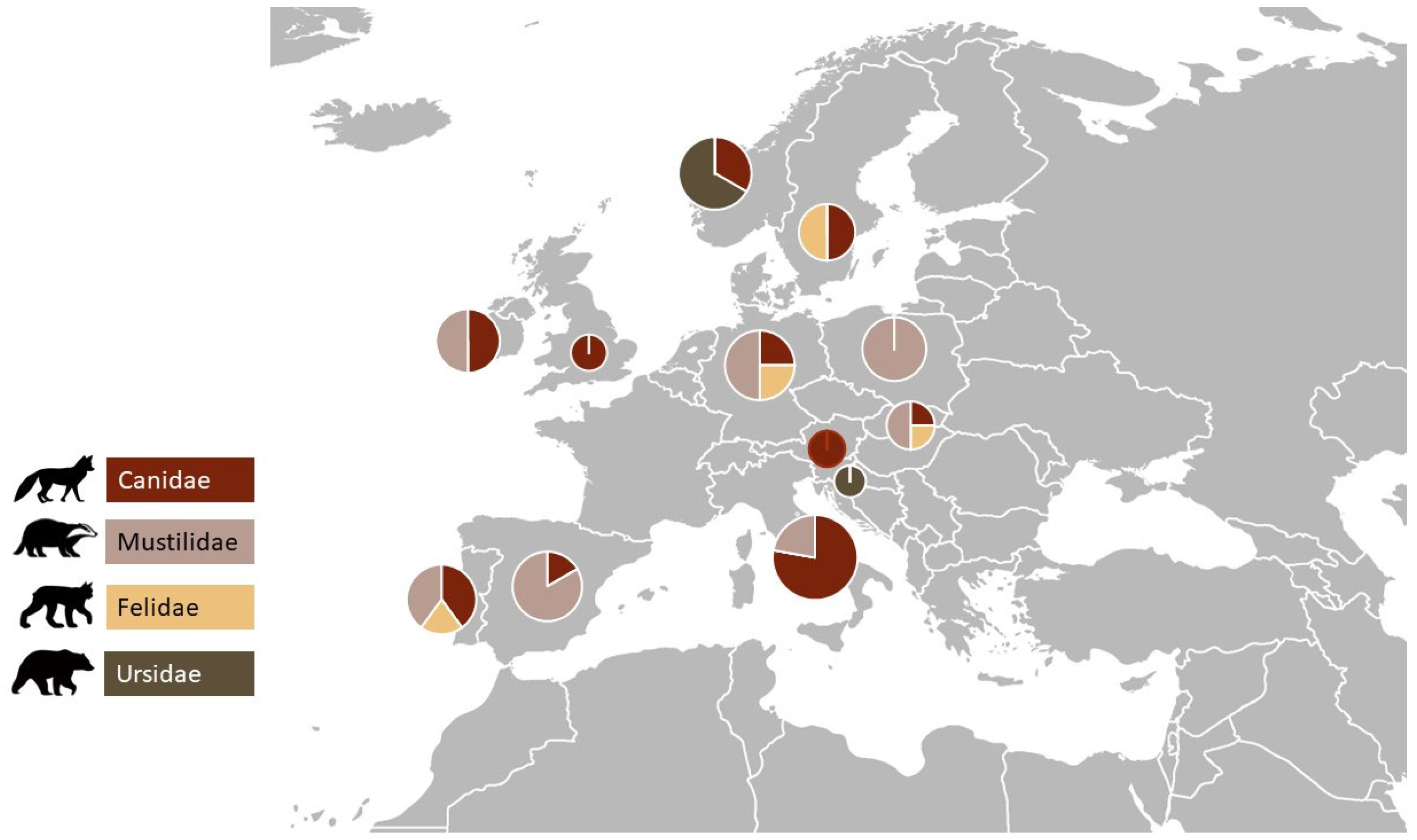
The main families of carnivores where studies were carried out, in descending order, were as follows: 11.1% (n = 4) Ursidae, 16.6% (n = 6) Felidae, 19.4% (n = 7) Canidae, and 52.7% (n = 19) Mustilidae. The species with the most AMR studies was Vulpes vulpes with 12 studies, followed by Lutra lutra with 11 studies. The countries where the studies were carried out, in ascending order, were as follows: 41.6% (n = 15) Portugal, 25% (n = 9) Italy, 8.3% (n = 3) Norway, 8.3% (n = 3) Germany, 8.3% (n = 3) Slovakia, 5.5% (n = 2) Ireland, 5.5% (n = 2) Slovenia, 5.5% (n = 2) Poland, 2.7% (n = 1) Austria, 2.7% (n = 1) Sweden, 2.7% (n = 1) United Kingdom. Figure 2 represents the number of studies by carnivorous species in each country included in this research.
Figure 2.
Distribution of the studies in the different European countries by carnivorous family group.
3.2. Bacteria, Antibiotic Resistance Pattern, and Resistance Genes
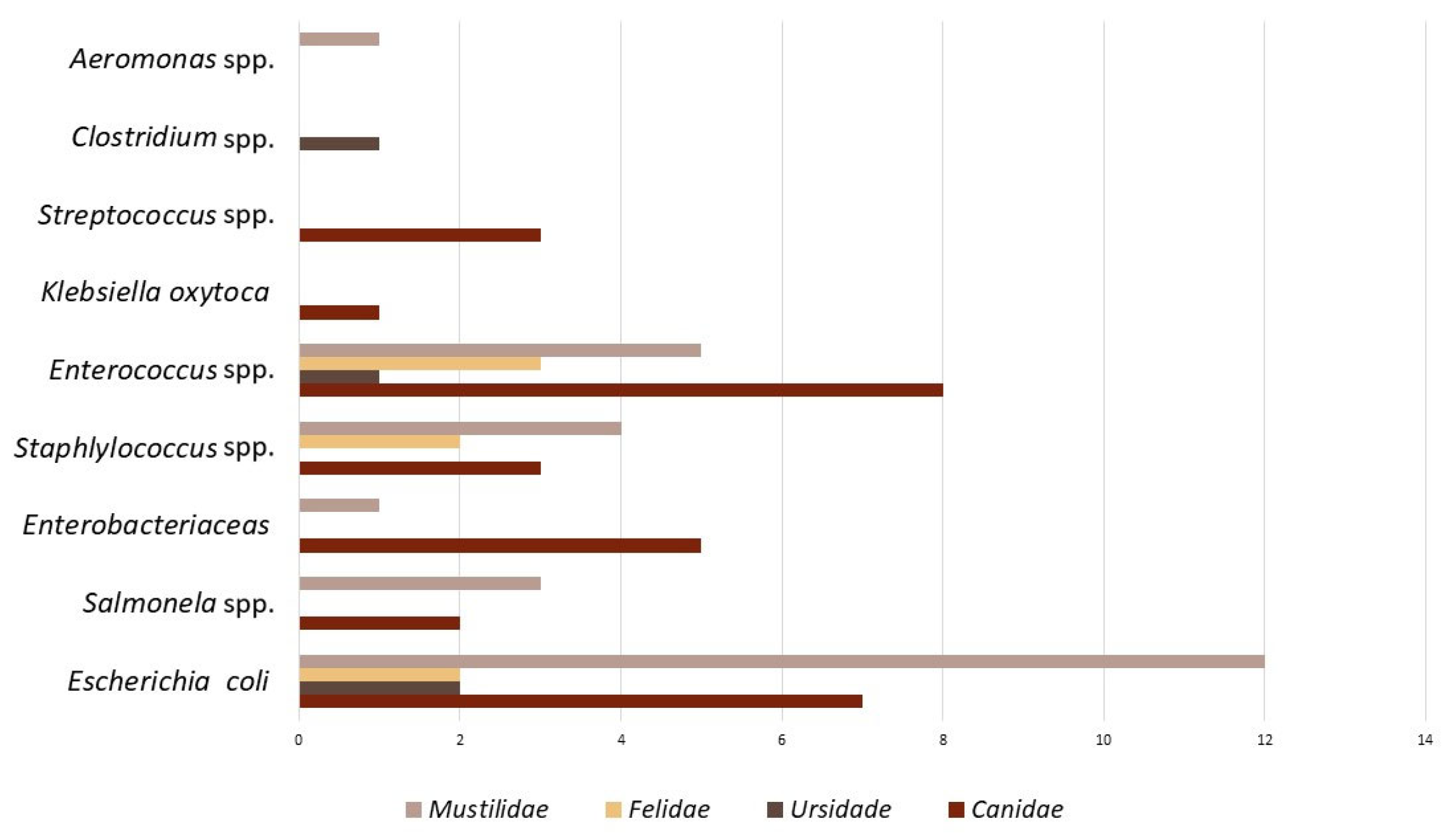
Most of the studies were performed in fecal samples or rectal swabs; therefore, the bacteria isolated mostly were microbiota from the gut microflora (Figure 3).
Figure 3.
Bacteria species that predominate in the 36 studies in antibiotic resistance in wild carnivores.

Figure 4. Occurrence of phenotypic antimicrobial resistance profile of bacteria in wild carnivores based on the articles included in this research (AMC: amoxicillin/clavulanic acid; AMP: ampicillin; STR: streptomycin; E: erythromycin; ENR: enrofloxacin; E: erythromycin; BE: benzylpenicillin; C: chloramphenicol; CD: clindamycin; CEF: ceftiofur; CEP: cephalothin; CN: gentamicin; CPN: cephalexin; CRO: ceftriaxone; CTX: cefotaxime; DXT: doxycycline; F: nitrofurantoin; IMI: imipenem; INN: cefovecin; KF: cephalothin; MAR: marbofloxacin; NEO: neomycin; PRA: pradofloxacin; PX: cefpodoxime; SXT: trimethoprim/sulfamethoxazole; TE: tetracycline; N: nalidixic acid; CIP: ciprofloxacin; KAN: kanamycin; VAN: vancomycin; Q–D: quinupristin–dalfopristin; CZA: ceftazidime; FEP: cefepime; FOX: cefoxitin: FA: fusidic acid; P: penicillin; T: tobramycin).
4. Carnivores and Antibiotic Resistance
Antibiotic-resistant bacteria can be acquired by carnivorous species in several ways, mainly through direct and indirect exposure to these resistant strains from anthropogenic sources and domestic animals [10,62][10][62]. Normally, these animals are not treated with antibiotic therapy, except in some particular cases in which some individuals are admitted to wild animal rehabilitation centers due to illness or trauma. However, even in these cases, exposure to these agents is brief [64,65][64][65]. Major predators can generally travel great distances across the territory for food. They can disperse AMR over large areas, a key element of AMR dynamics in the ecosystem [66]. Some species of animals are natural carriers of AMR bacteria. For example, European hedgehogs (Erinaceus europaeus) are natural carriers of MRSA that have been selected as a response to the presence of b-lactam-producing microorganisms (Trichophyton erinacei) in the microbiome of this animal [67]. The greatest problem is the contamination of the environment with antibiotic resistance determinants and resistance drivers (e.g., antibiotic residues, pesticides, heavy metals) from agriculture, waste disposal, or the disposal of wastewater of human and veterinary origin [8]. The dispersion of these agents in the environment is a public health problem, as it can lead to the emergence and proliferation of pathogens that are difficult or impossible to treat [8,63][8][63]. The dispersion of these agents has negative economic and health consequences for humans and animals [68]. Several studies have already been carried out on the presence and impact of antibiotic-resistant bacteria in wildlife across various vertebrates, from birds to reptiles [69,70][69][70]. Based on already available data, the prevalence of antibiotic-resistant bacteria depends on multiple factors, such as habitat use, the foraging strategy of the species, behavior, and territory [8]. Unfortunately, not all European carnivore species are represented in this research, as no data are available for some of them [62]. This article’s main limitation is that it does not allow a realistic comparison between different species. This demonstrates that it is necessary to collect more data on other species and in different regions, in the long term, to compare the impact that the use and abuse of antibiotics have on these animals. Most of the studies included in this research were conducted in Southern and Eastern European countries (Figure 3). This fact may be partly associated with the greater diversity of animals in these regions. However, it is also possible that it is related to the fact that several Southern and Eastern European countries have reported higher levels of antibiotic resistance in livestock, often linked to differences in agricultural practices, regulations, and intensive livestock production. In addition, many of these regions are highly industrialized [71,72][71][72]. Concerning the available data, it is possible to observe that most studies were conducted on small mammals (Figure 3), mainly from the Mustilidae family. This may be associated with the fact that large carnivore populations (wolf, bear, wolverine, lynx) have declined in Europe and their numbers are very small [62,73][62][73]. Most of these populations are threatened and protected by law [34,52][34][52]; therefore, it is necessary to access samples from these individuals to carry out studies [37,74][37][74]. In addition to being threatened, some species, such as polar bears, live in very remote areas, difficult to access and with harsh climates [25,50][25][50]. One of the most represented species is the red fox (Vulpes vulpes). This may be associated with its omnivorous diet and adaptability to urban centers. Currently, these animals can be easily found in several European cities, feeding on human waste and in close contact with domestic animals [26,42][26][42]. Due to this behavior, they can be excellent bioindicators of the presence of AMR in the environment [47,75][47][75]. Animals such as foxes, which live close to humans and often depend on their waste for food, are more susceptible to these agents. Similar studies in birds in Southern France detected carbapenem-resistant E. coli isolates in yellow-legged gulls (Larus michahellis) feeding in landfills. However, no isolates were obtained from slender-billed gulls (Chroicocephalus genie) provided from deep-sea fish [76]. Another source of contamination may be the prey of small species, such as rodents or insects, which may represent a link between humans/domestic animals and predators [76]. In the case of flies, these are usually found in contaminated waste and can travel quite a long distance as vectors of AMR bacteria, infecting wild/domestic animals and humans [66]. Moreover, scavenging contaminated carcasses or consuming peridomestic prey may promote exposure to AMR [8]. The most observed species of bacteria were E. coli and Enterococcus spp. (Figure 3) in general in the four families of carnivores. E. coli prevailed in all families except Felidae, where Enterococcus spp. was the most prominent bacterial species. The results were expected since most samples originated from feces or rectal swabs [52,72][52][72]. Although the use of antibiotics in livestock farming has been reduced to minimal use under EU regulations [1], sulfamethoxazole, ampicillin, and tetracycline were the primary resistance types reported in livestock animals [77,78][77][78]. Tetracycline and ampicillin are also some of the most commonly used antibiotics in human medicine, and resistance to these isolates is frequently reported [79]. In the data collection, almost all carnivore families have resistance to ampicillin, tetracyclines, and sulfonamides. This may be an indication that livestock and humans may be the potential sources of these forms of AMR in wild carnivores. Some resistant bacteria are more dangerous than others, as in the case of extended-spectrum beta-lactamase (ESBL)-producing bacteria, vancomycin-resistant Enterococci (VRE), and methicillin-resistant Staphylococcus aureus (MRSA) [44,62][44][62]. Infections with these bacteria are challenging to treat and can lead to severe complications [62,63][62][63]. The presence of ESBL has been reported in Iberian wolves [32[32][34],34], red foxes, badgers [58], and European otters [54,56][54][56]; VRE in Iberian wolves [36]; and MRSA in European otters and the European lynx [45,57][45][57] The mecA gene is the main genetic determinant responsible for methicillin resistance in Staphylococcus aureus. In the studies presented where MRSA was observed, it was isolated from mecC, which is homologous for mecA [45,57][45][57]. Vancomycin resistance in Enterococcus is commonly associated with two genes, vanA and vanB. These genes were isolated in the Iberian wolf [37]. Several genes are associated with ESBL production, such as TEM, SHV, and CTX-M. These genes have been identified in Iberian wolves [32[32][34],34], red foxes, badgers [58], and European otters [54,56][54][56]. Other important genes are blaCTX-M, blaCMY, tetM, and ermB, also isolated in several species, indicating that bacteria or resistance originated in human or domestic animals [78,79][78][79]. The correlation between AMR and the United Nations Sustainable Development Goals (SDGs) is a global health concern and has specific implications for wildlife populations, including carnivores in Europe [80]. As these animals play vital roles in ecosystems, their health is interconnected with the broader environmental and human health goals outlined in the SDGs. In the context of carnivores, AMR can have cascading effects on ecosystems. For example, the use of antibiotics in domestic animals, which is linked to AMR, can indirectly impact carnivores through food chain dynamics [81]. Additionally, the spread of antibiotic-resistant bacteria in the environment, including water bodies, can affect carnivores that rely on these resources. This aligns with SDG 15 (Life on Land) and SDG 14 (Life Below Water), emphasizing the importance of safeguarding terrestrial and aquatic ecosystems. Moreover, the potential transmission of antibiotic-resistant bacteria between wildlife, livestock, and humans underscores the interconnectedness of SDG 3 (Good Health and Well-Being). Efforts to mitigate AMR in carnivores involve understanding and addressing the factors contributing to the spread of resistance, emphasizing the need for interdisciplinary approaches that span the environmental, veterinary, and human health domains. In the broader context of SDG 17 (Partnerships for the Goals), collaboration between environmental scientists, veterinarians, public health experts, and policymakers becomes crucial [80]. Shared knowledge and coordinated efforts are necessary to develop strategies that protect carnivores, ecosystems, and public health from the threats posed by AMR [81]. Addressing AMR in carnivores aligns with the holistic and interconnected approach of the SDGs, recognizing that the health of wildlife is intrinsically linked to broader sustainability and well-being goals for the planet and its inhabitants [82].References
- Mo, S.S.; Urdahl, A.M.; Madslien, K.; Sunde, M.; Nesse, L.L.; Slettemeås, J.S.; Norström, M. What Does the Fox Say? Monitoring Antimicrobial Resistance in the Environment Using Wild Red Foxes as an Indicator. PLoS ONE 2018, 13, e0198019.
- Costa, D.; Poeta, P.; Sáenz, Y.; Vinué, L.; Coelho, A.C.; Matos, M.; Rojo-Bezares, B.; Rodrigues, J.; Torres, C. Mechanisms of Antibiotic Resistance in Escherichia coli Isolates Recovered from Wild Animals. Microb. Drug Resist. 2008, 14, 71–77.
- Sousa, M.; Gonçalves, A.; Silva, N.; Serra, R.; Alcaide, E.; Zorrilla, I.; Torres, C.; Caniça, M.; Igrejas, G.; Poeta, P. Acquired Antibiotic Resistance among Wild Animals: The Case of Iberian Lynx (Lynx pardinus). Vet. Q. 2014, 34, 105–112.
- Suárez-Pérez, A.; Corbera, J.A.; González-Martín, M.; Tejedor-Junco, M.T. Multidrug-Resistant Phenotypes of Escherichia coli Isolates in Wild Canarian Egyptian Vultures (Neophron percnopterus majorensis). Animals 2021, 11, 1692.
- Baros Jorquera, C.; Moreno-Switt, A.I.; Sallaberry-Pincheira, N.; Munita, J.M.; Flores Navarro, C.; Tardone, R.; González-Rocha, G.; Singer, R.S.; Bueno, I. Antimicrobial Resistance in Wildlife and in the Built Environment in a Wildlife Rehabilitation Center. One Health 2021, 13, 100298.
- CDC. One Health. Available online: https://www.who.int/news-room/questions-and-answers/item/one-health (accessed on 11 April 2023).
- Smoglica, C.; Di Francesco, C.E.; Angelucci, S.; Antonucci, A.; Innocenti, M.; Marsilio, F. Occurrence of the Tetracycline Resistance Gene tetA(P) in Apennine Wolves (Canis lupus italicus) from Different Human–Wildlife Interfaces. J. Glob. Antimicrob. Resist. 2020, 23, 184–185.
- Ramey, A.M.; Ahlstrom, C.A. Antibiotic resistant bacteria in wildlife: Perspectives on trends, acquisition and dissemination, data gaps, and future directions. J. Wildl. Dis. 2019, 56, 1–15.
- Sens-Junior, H.; Trindade, W.A.; Oliveira, A.F.; Zaniolo, M.M.; Serenini, G.F.; Araujo-Ceranto, J.B.; Gonçalves, D.D.; Germano, R.M. Bacterial Resistance in Bats from the Phyllostomidae Family and Its Relationship with Unique Health. Pesq. Vet. Bras. 2018, 38, 1207–1216.
- Sherley, M.; Gordon, D.M.; Collignon, P.J. Variations in Antibiotic Resistance Profile in Enterobacteriaceae Isolated from Wild Australian Mammals. Env. Microbiol 2000, 2, 620–631.
- Gharout-Sait, A.; Touati, A.; Ahmim, M.; Brasme, L.; Guillard, T.; Agsous, A.; de Champs, C. Occurrence of Carbapenemase-Producing Klebsiella pneumoniae in Bat Guano. Microb. Drug Resist. 2019, 25, 1057–1062.
- Blanco, G.; Lemus, J.A.; Grande, J.; Gangoso, L.; Grande, J.M.; Donázar, J.A.; Arroyo, B.; Frías, O.; Hiraldo, F. Retracted Geographical Variation in Cloacal Microflora and Bacterial Antibiotic Resistance in a Threatened Avian Scavenger in Relation to Diet and Livestock Farming Practices. Environ. Microbiol. 2007, 9, 1738–1749.
- Oliveira, M.; Pedroso, N.; Sales-Luís, T.; Santos-Reis, M.; Tavares, L.; Vilela, C. Evidence of Antimicrobial Resistance in Eurasian Otter (Lutra lutra Linnaeus, 1758) Fecal Bacteria in Portugal. In Wildlife: Destruction, Conservation and Biodiversity; Nova Science Publishers: New York, NY, USA, 2009; pp. 201–221. ISBN 978-1-60692-974-2.
- Dwi Ash-Santri, A.; Cantya Prakasita, V.; Kristian Adi, Y.; Budipitojo, T.; Endang Tri Hastuti Wahyuni, A. Isolation, Identification, and Antimicrobial Susceptibility Test of Bacteria from Vulva Swab of African Pygmy Hedgehog (Atelerix albiventris) and Sunda Porcupine (Hystrix javanica). BIO Web Conf. 2021, 33, 06009.
- Baker, P.J.; Harris, S. Urban Mammals: What Does the Future Hold? An Analysis of the Factors Affecting Patterns of Use of Residential Gardens in Great Britain. Mammal Rev. 2007, 37, 297–315.
- Oliveira, M.; Pedroso, N.; Sales-Luís, T.; Santos-Reis, M.; Tavares, L.; Vilela, C. Antimicrobial-Resistant Salmonella Isolated from Eurasian Otters (Lutra Lutra Linnaeus, 1758) in Portugal. J. Wildl. Dis. 2010, 46, 1257–1261.
- Bellani, G.G. Chapter 1—Order of Carnivores (Carnivora). In Felines of the World; Bellani, G.G., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 1–12. ISBN 978-0-12-816503-4.
- Clark, T.W.; Curlee, A.P.; Reading, R.P. Crafting Effective Solutions to the Large Carnivore Conservation Problem. Conserv. Biol. 1996, 10, 940–948.
- Carnivore-Mammal Classification|Britannica. Available online: https://www.britannica.com/animal/carnivore-mammal (accessed on 31 October 2023).
- Farris, Z.J.; Golden, C.D.; Karpanty, S.; Murphy, A.; Stauffer, D.; Ratelolahy, F.; Andrianjakarivelo, V.; Holmes, C.M.; Kelly, M.J. Hunting, Exotic Carnivores, and Habitat Loss: Anthropogenic Effects on a Native Carnivore Community, Madagascar. PLoS ONE 2015, 10, e0136456.
- Trouwborst, A. Managing the Carnivore Comeback: International and EU Species Protection Law and the Return of Lynx, Wolf and Bear to Western Europe. J. Environ. Law 2010, 22, 347–372.
- Martes foina. Britannica. Available online: https://naturdata.com/especie/ (accessed on 1 November 2023).
- Mustela putorius. Britannica. Available online: https://naturdata.com/especie/ (accessed on 1 November 2023).
- Dewey, T.; Ballenger, L. Ursus arctos (Brown Bear). Available online: https://animaldiversity.org/accounts/Ursus_arctos/ (accessed on 1 November 2023).
- Jirik, K. Polar Bear (Ursus maritimus). Available online: https://ielc.libguides.com/sdzg/factsheets/polarbear/summary (accessed on 1 November 2023).
- Letková, V.; Lazar, P.; Čurlík, J.; Goldová, M.; Košuthová, L.; Mojžišová, J. The Red Fox (Vulpes vulpes L.) as a Source of Zoonoses. Vet. Arh. 2006, 76, 73–81.
- Rafferty, R. Badger. Available online: https://www.britannica.com/animal/badger (accessed on 29 November 2023).
- Fusillo, R.; Romanucci, M.; Marcelli, M.; Massimini, M.; Della Salda, L. Health and Mortality Monitoring in Threatened Mammals: A First Post Mortem Study of Otters (Lutra lutra L.) in Italy. Animals 2022, 12, 609.
- Canis lupus subsp. italicus Altobello. 1921. Available online: https://www.gbif.org/species/165635864 (accessed on 2 November 2023).
- Canis lupus signatus. Britannica. Available online: https://naturdata.com/especie/ (accessed on 2 November 2023).
- Lynx pardinus. Britannica. Available online: https://naturdata.com/especie/ (accessed on 2 November 2023).
- Ivory, A. Canis aureus (Golden Jackal). Available online: https://animaldiversity.org/accounts/Canis_aureus/ (accessed on 2 November 2023).
- Foti, M.; Fisichella, V. Study of the Spread of the Antibiotic Resistance Phenomenon in a Wolf Population (Canis lupus, Linneaus 1758) in the Aspromonte National Park; Iris: San Francisco, CA, USA, 2017.
- Smoglica, C.; Angelucci, S.; Di Tana, F.; Antonucci, A.; Marsilio, F.; Di Francesco, C. Antibiotic Resistance in the Apennine Wolf (Canis lupus italicus): Implications for Wildlife and Human Health. Antibiotics 2023, 12, 950.
- Simões, R.; Ferreira, C.; Gonçalves, J.; Álvares, F.; Rio-Maior, H.; Roque, S.; Brandão, R.; Martins da Costa, P. Occurrence of Virulence Genes in Multidrug-Resistant Escherichia coli Isolates from Iberian Wolves (Canis lupus signatus) in Portugal. Eur. J. Wildl. Res. 2012, 58, 677–684.
- Gonçalves, A.; Igrejas, G.; Radhouani, H.; Santos, T.; Monteiro, R.; Pacheco, R.; Alcaide, E.; Zorrilla, I.; Serra, R.; Torres, C.; et al. Detection of Antibiotic Resistant Enterococci and Escherichia coli in Free Range Iberian Lynx (Lynx pardinus). Sci. Total Environ. 2013, 456–457, 115–119.
- Gonçalves, A.; Igrejas, G.; Radhouani, H.; López, M.; Guerra, A.; Petrucci-Fonseca, F.; Alcaide, E.; Zorrilla, I.; Serra, R.; Torres, C.; et al. Detection of Vancomycin-Resistant Enterococci from Faecal Samples of Iberian Wolf and Iberian Lynx, Including Enterococcus faecium Strains of CC17 and the New Singleton ST573. Sci. Total Environ. 2011, 410–411, 266–268.
- Di Francesco, A.; Salvatore, D.; Gobbi, M.; Morandi, B. Antimicrobial Resistance Genes in a Golden Jackal (Canis aureus L. 1758) from Central Italy. Vet. Res. Commun. 2023, 1–5.
- Radhouani, H.; Igrejas, G.; Gonçalves, A.; Pacheco, R.; Monteiro, R.; Sargo, R.; Brito, F.; Torres, C.; Poeta, P. Antimicrobial Resistance and Virulence Genes in Escherichia coli and Enterococci from Red Foxes (Vulpes vulpes). Anaerobe 2013, 23, 82–86.
- Radhouani, H.; Igrejas, G.; Carvalho, C.; Pinto, L.; Gonçalves, A.; Lopez, M.; Sargo, R.; Cardoso, L.; Martinho, A.; Rego, V.; et al. Clonal Lineages, Antibiotic Resistance and Virulence Factors in Vancomycin-Resistant Enterococci Isolated from Fecal Samples of Red Foxes (Vulpes vulpes). J. Wildl. Dis. 2011, 47, 769–773.
- O’Hagan, M.J.H.; Pascual-Linaza, A.V.; Couzens, C.; Holmes, C.; Bell, C.; Spence, N.; Huey, R.J.; Murphy, J.A.; Devaney, R.; Lahuerta-Marin, A. Estimation of the Prevalence of Antimicrobial Resistance in Badgers (Meles meles) and Foxes (Vulpes vulpes) in Northern Ireland. Front. Microbiol. 2021, 12, 596891.
- Dias, D.; Hipólito, D.; Figueiredo, A.; Fonseca, C.; Caetano, T.; Mendo, S. Unravelling the Diversity and Abundance of the Red Fox (Vulpes vulpes) Faecal Resistome and the Phenotypic Antibiotic Susceptibility of Indicator Bacteria. Animals 2022, 12, 2572.
- Bertelloni, F.; Cagnoli, G.; Biagini, F.; Poli, A.; Bibbiani, C.; Ebani, V.V. Virulence Genes of Pathogenic Escherichia coli in Wild Red Foxes (Vulpes vulpes). Animals 2022, 12, 1959.
- Botti, V.; Navillod, F.V.; Domenis, L.; Orusa, R.; Pepe, E.; Robetto, S.; Guidetti, C. Salmonella spp. and Antibiotic-Resistant Strains in Wild Mammals and Birds in North-Western Italy from 2002 to 2010. Vet. Ital. 2013, 94, 195–202.
- Monecke, S.; Gavier-Widen, D.; Mattsson, R.; Rangstrup-Christensen, L.; Lazaris, A.; Coleman, D.C.; Shore, A.C.; Ehricht, R. Detection of mecC-Positive Staphylococcus aureus (CC130-MRSA-XI) in Diseased European Hedgehogs (Erinaceus europaeus) in Sweden. PLoS ONE 2013, 8, e66166.
- Hamarova, L.; Kopcakova, A.; Kocianova-Adamcova, M.; Piknova, M.; Javorsky, P.; Pristas, P. Antimicrobial Resistance of Enterococci from Wild Animals in Slovakia. Pol. J. Environ. Stud. 2021, 30, 2085–2091.
- Carson, M.; Meredith, A.L.; Shaw, D.J.; Giotis, E.S.; Lloyd, D.H.; Loeffler, A. Foxes As a Potential Wildlife Reservoir for mecA-Positive Staphylococci. Vector-Borne Zoonotic Dis. 2012, 12, 583–587.
- Gambino, D.; Vicari, D.; Vitale, M.; Schirò, G.; Mira, F.; Giglia, M.L.; Riccardi, A.; Gentile, A.; Giardina, S.; Carrozzo, A.; et al. Study on Bacteria Isolates and Antimicrobial Resistance in Wildlife in Sicily, Southern Italy. Microorganisms 2021, 9, 203.
- Rajabi, M. An Investigation Study on Antimicrobial Resistance in Arctic Environments. Bull. Pure Appl. Sci.-Bot. 2014, 33b, 37.
- Glad, T.; Bernhardsen, P.; Nielsen, K.M.; Brusetti, L.; Andersen, M.; Aars, J.; Sundset, M.A. Bacterial Diversity in Faeces from Polar Bear (Ursus maritimus) in Arctic Svalbard. BMC Microbiol. 2010, 10, 10.
- Vadnov, M.; Barbič, D.; Žgur-Bertok, D.; Erjavec, M.S. Escherichia coli Isolated from Feces of Brown Bears (Ursus arctos) Have a Lower Prevalence of Human Extraintestinal Pathogenic E. coli Virulence-Associated Genes. Can. J. Vet. Res. 2017, 81, 59–63.
- Gonçalves, A.; Igrejas, G.; Radhouani, H.; Santos, T.; Monteiro, R.; Marinho, C.; Perez, M.J.; Canales, R.; Mendonza, J.L.; Serra, R.; et al. Iberian Lynx (Lynx pardinus) from the Captive Breeding Program as Reservoir of Antimicrobial Resistant Enterococci and Escherichia coli Isolates. J. Integr. OMICS 2013, 3, 138–144.
- Semedo-Lemsaddek, T.; Nóbrega, C.S.; Ribeiro, T.; Pedroso, N.M.; Sales-Luís, T.; Lemsaddek, A.; Tenreiro, R.; Tavares, L.; Vilela, C.L.; Oliveira, M. Virulence Traits and Antibiotic Resistance among Enterococci Isolated from Eurasian Otter (Lutra lutra). Vet. Microbiol. 2013, 163, 378–382.
- Semedo-Lemsaddek, T.; Pedroso, N.M.; Freire, D.; Nunes, T.; Tavares, L.; Verdade, L.M.; Oliveira, M. Otter Fecal Enterococci as General Indicators of Antimicrobial Resistance Dissemination in Aquatic Environments. Ecol. Indic. 2018, 85, 1113–1120.
- Oliveira, M.; Sales-Luís, T.; Semedo-Lemsaddek, T.; Ribeiro, T.; Pedroso, N.; Tavares, L.; Vilela, C. Chapter 6—Antimicrobial Resistant Aeromonas Isolated from Eurasian Otters (Lutra lutra Linnaeus, 1758) in Portugal. In Animal Diversity, Natural History and Conservation; Daya Publishing House: New Delhi, India, 2011; Volume 1, pp. 123–143.
- Mengistu, T.S.; Garcias, B.; Castellanos, G.; Seminati, C.; Molina-López, R.A.; Darwich, L. Occurrence of Multidrug Resistant Gram-Negative Bacteria and Resistance Genes in Semi-Aquatic Wildlife-Trachemys scripta, Neovison vison and Lutra lutra-as Sentinels of Environmental Health. Sci. Total Environ. 2022, 830, 154814.
- Loncaric, I.; Kübber-Heiss, A.; Posautz, A.; Stalder, G.; Hoffmann, D.; Rosengarten, D.; Walzer, C. Characterization of Methicillin-Resistant Staphylococcus spp. Carrying the mecC Gene, Isolated from Wildlife. J. Antimicrob. Chemother. 2013, 68, 2222–2225.
- Vingino, A.; Roberts, M.; Wainstein, M.; West, J.; Norman, S.; Lambourn, D.; Lahti, J.; Ruiz, R.; D’angeli, M.; Weissman, S.; et al. Antibiotics Surveillance for Antibiotic-Resistant E. coli in the Salish Sea Ecosystem. Antibiotics 2021, 10, 1201.
- Alonso, C.A.; Alcalá, L.; Simón, C.; Torres, C. Novel Sequence Types of Extended-Spectrum and Acquired AmpC Beta-Lactamase Producing Escherichia coli and Escherichia Clade V Isolated from Wild Mammals. FEMS Microbiol. Ecol. 2017, 93, fix097.
- Wilson, J.S.; Hazel, S.M.; Williams, N.J.; Phiri, A.; French, N.P.; Hart, C.A. Nontyphoidal salmonellae in United Kingdom badgers: Prevalence and spatial distribution. Appl. Environ. Microbiol. 2003, 69, 4312–4315.
- Darwich, L.; Vidal, A.; Seminati, C.; Albamonte, A.; Casado, A.; López, F.; Molina-López, R.A.; Migura-Garcia, L. High Prevalence and Diversity of Extended-Spectrum β-Lactamase and Emergence of OXA-48 Producing Enterobacterales in Wildlife in Catalonia. PLoS ONE 2019, 14, e0210686.
- Osińska, M.; Nowakiewicz, A.; Zięba, P.; Gnat, S.; Łagowski, D.; Trościańczyk, A. Wildlife Carnivorous Mammals As a Specific Mirror of Environmental Contamination with Multidrug-Resistant Escherichia coli Strains in Poland. Microb. Drug Resist. 2020, 26, 1120–1131.
- García, L.A.; Torres, C.; López, A.R.; Rodríguez, C.O.; Espinosa, J.O.; Valencia, C.S. Staphylococcus spp. from Wild Mammals in Aragón (Spain): Antibiotic Resistance Status. J. Vet. Res. 2020, 64, 373–379.
- Steele, C.M.; Brown, R.N.; Botzler, R.G. Prevalences of Zoonotic Bacteria Among Seabirds in Rehabilitation Centers Along the Pacific Coast of California and Washington, USA. J. Wildl. Dis. 2005, 41, 735–744.
- Garcês, A. Why Do Antibiotics Fail? A Veterinary Perspective. J. Small Anim. Adv. 2022, 1, 10–15.
- Jacobsen, L.; Wilcks, A.; Hammer, K.; Huys, G.; Gevers, D.; Andersen, S.R. Horizontal Transfer of tet(M) and erm(B) Resistance Plasmids from Food Strains of Lactobacillus plantarum to Enterococcus faecalis JH2-2 in the Gastrointestinal Tract of Gnotobiotic Rats. FEMS Microbiol. Ecol. 2007, 59, 158–166.
- Bengtsson, B.; Persson, L.; Ekström, K.; Unnerstad, H.; Uhlhorn, H.; Börjesson, S. High Occurrence of mecC-MRSA in Wild Hedgehogs (Erinaceus europaeus) in Sweden. Vet. Microbiol. 2017, 207, 103–107.
- Aslam, B.; Khurshid, M.; Arshad, M.I.; Muzammil, S.; Rasool, M.; Yasmeen, N.; Shah, T.; Chaudhry, T.H.; Rasool, M.H.; Shahid, A.; et al. Antibiotic Resistance: One Health One World Outlook. Front. Cell. Infect. Microbiol. 2021, 11, 1153.
- Bonnedahl, J.; Järhult, J.D. Antibiotic Resistance in Wild Birds. Upsala J. Med. Sci. 2014, 119, 113–116.
- Gorski, L.; Jay-Russell, M.T.; Liang, A.S.; Walker, S.; Bengson, Y.; Govoni, J.; Mandrell, R.E. Diversity of Pulsed-Field Gel Electrophoresis Pulsotypes, Serovars, and Antibiotic Resistance Among Salmonella Isolates from Wild Amphibians and Reptiles in the California Central Coast. Foodborne Pathog. Dis. 2013, 10, 540–548.
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Molecules 2018, 23, 795.
- Joosten, P.; Ceccarelli, D.; Odent, E.; Sarrazin, S.; Graveland, H.; Van Gompel, L.; Battisti, A.; Caprioli, A.; Franco, A.; Wagenaar, J.A.; et al. Antimicrobial Usage and Resistance in Companion Animals: A Cross-Sectional Study in Three European Countries. Antibiotics 2020, 9, 87.
- Kruuk, H. Carnivores. In Encyclopedia of Biodiversity; Levin, S.A., Ed.; Elsevier: New York, NY, USA, 2001; pp. 629–640. ISBN 978-0-12-226865-6.
- Amusa, C.; Rothman, J.; Odongo, S.; Matovu, H.; Ssebugere, P.; Baranga, D.; Sillanpää, M. The endangered African Great Ape: Pesticide residues in soil and plants consumed by Mountain Gorillas (Gorilla beringei) in Bwindi Impenetrable National Park, East Africa. Sci. Total Environ. 2021, 758, 143692.
- Campbell, S.J.; Ashley, W.; Gil-Fernandez, M.; Newsome, T.M.; Di Giallonardo, F.; Ortiz-Baez, A.S.; Mahar, J.E.; Towerton, A.L.; Gillings, M.; Holmes, E.C.; et al. Red Fox Viromes in Urban and Rural Landscapes. Virus Evol. 2020, 6, veaa065.
- Vittecoq, M.; Laurens, C.; Brazier, L.; Durand, P.; Elguero, E.; Arnal, A.; Thomas, F.; Aberkane, S.; Renaud, N.; Prugnolle, F.; et al. VIM-1 Carbapenemase-Producing Escherichia coli in Gulls from Southern France. Ecol. Evol. 2017, 7, 1224–1232.
- Blanco, G.; Junza, A.; Segarra, D.; Barbosa, J.; Barrón, D. Wildlife Contamination with Fluoroquinolones from Livestock: Widespread Occurrence of Enrofloxacin and Marbofloxacin in Vultures. Chemosphere 2016, 144, 1536–1543.
- Smoglica, C.; Vergara, A.; Angelucci, S.; Festino, A.R.; Antonucci, A.; Marsilio, F.; Di Francesco, C.E. Antibiotic-Resistant Bacteria Dissemination in the Wildlife, Livestock, and Water of Maiella National Park, Italy. Animals 2023, 13, 432.
- Ulstad, C.R.; Solheim, M.; Berg, S.; Lindbæk, M.; Dahle, U.R.; Wester, A.L. Carriage of ESBL/AmpC-Producing or Ciprofloxacin Non-Susceptible Escherichia coli and Klebsiella spp. in Healthy People in Norway. Antimicrob. Resist. Infect. Control 2016, 5, 1–11.
- Grijjs, J. A Guide to SDG Interactions: From Science to Implementation; International Council for Science (ICSU): Paris, France, 2017.
- Gajdács, M.; Urbán, E.; Stájer, A.; Baráth, Z. Antimicrobial Resistance in the Context of the Sustainable Development Goals: A Brief Review. Eur. J. Investig. Health Psychol. Educ. 2021, 11, 71–82.
- WHO Study Group. Antimicrobial Resistance and the United Nations Sustainable Development Cooperation Framework: Guidance for United Nations Country Teams. Available online: https://www.who.int/publications-detail-redirect/9789240036024 (accessed on 28 November 2023).
More
