Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Francisco Andrés Monsalve.
The study of adipose tissue has received considerable attention due to its importance not just in maintaining body energy homeostasis but also in playing a role in a number of other physiological processes. Beyond storing energy, adipose tissue is important in endocrine, immunological, and neuromodulatory functions, secreting hormones that participate in the regulation of energy homeostasis.
- adipose tissue
- adipocytokines
- overweight
- obesity
1. Adipose Tissue
Beyond storing energy, adipose tissue is important in several processes, such as the modulation of energy homeostasis, metabolism, and the regulation of the immune system [1]. Adipose tissue is considered an endocrine organ since it produces and secrets molecules that can exert their action in surrounding or distant tissues [2]. Adipose tissue is composed of adipocytes, pre-adipocytes, endothelial cells, fibroblasts, and some immune cells such as macrophages, dendritic cells, and T cells that contribute to the release of metabolites, lipids, cytokines, and adipocytokines [3]. Hormones and adipocytokines produced by adipocytes affect the central nervous system, skeletal muscle, liver, bone, and other tissues, which have been studied extensively in the last two decades, establishing that these factors play a preponderant role in the homeostasis of body glucose, through endocrine, autocrine, and paracrine mechanisms. Adipocytokines are essential for the balance between appetite and satiety, body fat reserve and energy expenditure, glucose tolerance, insulin release and sensitivity, cell growth, inflammation, angiogenesis, and reproduction [4].
Under physiological conditions, adipose tissue plays a central role in keeping the homeostasis of the entire body serving as the main storage for excess energy; namely energy that is used during fasting, thus preserving proteins, regulating metabolism, satiety, reproduction, and enhancing the immune response in pathogenic invasion [1].
Adipocytes that have a large drop of lipids, called unilocular adipose cells, form part of the white adipose tissue and cells with multiple small drops of lipids, called multilocular adipose cells, constitute the brown adipose tissue [5]. White adipose tissue is the most abundant and is distributed throughout the body, mainly as perivascular and visceral fat [3]. It produces and secretes adipocytokines, glucocorticoids, and sex hormones [6]. Brown adipose tissue is considered thermogenic and its color reflects its numerous mitochondria [7]. UCP1 decoupling protein is responsible for modifying oxidative phosphorylation in mitochondria, causing a decrease in ATP production and increasing heat production by this tissue [8]. Brown adipose tissue has a regulatory function in body temperature via adaptive thermogenesis, regulating the concentration of circulating triglycerides, storing glucose, and secreting prostaglandins, nitric oxide, adipsin, and other adipocytokines [9].
The beige adipose tissue has an intermediate diameter between white and brown adipose tissue [7]. Originally, it was observed mainly in response to cold [10]. However, factors such as diet, physical, pre and probiotic activity, and drugs, among others, are able to induce the transdifferentiation (to beige or brown) of white adipose tissue. This tissue has the function of storage or energy expenditure, according to physiological needs [8].
The excessive adiposity that occurs in obesity (excess of white adipose tissue) disrupts the metabolic balance of the adipose tissue, producing a negative impact on the homeostasis of the human body. Therefore, obesity is the causality of a group of chronic and complex diseases such as cardiovascular diseases, metabolic syndrome, type 2 diabetes [11], and even some types of cancer [12].
2. Adipose Tissue as an Endocrine Organ
Adipose tissue, besides being an energy reservoir, is an important endocrine organ as it produces several hormones that participate in the regulation of homeostasis. A positive imbalance of adipose tissue (accumulation of fatty acids over a BMI 24.9 kg/m2) generates structural and functional changes in this tissue, favoring the secretion of deleterious adipocytokines related to insulin signaling and those that benefit a pro-inflammatory state, which could promote the development of metabolic and cardiovascular diseases (see Figure 1) [13]. One of the first of these hormones discovered was leptin [14], which suppresses food intake by inducing satiety, along with increasing energy expenditure. Leptin levels are positively correlated with the amount of adipose tissue and it is secreted mainly by visceral white adipose tissue [15,16][15][16]. Adiponectin is a hormone secreted by subcutaneous white adipose tissue which has anti-inflammatory and insulin-sensitizing functions [17]. In overweight or obese people with insulin resistance, plasma adiponectin levels are low [18]. Resistin is another hormone secreted by adipose tissue and has a close relationship with obesity and diabetes, notably contributing to insulin resistance and vascular inflammation [19]. Fibroblast growth factor 21 (FGF21) is a protein produced by adipose and other tissues, with thermogenic effects that promote the transdifferentiation from beige to brown adipose tissue. Like adiponectin, it has insulin-sensitizing effects and in overweight or obese patients, plasmatic concentrations are elevated [13]. Vaspin (also serpin A12) is an inhibitor of serine protease, which acts as an insulin-sensitizing adipocytokine [20,21][20][21], that is increased in obese patients, promoting insulin resistance and decreasing glucose tolerance [22]. Visfatin is another hormone involved in glucose homeostasis and, like acylation-stimulating protein (ASP), is preferentially involved in fat storage [23].
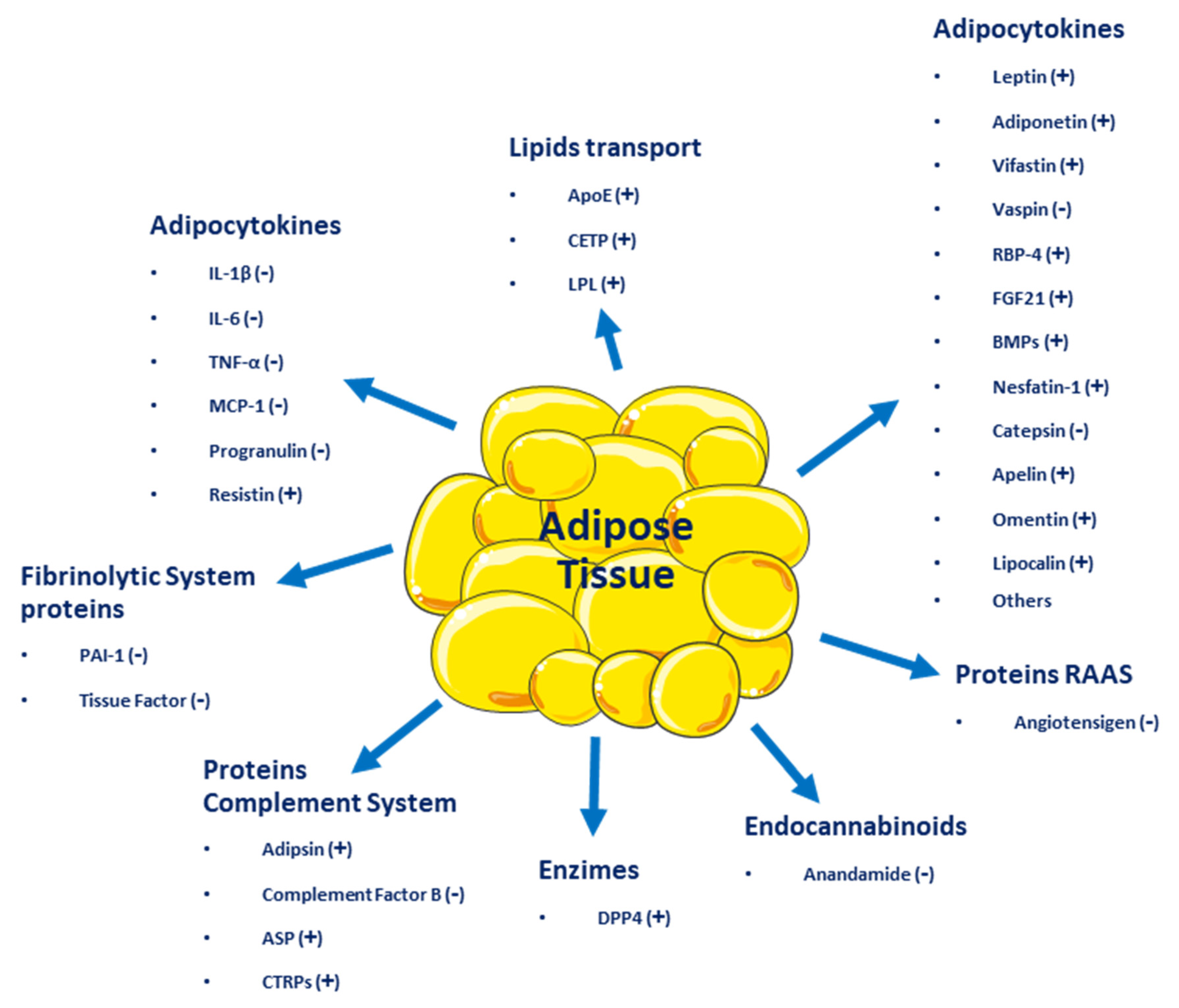
Figure 1. Adipocytokines and other molecules are secreted by adipose tissue. (+) Beneficial effect on energy homeostasis; (−) Negative effect on energy homeostasis.
Adipose tissue secretes inflammatory cytokines such as interleukin-6 (IL-6), IL-8, interferon-γ (INF-γ), and plasminogen activator inhibitor-1 (PAI-1) [24]. Other molecules secreted by adipose tissue include retinol-binding protein 4 (RBP4), omentin, angiotensinogen, macrophage migration inhibitory factor (MIF), lipoprotein lipase (LPL), prostaglandins, estrogens, and glucocorticoids [14]. All these molecules influence homeostatic processes, affecting health positively or negatively and resulting in the development of several diseases, such as type 2 diabetes mellitus (T2DM), metabolic syndrome, and several types of cancer (breast, cervical, endometrial, renal, and gastrointestinal). Even more, adipose tissue dysfunction can lead to psychiatric disorders, such as depression, dementia, insomnia, and many other [1].
3. Definition and Incidence of Overweight and Obesity
The Body Mass Index [BMI (kg/m2, weight of the person divided by the square of their height)] is used to define and diagnose obesity according to the clinical guidelines of the World Health Organization (WHO) [25]. In adults, the WHO defines overweight as a BMI of 25.0 to 29.9 kg/m2 and obese as a BMI > 30.0 kg/m2. In addition, obesity is classified into three levels of severity: class I (BMI 30.0–34.9 kg/m2), class II (BMI 35.0–39.9 kg/m2), and class III (BMI > 40.0 kg/m2) [26]. For every 5-unit increase in BMI above 25.0 kg/m2, overall mortality increases by 29%, vascular mortality by 41%, and diabetes-related mortality by 210% [27].
Obesity is often stigmatized and associated with a false perception that it is primarily caused by a lack of willpower, leading to inappropriate dietary choices and physical inactivity. However, there is abundant literature-based evidence presenting obesity as a complicated chronic medical condition caused by multiple genetic, environmental, metabolic, and behavioral factors [28].
Obesity increases the probability of developing several other diseases and pathological conditions that are linked to increased mortality, such as type 2 diabetes mellitus (T2DM), cardiovascular disease (CVD), metabolic syndrome (MetS), chronic kidney disease (CKD), hyperlipidemia, hypertension, non-alcoholic fatty liver disease (NAFLD), certain types of cancers, obstructive sleep apnea, osteoarthritis, depression, and neurodegenerative disorders [29].
The pathogenesis of obesity is complex, with environmental, socio-cultural, physiological, medical, genetic, epigenetic, and numerous other factors contributing to the cause and persistence of this condition [30].
4. Causes or Mechanisms of Obesity
4.1. Genetic Factors
Data show that around 40 to 70% of the variations in obesity in humans are the result of genetic factors [31]. While environmental changes have increased the rate of obesity, genetic factors play a key role in the development of this condition, with nearly 100 genes related to obesity and fat distribution [28,32][28][32]. It is clear that in the same environment, some people become obese and others do not. Genetic causes of obesity can be classified as (A) monogenic causes that result in a single mutation located mainly in the leptin–melanocortin pathway. Many of the genes, such as AgRP (Agouti-related peptide), PYY (peptide tyrosine tyrosine, and orexigenic), or MC4R (melanocortin-4 receptor), were identified as causing monogenic obesity and deregulating appetite and body weight control systems, where hormonal signaling (ghrelin, leptin, and insulin) is sensed by receptors located in the hypothalamus (arcuate nucleus) [33]. (B) Syndromic obesity, as the result of severe obesity due to neurodevelopmental abnormalities and other organ/system malformations. This can be caused by mutations in a single gene or a chromosomal region that spans multiple genes [34]. (C) Polygenic obesity, caused by the cumulative contribution of several genetic alterations. The presence of these types of alterations causes an increase in caloric intake, an increase in appetite, a reduced control of satiety, and a higher tendency to store body fat and to a sedentary lifestyle [35]. There are also several genetic, neuroendocrine, and chromosomal syndromes that cause obesity, such as Prader-Willi syndrome (PWS), which is a neurodevelopmental disorder involving hypothalamic dysfunction and leading to impaired secretion of several hormones [36] and polycystic ovary syndrome, an endocrine disorder that results in increased body fat mass [37] associated with deletions such as 16p11.2, 2q37 (brachydactylic mental retardation syndrome), 1p36 (monosomy 1p36 syndrome), 9q34 (Kleefstra syndrome), 6q16 (PWS-like syndrome), 17p11.2 (Smith Magenis syndrome), and 11p13 (WAGR syndrome) [38]. All of these conditions show an energy imbalance between calories intake and expenditure as the main cause of obesity [39].4.2. Fat Cells
The excess of calories from food intake results in an accumulation of fat in adipocytes [28]. This enlargement and/or increase in the number of fat cells to adapt to increased fat storage establish the initial pathological lesion in obesity. The accumulation of ectopic fat, such as visceral, cardiac, and muscle fat, is associated with several factors when adipocytes have already reached their maximal storage capacity [40]. Nevertheless, as the group of Scherer showed, a complete abolition of adipose tissue involved in inflammation was associated with several adverse metabolic readouts [41]. In other words, inflammation in a healthy dose is important during adipose expansion. The increase in the size of the adipocyte eventually generates an inflammatory microenvironment due to an alteration of the homeostasis between the adipocyte and the surrounding cells, mainly resident macrophages [4]. The alteration of a healthy expansion in adipocyte size produces an increase in the secretion of several inflammatory adipocytokines, such as leptin, IL-6, TNF-α, angiotensinogen, adipsin, free fatty acids, and lactate, while the levels of anti-inflammatory molecules secreted, such as adiponectin, decreases [42].4.3. Dysregulation of Energy Balance
Genes and the environment interact in a complex manner in physiological processes that regulate energy balance and body weight [43]. Two groups of neurons located in the arcuate nucleus of the hypothalamus are inhibited or stimulated by ghrelin and leptin, they are thus hormones that control energy balance by regulating food intake and energy expenditure, namely AgRP and POMC neurons. Brain regions external to the hypothalamus also contribute to the regulation of energy balance through sensory signals, cognitive processes, memory, and attention [44]. Reducing food intake or increasing physical activity generates a negative energy balance, activating adaptive compensatory mechanisms that preserve vital functions [45]. Conversely, at rest, there is a relative reduction in energy expenditure, seeking food and metabolic processes that depend on the magnitude and duration of caloric restriction [46]. An increase in the stimulation of the orexigenic center could explain a subtle and often inappropriate increase in appetite and food intake, limiting weight loss associated with interventions such as physical exercise programs. It is important to always consider obesity as a chronic disease, that requires long-term monitoring and weight control since there is a high relapse rate in those people who have managed to lose weight [30].4.4. Metabolic and Physiological Effects
As mentioned, adipocytes synthesize signaling molecules (adipocytokines) and hormones and their secretion and effects are influenced by the distribution and amount of adipose tissue in the body [47]. The excessive secretion of proinflammatory adipocytokines by adipocytes and macrophages within adipose tissue leads to low-grade systemic inflammation in people with obesity [30]. Triglyceride breakdown in adipocytes leads to the release of free fatty acids which are then transported in the plasma to sites where they can be metabolized. In overweight people and to a greater extent in those with obesity, free fatty acid levels are often elevated, reflecting the increased mass of adipose tissue [47]. Lipids are not only stored in adipose tissue; they are also stored in other cell types in organelles called liposomes, located near mitochondria [48]. For example, liposomes in hepatocytes increase in size, forming large vacuoles, which are observed in a series of pathological states such as non-alcoholic fatty liver disease, steatohepatitis, and cirrhosis [49], generating cell dysfunction and apoptosis [30]. Elevated levels of free fatty acids, proinflammatory cytokines, and intermediary lipids, such as ceramides, in non-adipose tissues, contribute to impaired insulin signaling and a state of insulin resistance [50]. All these metabolic and anatomical findings are some of the pathophysiological mechanisms caused by dyslipidemia in obesity, type 2 diabetes, obesity-related liver disease, and osteoarthritis; they are also implicated in the development of some cancers, likely owing to the association with elevated levels of tumor-promoting molecules [12]. There is cumulative evidence showing a complex interplay between obesity and both the central nervous system and the peripheral nervous system. These associations are quite complex because they not only involve the so-called organokines (adipokines, myokines, and hepatokines) acting on the nervous system but they also involve hormones and factors secreted by the nervous tissue acting on other organs. Furthermore, there is also evidence that associates inflammation-related obesity to a leaky gut [51,52,53][51][52][53], with alterations in the gut microbiota, through which intact Gram (−) bacteria or broken-down products of its wall pass through the intestinal epithelium and reach the bloodstream [54]. The wall of Gram (−) bacteria is rich in lipopolysaccharides, also called endotoxin, which is a Pathogen Associated Molecular Pattern (PAMP) recognized by the Toll-like receptor 4, a member of the Toll family of receptors, involved in triggering pro-inflammatory signaling through NF-kappa B transcription factor [55] that activates the expression of several genes associated with inflammation, like cytokines and chemokines. Of note, humans are significantly more sensitive to LPS than other species [56]. Finally, sympathetic nervous system hyperactivity in some overweight or obese individuals might produce multiple pathophysiological processes (see Figure 2) such as arterial hypertension, heart disease, a heart attack, and chronic kidney disease, all associated with insulin resistance, dyslipidemia, and type 2 diabetes [57].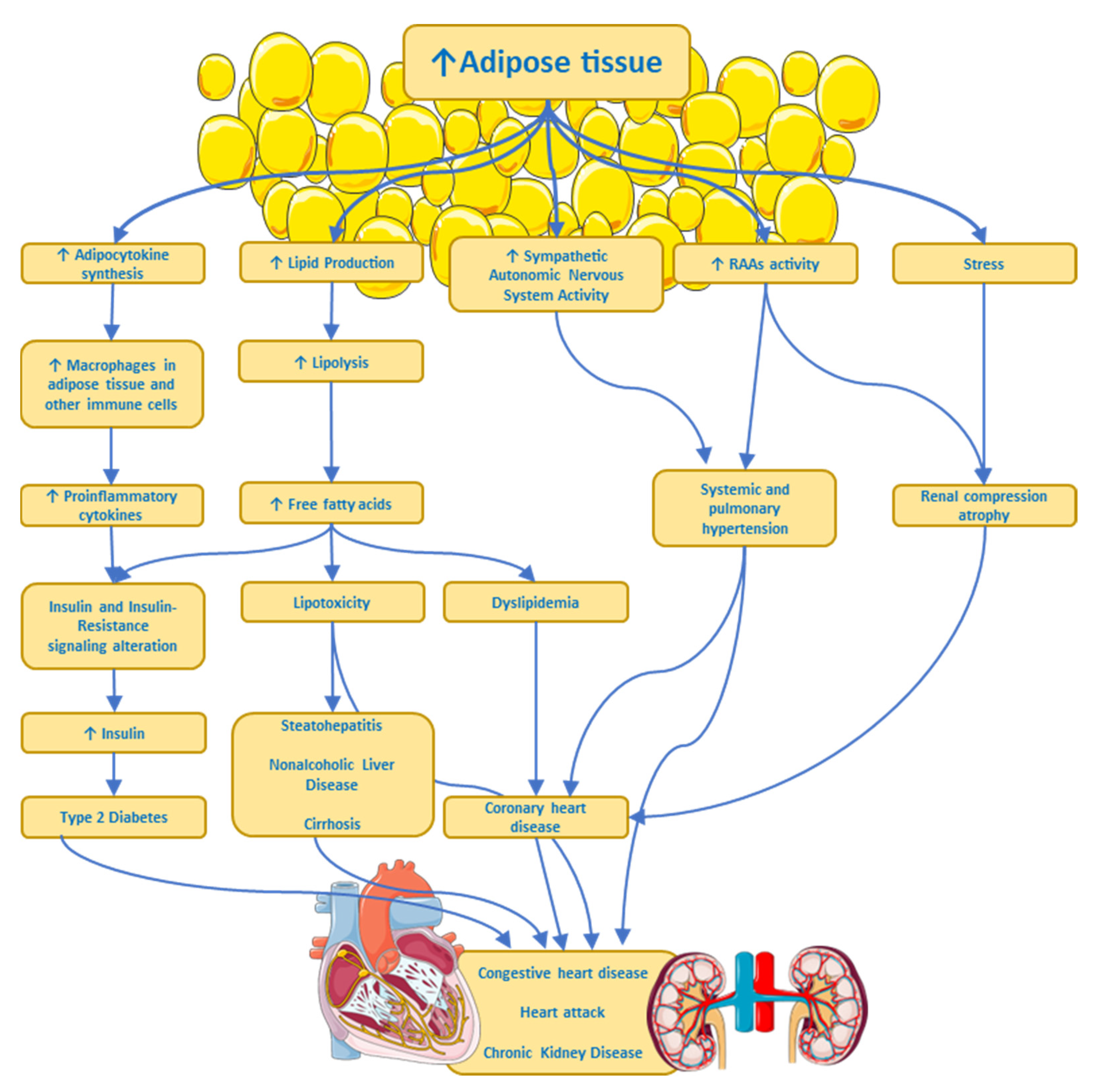
Figure 2. Some pathways by which excess adipose tissue produces risk factors or chronic diseases. ANS, Autonomic Nervous System; RAAs, Renin–Angiotensin–Aldosterone System. ↑ imply an increase.
References
- Booth, A.; Magnuson, A.; Fouts, J.; Foster, M.T. Adipose tissue: An endocrine organ playing a role in metabolic regulation. Horm. Mol. Biol. Clin. Investig. 2016, 26, 25–42.
- Gimeno, R.E.; Klaman, L.D. Adipose tissue as an active endocrine organ: Recent advances. Curr. Opin. Pharmacol. 2005, 5, 122–128.
- Zwick, R.K.; Guerrero-Juarez, C.F.; Horsley, V.; Plikus, M.V. Anatomical, Physiological, and Functional Diversity of Adipose Tissue. Cell Metab. 2018, 27, 68–83.
- Ringel, A.E.; Drijvers, J.M.; Baker, G.J.; Catozzi, A.; García-Cañaveras, J.C.; Gassaway, B.M.; Miller, B.C.; Juneja, V.R.; Nguyen, T.H.; Joshi, S.; et al. Obesity Shapes Metabolism in the Tumor Microenvironment to Suppress Anti-Tumor Immunity. Cell 2020, 183, 1848–1866.e26.
- Cinti, S. The adipose organ at a glance. Dis. Model Mech. 2012, 5, 588–594.
- Wang, F.; Vihma, V.; Soronen, J.; Turpeinen, U.; Hämäläinen, E.; Savolainen-Peltonen, H.; Mikkola, T.S.; Naukkarinen, J.; Pietiläinen, K.H.; Jauhiainen, M.; et al. 17β estradiol and estradiol fatty acyl esters and estrogen-converting enzyme expression in adipose tissue in obese men and women. J. Clin. Endochrinol. Metab. 2013, 98, 4923–4931.
- Kajimura, S.; Spiegelman, B.M.; Seale, P. Brown and Beige Fat: Physiological Roles beyond Heat Generation. Cell Metab. 2015, 22, 546–559.
- Vega-Robledo, G.; Rico-Rosillo, M.G. Adipose tissue: Immune function and alterations caused by obesity. Rev. Alerg. Mex. 2016, 66, 340–353.
- Svensson, K.J.; Long, J.Z.; Jedrychowski, M.P.; Cohen, P.; Lo, J.C.; Serag, S.; Kir, S.; Shinoda, K.; Tartaglia, J.A.; Rao, R.R.; et al. A secreted Slit2 fragment regulates adipose tissue thermogenesis and metabolic function. Cell Metab. 2016, 23, 454–466.
- Wu, J.; Boström, P.; Sparks, L.M.; Ye, L.; Choi, J.H.; Giang, A.H.; Khandekar, M.; Virtanen, K.A.; Nuutila, P.; Schaart, G.; et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012, 150, 366–376.
- O’Neill, S.; O’Driscoll, L. Metabolic syndrome: A closer look at the growing epidemic and its associated pathologies. Obes. Rev. 2015, 16, 1–12.
- Rathmell, J.C. Obesity, Immunity, and Cancer. N. Engl. J. Med. 2021, 384, 1160–1162.
- Fasshauer, M.; Blüher, M. Adipokines in health and disease. Trends Pharmacol. Sci. 2015, 36, 461–470.
- Szablewski, L. Introductory Chapter: Adipose. In Tissue; IntechOpen: London, UK, 2019.
- Atzmon, G.; Yang, X.M.; Muzumdar, R.; Ma, X.H.; Gabriely, I.; Barzilai, N. Differential gene expression between visceral and subcutaneous fat depots. Horm. Metab. Res. 2002, 34, 622–628.
- Bastard, J.P.; Maachi, M.; Lagathu, C.; Kim, M.J.; Caron, M.; Vidal, H.; Capeau, J.; Feve, B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur. Cytokine Netw. 2006, 17, 4–12.
- Fain, J.N.; Madan, A.K.; Hiler, M.L.; Cheema, P.; Bahouth, S.W. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology 2004, 145, 2273–2282.
- Choi, H.M.; Doss, H.M.; Kim, K.S. Multifaceted Physiological Roles of Adiponectin in Inflammation and Diseases. Int. J. Mol. Sci. 2020, 21, 1219.
- Aida-Souki, A.-R.; Prieto-Fuenmayor, C.; Cano-Ponce, C. Aspectos Básicos en Obesidad; Ediciones Universidad Simón Bolívar: Barranquilla, Colombia, 2018; pp. 1–44.
- Hida, K.; Wada, J.; Eguchi, J.; Zhang, H.; Baba, M.; Seida, A.; Hashimoto, I.; Okada, T.; Yasuhara, A.; Nakatsuka, A.; et al. Visceral adipose tissue-derived serine protease inhibitor: A unique insulin-sensitizing adipocytokine in obesity. Proc. Natl. Acad. Sci. USA 2005, 102, 10610–10615.
- Ulbricht, D.; Pippel, J.; Schultz, S.; Meier, R.; Sträter, N.; Heiker, J.T. A unique serpin P1′ glutamate and a conserved β-sheet C arginine are key residues for activity, protease recognition and stability of serpinA12 (vaspin). Biochem. J. 2015, 470, 357–367.
- Pilarski, Ł.; Pelczyńska, M.; Koperska, A.; Seraszek-Jaros, A.; Szulińska, M.; Bogdański, P. Association of Serum Vaspin Concentration with Metabolic Disorders in Obese Individuals. Biomolecules 2023, 13, 508.
- Fukuhara, A.; Matsuda, M.; Nishizawa, M.; Segawa, K.; Tanaka, M.; Kishimoto, K.; Matsuki, Y.; Murakami, M.; Ichisaka, T.; Murakami, H.; et al. Visfatin: A protein secreted by visceral fat that mimics the effects of insulin. Science 2005, 307, 426–430.
- DeFuria, J.; Belkina, A.C.; Jagannathan-Bogdan, M.; Snyder-Cappione, J.; Carr, J.D.; Nersesova, Y.R.; Markham, D.; Strissel, K.J.; Watkins, A.A.; Zhu, M.; et al. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proc. Natl. Acad. Sci. USA 2013, 110, 5133–5138.
- WHO. Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation; World Health Organization Technical Report Series 894; WHO Consultation on Obesity: Geneva, Switzerland, 2000; 253p.
- Poirier, P.; Giles, T.D.; Bray, G.A.; Hong, Y.; Stern, J.S.; Pi-Sunyer, F.X.; Eckel, R.H. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 968–976.
- Apovian, C.M. Obesity: Definition, comorbidities, causes, and burden. Am. J. Manag. Care 2016, 22, s176–s185.
- Bray, G.A.; Kim, K.K.; Wilding, J.P.H.; Federation, W.O. Obesity: A chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes. Rev. 2017, 18, 715–723.
- Swinburn, B.A.; Sacks, G.; Hall, K.D.; McPherson, K.; Finegood, D.T.; Moodie, M.L.; Gortmaker, S.L. The global obesity pandemic: Shaped by global drivers and local environments. Lancet 2011, 378, 804–814.
- Heymsfield, S.B.; Wadden, T.A. Mechanisms, Pathophysiology, and Management of Obesity. N. Engl. J. Med. 2017, 376, 1492.
- Wu, Y.; Duan, H.; Tian, X.; Xu, C.; Wang, W.; Jiang, W.; Pang, Z.; Zhang, D.; Tan, Q. Genetics of Obesity Traits: A Bivariate Genome-Wide Association Analysis. Front. Genet. 2018, 9, 179.
- Shungin, D.; Winkler, T.W.; Croteau-Chonka, D.C.; Ferreira, T.; Locke, A.E.; Mägi, R.; Strawbridge, R.J.; Pers, T.H.; Fischer, K.; Justice, A.E.; et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature 2015, 518, 187–196.
- Thaker, V.V. Genetic and Epigenetic Causes of Obesity. Adolesc. Med. State Art Rev. 2017, 28, 379–405.
- Huvenne, H.; Dubern, B.; Clément, K.; Poitou, C. Rare Genetic Forms of Obesity: Clinical Approach and Current Treatments in 2016. Obes. Facts 2016, 9, 158–173.
- Koochakpour, G.; Esfandiar, Z.; Hosseini-Esfahani, F.; Mirmiran, P.; Daneshpour, M.S.; Sedaghati-Khayat, B.; Azizi, F. Evaluating the interaction of common FTO genetic variants, added sugar, and trans-fatty acid intakes in altering obesity phenotypes. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 474–480.
- Gupta, N.; Jain, V. Prader Willi Syndrome—A Common Epigenetic Cause of Syndromic Obesity. Indian J. Pediatr. 2017, 84, 809–810.
- Cena, H.; Chiovato, L.; Nappi, R.E. Obesity, Polycystic Ovary Syndrome, and Infertility: A New Avenue for GLP-1 Receptor Agonists. J. Clin. Endocrinol. Metab. 2020, 105, e2695–e2709.
- D’Angelo, C.S.; Koiffmann, C.P. Copy number variants in obesity-related syndromes: Review and perspectives on novel molecular approaches. J. Obes. 2012, 2012, 845480.
- Lin, X.; Li, H. Obesity: Epidemiology, Pathophysiology, and Therapeutics. Front. Endocrinol. 2021, 12, 706978.
- Rosenbaum, M.; Knight, R.; Leibel, R.L. The gut microbiota in human energy homeostasis and obesity. Trends Endocrinol. Metab. 2015, 26, 493–501.
- Wernstedt Asterholm, I.; Tao, C.; Morley, T.S.; Wang, Q.A.; Delgado-Lopez, F.; Wang, Z.V.; Scherer, P.E. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab. 2014, 20, 103–118.
- Arita, Y.; Kihara, S.; Ouchi, N.; Takahashi, M.; Maeda, K.; Miyagawa, J.; Hotta, K.; Shimomura, I.; Nakamura, T.; Miyaoka, K.; et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 1999, 257, 79–83.
- Pigeyre, M.; Yazdi, F.T.; Kaur, Y.; Meyre, D. Recent progress in genetics, epigenetics and metagenomics unveils the pathophysiology of human obesity. Clin. Sci. 2016, 130, 943–986.
- van der Klaauw, A.A.; Farooqi, I.S. The hunger genes: Pathways to obesity. Cell 2015, 161, 119–132.
- MacLean, P.S.; Higgins, J.A.; Giles, E.D.; Sherk, V.D.; Jackman, M.R. The role for adipose tissue in weight regain after weight loss. Obes. Rev. 2015, 16 (Suppl. S1), 45–54.
- Ochner, C.N.; Tsai, A.G.; Kushner, R.F.; Wadden, T.A. Treating obesity seriously: When recommendations for lifestyle change confront biological adaptations. Lancet Diabetes Endocrinol. 2015, 3, 232–234.
- Tchkonia, T.; Thomou, T.; Zhu, Y.; Karagiannides, I.; Pothoulakis, C.; Jensen, M.D.; Kirkland, J.L. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab. 2013, 17, 644–656.
- Heymsfield, S.B.; Hu, H.H.; Shen, W.; Carmichael, O. Emerging Technologies and their Applications in Lipid Compartment Measurement. Trends Endocrinol. Metab. 2015, 26, 688–698.
- McCullough, A.J. The clinical features, diagnosis and natural history of nonalcoholic fatty liver disease. Clin. Liver Dis. 2004, 8, 521–533.
- Kaur, J. A comprehensive review on metabolic syndrome. Cardiol. Res. Pract. 2014, 2014, 943162.
- Schéle, E.; Grahnemo, L.; Anesten, F.; Hallén, A.; Bäckhed, F.; Jansson, J.O. The gut microbiota reduces leptin sensitivity and the expression of the obesity-suppressing neuropeptides proglucagon (Gcg) and brain-derived neurotrophic factor (Bdnf) in the central nervous system. Endocrinology 2013, 154, 3643–3651.
- Jiang, L.; Su, H.; Wu, X.; Shen, H.; Kim, M.H.; Li, Y.; Myers, M.G.; Owyang, C.; Rui, L. Leptin receptor-expressing neuron Sh2b1 supports sympathetic nervous system and protects against obesity and metabolic disease. Nat. Commun. 2020, 11, 1517.
- Ochoa-Repáraz, J.; Kasper, L.H. The Second Brain: Is the Gut Microbiota a Link Between Obesity and Central Nervous System Disorders? Curr. Obes. Rep. 2016, 5, 51–64.
- Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut microbiota, intestinal permeability, and systemic inflammation: A narrative review. Intern. Emerg. Med. 2023.
- Fitzgerald, K.A.; Rowe, D.C.; Barnes, B.J.; Caffrey, D.R.; Visintin, A.; Latz, E.; Monks, B.; Pitha, P.M.; Golenbock, D.T. LPS-TLR4 signaling to IRF-3/7 and NF-kappaB involves the toll adapters TRAM and TRIF. J. Exp. Med. 2003, 198, 1043–1055.
- Munford, R.S. Murine responses to endotoxin: Another dirty little secret? J. Infect. Dis. 2010, 201, 175–177.
- Hall, J.E.; da Silva, A.A.; do Carmo, J.M.; Dubinion, J.; Hamza, S.; Munusamy, S.; Smith, G.; Stec, D.E. Obesity-induced hypertension: Role of sympathetic nervous system, leptin, and melanocortins. J. Biol. Chem. 2010, 285, 17271–17276.
More
