Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Wendy Huang and Version 1 by Boniface Ndayambaza.
Water is a vital resource in the agricultural systems of countries impacted by aridity and salinity. Worldwide efforts to reduce quantitative yield losses on Populus euphratica by adapting tree plant production to unfavorable environmental conditions have been made in response to the responsiveness of the increasing control of water stress. Although there has been much advancement in identifying the genes that resist abiotic stresses, little is known about how plants such as P. euphratica deal with numerous abiotic stresses. P. euphratica is a varied riparian plant that can tolerate drought, salinity, low temperatures, and climate change, and has a variety of water stress adaptability abilities.
- P. euphratica
- salt stress
- drought stress
- soil
- genes
- photosynthesis
1. Introduction
P. euphratica Oliv. (P. euphratica), the Euphrates poplar or desert poplar, is non-halophyte and mesophyte in its morphology, but has a high abiotic stress tolerance [1] and is an important component of riparian ecosystems in arid regions. This poplar tree species is mainly distributed in Southwestern Europe, including Spain [2]; Western Asian countries, including Iran [3], Iraq, Syria, and Turkey [4]; Central Asia, including Kazakhstan [5]; Pakistan [6]; India [7]; and China’s western Inner Mongolia, Xinjiang, and other arid regions [8,9,10,11,12][8][9][10][11][12]. It is also found in many other countries outside of Asia, such as the Middle East and North Africa (Kenya and Morocco [13,14][13][14]). P. euphratica is mostly found in the Chinese province Xinjiang, the Tarim Basin area, where it covers 89.1% of its total territory and is dispersed along rivers [15,16][15][16]. Although not a halophyte, it can adapt to extreme conditions, from flooding to extremely dry, hot atmospheres, with temperatures ranging from +54 °C to –45 °C, and from normal soil to soil with very high salinity (up to 2~5%) [17]. The adaptation of P. euphratica to high saline concentrations and high pH levels could be due to its physiological capacity [18,19,20][18][19][20]. Deserts have some of the harshest ecosystems because they combine high temperatures and low rainfall. Abiotic stress is an important variable affecting agricultural production and reducing tree yields [21,22][21][22]. Globally, these pressures include heavy metals, high pH, salinity, extreme temperatures (hot and cold), and extreme water levels (drought) [23]. The variety of plant evolution within gene regulatory profiles helps to control the water and ion balance, sustain proper photosynthesis, and resist abiotic stress [24,25][24][25]. These regulatory genes involve several physiological, metabolic, and cellular activities, including transcription, signal transduction, photosynthesis, energy metabolism, and protein synthesis and breakdown [26,27][26][27]. P. euphratica is a deciduous tree that may reach a height of 15 m and belongs to the Salicaceae family. This species is a dioecy, pollinated by the wind, and has different individuals for its male and female flowers [28]. Its fruits are capsules of tiny seeds produced in enormous amounts with cotton-like appendages [29], where these seeds disperse efficiently via wind at great distances. In phreatophytes, vegetative regeneration is very significant. P. euphratica roots spread virtually horizontally within the top 0.6 m of the soil, and the suckers they create can reach a distance of up to 40 m from their parent trees [30]. A phreatophyte found in arid areas is P. euphratica [31] because it can survive salt, which makes it an excellent natural genetic resource for developing plants with salt tolerance. P. euphratica has acquired a variety of morphological characteristics to cope with the salinity of its environment [32], including a unique hydraulic system [33] and succulent leaves [34,35][34][35]. Desertification occurs frequently in the riparian forests along the Xinjiang and Inner Mongolia sites, which are suitable for P. euphratica establishment [36,37][36][37]. To clarify the adaptation mechanisms that allow this species to live in the seasonal change-prone riparian ecosystem, P. euphratica uses the soil and trunk as water reservoirs to manage water stress during brief drought periods [38,39,40][38][39][40]. The restoration of many species in water-scarce places has a scientific foundation thanks to several studies, which also helps to improve the soil water balance and maintain the stability of the vegetation ecology in arid areas of China [41,42][41][42]. Consequently, countless studies confirm that gene families play a decisive role in the effects of drought, salt, and low-temperature stress on physiological and biochemical processes, which impede photosynthesis and destroy cell membranes.
In the last two decades, genomic information from decoded plant genomes, including O. sativa and A. thaliana, has employed DNA microarray technology with a bioinformatics method to evaluate the expression of genome-scale transcripts (mRNA) or transcriptomes. For instance, several miRNAs, including miR395, miR398, and miR399 in P. tremula, were enhanced under salt stress in microarray studies on forestry species. When A. thaliana was exposed to salt stress, miR398 was significantly down-regulated [43]. Under salinity conditions, P. euphratica displayed substantial changes in the expression levels of MiR168, miR1444, and miR1446 [44]. Under low-temperature stress, genes and their functions were analyzed [45]. With the increasing growth of omics data, bioinformatics methods have advanced [46], including those of genome, transcriptome [47], and proteome, alongside the quick development of computer technology [48]. Bioinformatics is also built on the fundamental idea that any biological mechanism comprises many molecular events, and that knowing the interplay within and between distinct levels of genomic architecture is the only way to comprehend phenotypic features [49,50][49][50]. Due to their high throughput and genome-wide performance, P. trichocarpa genome draft and poplar DNA microarray methods contribute to the inconclusive identification of gene functions [51,52,53,54,55][51][52][53][54][55]. Functional understanding, including metabolic pathways, protein complexes, and stress responses, is predicted through co-expression scrutiny. P. euphratica has also been studied [36,55,56][36][55][56]; the genome sequencing data were uploaded at the NCBI [56,57,58][56][57][58] and co-expressed the functions that respond to biotic stress. P. euphratica shows transcriptional modification in signaling, photoprotection, oxidative stress detoxification, and the suppression of stomatal closure, potentially changing drought stress responses (as illustrated in Figure 1) [47]. The expression of particular gene sets in plants determines their susceptibility to stress and their level of resistance to it. P. euphratica proteins’ functions in plant growth, development, metabolic pathways, and stress responses require advanced study for their regulatory roles and functions.
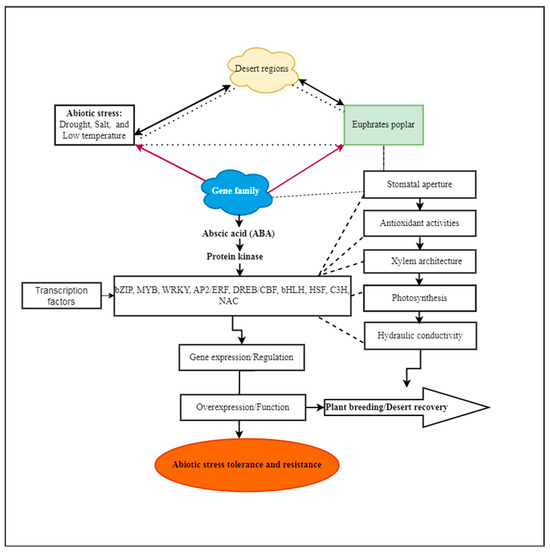
Figure 1. Abiotic stresses are perceived by P. euphratica throughout transcription regulation to ensure abiotic stress tolerance and resistance in arid areas.
2. Effect of Salt Stress in P. euphratica
Around 60% of the world’s land surface is exaggerated by salt, which spreads due to ineffective irrigation practices or water contaminated by salt. The total amount of soluble salts in the soil is measured as soil salinity, and high levels can kill or induce plant wilting. Salts, including NaCl, CaCl2, gypsum, magnesium sulfate, potassium chloride, and sodium sulfate, are frequently found in saline soils. As a result of salt growth in the cytoplasm, high salinity can harm and even kill leaves. Halophytes are salt-tolerant plants that can survive in environments with salinities higher than 400 mM. Terrestrial vascular plants depend on xylem water transfer and stomatal evaporation to survive in stressful environments. The leaves of P. euphratica are wax-coated, rigid, and thick, preventing oxidation-related damage. They grow more robust xylems with drought-induced cavitation due to their adaptation to hydraulic conductivity and embolism. P. euphratica grows in dry, hot climes; increases its photosynthetic rate and evaporation; accumulates salts; deepens its tape roots; and increases its salt content. The primary root penetrates one meter of soil vertically, while the lower end develops lateral feeder roots. However, salt stress has a major negative impact on plant development, growth, and reproduction. Understanding salt tolerance pathways is essential, as rapid exposure activates genes involved in ribosome activities, photosynthesis, cell development, and transport. Reactive oxygen species (ROS) in excess can harm organisms by degrading chlorophyll and causing membrane leakage. It is necessary to find tree and woody plant species that can withstand salt and improve their resistance. The P. euphratica tree is a good example of a tree with salt tolerance. P. euphratica has lengthy juvenile periods and frequently reproduces across several years. One of the main characteristics that sets P. euphratica apart from other plants is its secondary growth. It has the capacity to produce thickened vascular bundles that accumulate to create secondary xylem (dicots) or wood-like tissue (monocots), which allows them to improve their transport capacity when needed. This species has high outcrossing rates, long-distance pollen distribution, large effective populations, an arborescent stature, longevity, and late successional communities. These characteristics could make P. euphratica less susceptible to genetic bottlenecks and more resilient to habitat fragmentation and climatic changes. Tissue-specific differentially expressed genes (DEG) under salt stress has diverse functions, with membrane transporter activity being the most significant leaf function and the oxidation–reduction process being the most significant root function. Gene families like SOS, NHX, GolS, GPX, APX, RBHF, and CBL are involved in ionic homeostasis in P. euphratica seedling tissues [99][59]. DEGs, such as antioxidant genes, contribute to ROS scavenging and plant salinity tolerance by maintaining ionic and ROS homeostasis in tissues and improving ion uptake, transport, and compartmentalization [100,101][60][61]. The regulation of pathways, including plasma membrane and tonoplast Na+/H+ transporters, is crucial for salt stress tolerance. P. euphratica halophytes maintain a low Na+ influx and prevent Na+ accumulation [102,103][62][63]. Ionic homeostasis is accomplished through genes that code for pyrophosphatase, cation/proton antiporters, plasma membrane and vacuolar H+-ATPases, and salt tolerance systems (as illustrated in Figure 2) [104,105][64][65]. P. euphratica studies reveal genes controlling the cells’ salt tolerance, ion compartmentalization, xylem loading, and potassium levels [18,106,107][18][66][67]. P. euphratica has intricate and interrelated defense mechanisms that help it avoid or lessen environmental harm. As part of these systems, transcription factors (TFs) bind to cis-elements in the promoters of target genes or other useful modular structures to regulate how genes are expressed in response to abiotic stress. In P. euphratica, 2382 TFs (2382 loci) have been discovered and categorized into 58 families following the family assignment guidelines. The P. euphratica genome has a high content of TFs (http://planttfdb.gao-lab.org/index.php?sp=Peu, accessed on 6 July 2023) and various transcription factors, including DREB, bZIP, AP2/ERF, WRKY, and bHLH, that regulate plant responses to stress (as mentioned in Figure 1), including salt stress [108,109][68][69]. In non-woody plants, WRKY genes have just been discovered; nonetheless, the effects of salinity on these transcription factors in woody plants may be pertinent. Interestingly, researchers have revealed that salt stress inhibits the PalWRKY77 gene by reducing the salt tolerance in P alba var. pyramidalis [110][70]. The PalWRKY77 pathway receives a bad signal from this ABA regulatory mechanism, making poplar trees more vulnerable to salinity. However, the salt-induced transcriptional response of PeWRKY1 in P. euphratica reveals that WRKY1 binds to H+-ATPase promoters, improving gene expression and salt tolerance [72][71]. However, little is understood about the modifications that the P. euphratica xylem undergoes in response to salinity. Addressing the other transcription factor members is needed to facilitate a molecular revolution for tree breeding in other species.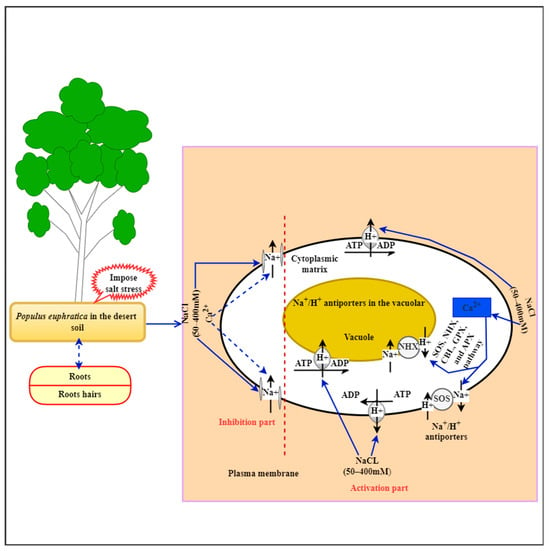
Figure 2.
A proposed diagram model of Na
+
/H
+
homeostasis in
P. euphratica
during NaCl stress tolerance in arid areas.
3. Effect of Drought Stress on the Physiology of P. euphratica
According to different studies, typical abiotic stress, known as drought, impacts cellular homeostasis, gas exchange, seed generation, plant development, and water relations [111,112][72][73]. Plants have evolved innate defenses against drought stress to adapt to harsh settings, such as closing stomata, decreasing transpiration, producing abscisic acid (ABA), and storing hydrogen peroxide (H2O2) [113][74]. Although drought can severely restrict a plant’s ability to grow and develop, family genes are thought to have a key role in how the plant reacts to various conditions. Drought impacts the physiological environment of the soil microbiota and plants. Auxins, cytokinins, gibberellins, and abscisic acid, among other phytohormones, are produced by bacteria and have been shown to increase drought resilience in P. euphratica [114,115][75][76]. As plant-growth factors, due to their capacity to produce endospores, which enable bacterial survival for lengthy periods under unfavorable environmental conditions, bacterium genera, including Bacillus sp., are frequently discovered in arid land [116][77]. At the same time, the various metabolisms and strong physical tolerance of Pseudomonas sp. isolates in P.euphratica cultivated in desolate and saline soil may explain their large abundance [117][78]. Plant rhizobacteria support plant development, biological regulation, and resistance to abiotic stress through direct and indirect processes [118][79]. The direct mechanisms involve phytohormone regulation, the release of volatile compounds, and an enhanced plant uptake of nutrients [119][80]. The indirectly beneficial effects include suppressing deleterious microorganisms and pathogens, competition for nutrients, inhibiting enzymes, and triggering host-induced systemic resistance [120,121][81][82]. The rhizosphere of both sexes suggests the presence of sex-specific variation in bacterial communities and their relative abundances [122][83]. In reaction to dryness, males have more drought-tolerant fungus and bacteria in their rhizospheres than females [123][84]. The altered bacterial and fungal community composition increase soil ammonification in the rhizosphere of female plants. The contribution of Rhizobium in biocontrol activities against pathogens and the alleviation of stresses play a decisive role in the P. euphratica. For instance, recent discoveries discussed the significance of rhizobia, which promote plant growth by reducing salt and osmotic stressors in contaminated soils (see [124][85] and references therein). Additionally, rhizobium occurrence and utilities in microbiomes of non-leguminous plants were reviewed to control the growth of various soilborne plant pathogens by focusing on the biological control of the different genera [125][86]. Rhizobium populi sp. nov., an endophytic bacterium recovered from P. euphratica, was particularly isolated from the storage fluids in the stems of P. euphratica trees, which is an interesting development [126][87]. The studies conducted on the pathogenic fungus in the pathogenic site of P. euphratica found that it increased its survival rate in arid regions [127][88]. Microbiome research indicates that planting P. euphratica may influence bacterial communities, possibly resulting in more infections. However, research on the relationship between pathogenic bacteria and a high rate of plant mortality is lacking. Different research revealed that the synergistic actions of the dioecious P. euphratica roots and coexisting microorganisms allow them to respond to and survive drought stress [128,129][89][90]. However, research on the identification of genes associated with microorganisms to cope with abiotic stresses remains scarce in this model tree plant. P. euphratica grows in deep water, relying on water table depth, but faces drought-induced cavitation, causing shoot cessation, stomatal closure, and reduced root growth [130,131][91][92]. Recent findings have also unveiled that long-term irrigation in P. euphratica plantations affects soil phosphorus fractions and microbial communities [132][93]. They found positive relationships between inorganic P and various bacteria, while negative associations were observed with Burkholderiaceae and soil phosphorus (soil P) in its inorganic form (Pi). The restudyearch suggests that water management techniques focusing on soil microbial recovery could improve soil quality. However, the increased mineralization of organic P in P. euphratica is linked to soil moisture, pH, and microorganism profiles, necessitating future research on foliar P fraction distribution. Based on the research conducted on the impact of cow dung and biochar on phosphorus efficiency in P. euphratica soil, bacterial communities, and functional genes (phoC, phoD, gcd, and pqqC; see [133][94] and references therein), the reseauthorchers found that returning cow dung improves soil properties, seedling growth, and phosphorus availability, which are up-taken throughout the roots of P. euphratica. Biochar, a carbonized form of cow manure, has a more definite cumulative phosphorus content and promotion of bacterial diversity in arid regions. However, the restudyearch suggests increasing biochar use in plantation management and conducting a long-term analysis to discover the utmost scientific addition technique for P. euphratica seedlings and cow dung. In desert environments, mycorrhizal associations and symbiotic interactions between fungi and plant roots increase plant resilience to drought stress [134,135][95][96]. These connections aid in the intake of nutrients, particularly phosphorus, which is necessary for plant growth [136][97]. These plants survive in arid conditions by developing specialized mycorrhizal associations, which enhance the soil’s surface area for the uptake of nutrients and water, as well as delivering carbon compounds from the host plant [137][98]. It is interesting to note that P. euphratica may also help us to fully comprehend the intricate mechanisms underpinning P. euphratica’s resilience to drought stress and salt stress, which are mediated via mycorrhizal connections in the desert. The restudyearch reveals that not all arbuscular mycorrhizal fungi (AMF) can infect and colonize plant roots. In extreme conditions, one species can become predominant. The alkaline P. euphratica rhizosphere soil favored G. mosseae growth, suggesting its selectivity and adaptability. Other AMF species may survive in the rhizosphere soil or colonize P. euphratica roots. There is, however, a small gap in this restudyearch that has to be filled [138][99]. Photosynthesis is one the most significant processes in a plant’s life, but drought stress may affect its mechanisms, which stops plant growth and development under severe ecological conditions. The direct impacts of drought stress on photosynthesis include fluctuations in photosynthetic metabolism and constraints on diffusion via the stomata and mesophyll. Secondary effects include oxidative damage brought on by the superposition of various stresses. Interestingly, a thorough investigation comparing salt and drought stress found that both conditions resulted in the down-regulation of several photosynthetic genes. In P. euphratica, for example, the roots and leaves suggested that during stress, protein concentration can be altered without affecting gene expression [131,139][92][100]. Importantly, at the transcriptome level, 27 photosynthesis genes were differentially expressed during drought stress, with the majority being down-regulated and six genes enhancing expression [47]. For examining the processes of abiotic tolerance in woody plants, Populus euphratica is a potential candidate species. For instance, under salt and drought stress conditions, PeGSTU58 overexpression lines showed increased expression of various stress-responsive genes, such as DREB2A, COR47, RD22, CYP8D11, and SOD1. Additionally, PebHLH35 has been demonstrated to be able to directly bind to the promoter region of PeGSTU58 and stimulate its expression in yeast one-hybrid experiments and luciferase studies. These findings suggested that PeGSTU58, whose expression was favorably controlled by PebHLH35, had a role in the tolerance to salt and drought stress by maintaining ROS homeostasis [140][101].References
- Chen, S.; Li, J.; Wang, S.; Hüttermann, A.; Altman, A. Salt, nutrient uptake and transport, and ABA of Populus euphratica; a hybrid in response to increasing soil NaCl. Trees 2001, 15, 186–194.
- Huang, X.; Lv, R.; Zhou, Z.; Fan, M.; Bai, Y.; Ding, Y.; Yang, G. CiteSpace Software Visualization Analyses of the Last Thirty Years of Research on Populus euphratica. Forests 2023, 14, 714.
- Tavakoli Neko, H.; Shirvani, A.; Assareh, M.H.; Morshedloo, M.R. Physiological Response to Salinity Stress in Various Populus euphratica Oliv. Ecotypes in Iran. Ecopersia 2019, 7, 97–103. Available online: http://ecopersia.modares.ac.ir/article-24-25997-en.html1 (accessed on 15 October 2023).
- Kansu, Ç.; Kaya, Z. Genetic diversity of marginal populations of Oliv. from highly fragmented river ecosystems. Silvae Genet. 2020, 69, 139–151.
- Wang, T.Y.; Wang, P.; Wang, Z.L.; Niu, G.Y.; Yu, J.J.; Ma, N.; Wu, Z.N.; Pozdniakov, S.P.; Yan, D.H. Drought adaptability of phreatophytes: Insight from vertical root distribution in drylands of China. J. Plant Ecol. 2021, 14, 1128–1142.
- Qiu, Q.; Ma, T.; Hu, Q.; Liu, B.; Wu, Y.; Zhou, H.; Wang, Q.; Wang, J.; Liu, J. Genome-scale transcriptome analysis of the desert poplar, Populus euphratica. Tree Physiol. 2011, 31, 452–461.
- Eusemann, P.; Petzold, A.; Thevs, N.; Schnittler, M. Growth patterns and genetic structure of Populus euphratica Oliv.(Salicaceae) forests in NW China–Implications for conservation and management. For. Ecol. Manag. 2013, 297, 27–36.
- Monda, Y.; Miki, N.; Yoshikawa, K. Stand structure and regeneration of Populus euphratica forest in the lower reaches of the Heihe River, NW China. Landsc. Ecol. Eng. 2008, 4, 115–124.
- Lam, T.Y.; Kleinn, C.; Coenradie, B. Double sampling for stratification for the monitoring of sparse tree populations: The example of Populus euphratica Oliv. forests at the lower reaches of Tarim River, Southern Xinjiang, China. Environ. Monit. Assess. 2011, 175, 45–61.
- Zhang, W.; Liu, P.; Feng, Q.; Wang, T.; Wang, T. The spatiotemporal responses of Populus euphratica to global warming in Chinese oases between 1960 and 2015. J. Geogr. Sci. 2018, 28, 579–594.
- Du, F.K.; Xu, F.; Qu, H.; Feng, S.; Tang, J.; Wu, R. Exploiting the transcriptome of Euphrates Poplar, Populus euphratica (Salicaceae) to develop and characterize new EST-SSR markers and construct an EST-SSR database. PLoS ONE 2013, 8, e61337.
- Gai, Z.; Zhai, J.; Chen, X.; Jiao, P.; Zhang, S.; Sun, J.; Qin, R.; Liu, H.; Wu, Z.; Li, Z. Phylogeography reveals geographic and environmental factors driving genetic differentiation of Populus sect. Turanga in Northwest China. Front. Plant Sci. 2021, 12, 5083.
- Phan, C.T.; Jörgensen, J.; Jouve, L.; Hausman, J.F.; Polle, A.; Teichmann, T. Micropropagation of Populus euphratica Olivier. Belg. J. Bot. 2004, 137, 175–180. Available online: https://www.jstor.org/stable/20794551 (accessed on 15 October 2023).
- Ma, T.; Wang, K.; Hu, Q.; Xi, Z.; Wan, D.; Wang, Q.; Feng, J.; Jiang, D.; Ahani, H.; Abbott, R.J.; et al. Ancient polymorphisms and divergence hitchhiking contribute to genomic islands of divergence within a poplar species complex. Proc. Natl. Acad. Sci. USA 2018, 115, E236–E243.
- Overdieck, D.; Ziche, D.; Yu, R. Gas exchange of Populus euphratica leaves in a riparian zone. J. Arid Land 2013, 5, 531–541.
- Lu, K.Q.; Qin, F.; Li, Y.; Xie, G.; Li, J.F.; Cui, Y.-M.; Ferguson, D.K.; Yao, Y.F.; Wang, G.H.; Wang, Y.F. A new approach to interpret vegetation and ecosystem changes through time by establishing a correlation between surface pollen and vegetation types in the eastern central Asian desert. Palaeogeography 2020, 551, 109762.
- Zhang, P.; Deng, X.; Long, A.; Xu, H.; Ye, M.; Li, J. Change in spatial distribution patterns and regeneration of Populus euphratica under different surface soil salinity conditions. Sci. Rep. 2019, 9, 9123.
- Chen, S.; Polle, A. Salinity tolerance of Populus. Plant Biol. 2010, 12, 317–333.
- Junghans, U.; Polle, A.; Düchting, P.; Weiler, E.; Kuhlman, B.; Gruber, F.; Teichmann, T. Adaptation to high salinity in poplar involves changes in xylem anatomy and auxin physiology. Plant Cell Environ. 2006, 29, 1519–1531.
- Yue, H.; Zhao, L.; Yang, D.; Zhang, M.; Wu, J.; Zhao, Z.; Xing, X.; Zhang, L.; Qin, Y.; Guo, F. Comparative analysis of the endophytic bacterial diversity of Populus euphratica oliv. in environments of different salinity intensities. Microbiol. Spectr. 2022, 10, e00500–e00522.
- Tavakoli Neko, H.; Shirvany, A.; Assareh, M.; Naghavi, M.; Pessarakli, M.; Pourmeidani, A. Effects of NaCl on growth, yield and ion concentration of various Populus euphratica Oliv. ecotypes in Iran. Desert 2018, 23, 189–198. Available online: https://jdesert.ut.ac.ir/article_69116_072933987c764fffcebcd5fe41363399.pdf (accessed on 15 September 2023).
- Rajput, V.D.; Minkina, T.; Yaning, C.; Sushkova, S.; Chapligin, V.A.; Mandzhieva, S. A review on salinity adaptation mechanism and characteristics of Populus euphratica, a boon for arid ecosystems. Acta Ecol. Sin. 2016, 36, 497–503.
- Polle, A.; Chen, S.L.; Eckert, C.; Harfouche, A. Engineering Drought Resistance in Forest Trees. Front. Plant Sci. 2018, 9, 1875.
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119.
- Loredana, F.C.; Pasqualina, W.; Amodio, F.; Giovanni, P.; Petronia, C. Plant Genes for Abiotic Stress; IntechOpen: Rijeka, Crotia, 2011; pp. 285–308.
- Saibo, N.J.; Lourenço, T.; Oliveira, M.M. Transcription factors and regulation of photosynthetic and related metabolism under environmental stresses. Ann. Bot. 2009, 103, 609–623.
- Carthew, R.W. Gene Regulation and Cellular Metabolism: An Essential Partnership. Trends Genet. 2021, 37, 389–400.
- Dong, X.; Chen, X.; Gai, Z.; Zhai, J.; Guo, X.; Han, X.; Zhang, S.; Wu, Z.; Li, Z. Phenotypic Diversity and Variation in Natural Populus euphratica Populations Shaped by Environmental Factors. Contemp. Probl. Ecol. 2023, 16, 230–252.
- Soleimani, A.; Etemad, V.; Calagari, M.; Namiranian, M.; Shirvany, A. Influence of climatic factors on fruit morphological traits in Populus euphratica Oliv. Ann. For. Res. 2014, 57, 31–38.
- Wiehle, M.; Eusemann, P.; Thevs, N.; Schnittler, M. Root suckering patterns in Populus euphratica (Euphrates poplar, Salicaceae). Trees 2009, 23, 991–1001.
- Thomas, F.M. Ecology of Phreatophytes; Springer: Berlin, Germany, 2013; pp. 335–375.
- Zhai, J.; Li, Z.; Si, J.; Zhang, S.; Han, X.; Chen, X. Structural and Functional Responses of the Heteromorphic Leaves of Different Tree Heights on Populus euphratica Oliv. to Different Soil Moisture Conditions. Plants 2022, 11, 2376.
- Li, D.; Si, J.; Zhang, X.; Gao, Y.; Wang, C.; Luo, H.; Qin, J.; Gao, G. Hydraulic characteristics of Populus euphratica in an arid environment. Forests 2019, 10, 407.
- Yu, L.; Dong, H.; Li, Z.; Han, Z.; Korpelainen, H.; Li, C. Species-specific responses to drought, salinity and their interactions in Populus euphratica and P. pruinosa seedlings. Plant Ecol. 2020, 13, 563–573.
- Chen, Y.; Chen, Y.; Zhou, H.; Hao, X.; Zhu, C.; Fu, A.; Yang, Y.; Li, W. Research advances in plant physiology and ecology of desert riparian forests under drought stress. Forests 2022, 13, 619.
- Jia, H.; Liu, G.; Li, J.; Zhang, J.; Sun, P.; Zhao, S.; Zhou, X.; Lu, M.; Hu, J. Genome resequencing reveals demographic history and genetic architecture of seed salinity tolerance in Populus euphratica. J. Exp. Bot. 2020, 71, 4308–4320.
- Zhang, Z.; Huisingh, D. Combating desertification in China: Monitoring, control, management and revegetation. J. Clean. Prod. 2018, 182, 765–775.
- Ma, J.X.; Huang, X.; Li, W.H.; Zhu, C.G. Sap flow and trunk maximum daily shrinkage (MDS) measurements for diagnosing water status of Populus euphratica in an inland river basin of Northwest China. Ecohydrology 2013, 6, 994–1000.
- Zhang, Y.; Hao, X.; Sun, H.; Hua, D.; Qin, J. How Populus euphratica utilizes dew in an extremely arid region. Plant Soil 2019, 443, 493–508.
- Fan, X.; Hao, X.; Zhang, S.; Zhao, Z.; Zhang, J.; Li, Y. Populus euphratica counteracts drought stress through the dew coupling and root hydraulic redistribution processes. Ann. Bot. 2023, 131, 451–461.
- Shengyue, F.; Lihua, Z. Desertification control in China: Possible solutions. Ambio 2001, 30, 384–385.
- Wang, L.; Zhu, G.; Lin, X.; Liu, Y.; Zhao, K.; Sang, L.; Zhang, W.; Qiu, D.; Zhang, Z.; Sun, Z. Water use patterns of dominant species of riparian wetlands in arid areas. Hydrol. Process. 2023, 37, e14835.
- Li, J.; Song, Q.; Zuo, Z.-F.; Liu, L. MicroRNA398: A Master Regulator of Plant Development and Stress Responses. Int. J. Mol. Sci. 2022, 23, 10803.
- Lotfi, A.; Pervaiz, T.; Jiu, S.; Faghihi, F.; Jahanbakhshian, Z.; Khorzoghi, E.G.; Fang, J.; Seyedi, S.M. Role of microRNAs and their target genes in salinity response in plants. Plant Growth Regul. 2017, 82, 377–390.
- Chen, J.; Tian, Q.; Pang, T.; Jiang, L.; Wu, R.; Xia, X.; Yin, W. Deep-sequencing transcriptome analysis of low temperature perception in a desert tree, Populus euphratica. BMC Genom. 2014, 15, 326.
- Wu, Z.; Jiang, Z.; Li, Z.; Jiao, P.; Zhai, J.; Liu, S.; Han, X.; Zhang, S.; Sun, J.; Gai, Z. Multi-omics analysis reveals spatiotemporal regulation and function of heteromorphic leaves in Populus. Plant Physiol. 2023, 192, 188–204.
- Tang, S.; Liang, H.; Yan, D.; Zhao, Y.; Han, X.; Carlson, J.E.; Xia, X.; Yin, W. Populus euphratica: The transcriptomic response to drought stress. Plant Mol. Biol. 2013, 83, 539–557.
- Tahmasebi, A.; Niazi, A.; Akrami, S. Integration of meta-analysis, machine learning and systems biology approach for investigating the transcriptomic response to drought stress in Populus species. Sci. Rep. 2023, 13, 847.
- Cobb, J.N.; Juma, R.U.; Biswas, P.S.; Arbelaez, J.D.; Rutkoski, J.; Atlin, G.; Hagen, T.; Quinn, M.; Ng, E.H. Enhancing the rate of genetic gain in public-sector plant breeding programs: Lessons from the breeder’s equation. Theor. Appl. Genet. 2019, 132, 627–645.
- Zhao, C.; Zhang, Y.; Du, J.; Guo, X.; Wen, W.; Gu, S.; Wang, J.; Fan, J. Crop phenomics: Current status and perspectives. Front. Plant Sci. 2019, 10, 714.
- Yan, D.H.; Fenning, T.; Tang, S.; Xia, X.; Yin, W. Genome-wide transcriptional response of Populus euphratica to long-term drought stress. Plant Sci. 2012, 195, 24–35.
- Rosso, L.; Cantamessa, S.; Bergante, S.; Biselli, C.; Fricano, A.; Chiarabaglio, P.M.; Gennaro, M.; Nervo, G.; Secchi, F.; Carra, A. Responses to Drought Stress in Poplar: What Do We Know and What Can We Learn? Life 2023, 13, 533.
- Tuskan, G.A.; Difazio, S.; Jansson, S.; Bohlmann, J.; Grigoriev, I.; Hellsten, U.; Putnam, N.; Ralph, S.; Rombauts, S.; Salamov, A. The genome of black cottonwood, Populus trichocarpa (Torr. & Gray). Science 2006, 313, 1596–1604.
- Evans, L.M.; Slavov, G.T.; Rodgers-Melnick, E.; Martin, J.; Ranjan, P.; Muchero, W.; Brunner, A.M.; Schackwitz, W.; Gunter, L.; Chen, J.-G.; et al. Population genomics of Populus trichocarpa identifies signatures of selection and adaptive trait associations. Nat. Genet. 2014, 46, 1089–1096.
- Slavov, G.T.; DiFazio, S.P.; Martin, J.; Schackwitz, W.; Muchero, W.; Rodgers-Melnick, E.; Lipphardt, M.F.; Pennacchio, C.P.; Hellsten, U.; Pennacchio, L.A.; et al. Genome resequencing reveals multiscale geographic structure and extensive linkage disequilibrium in the forest tree Populus trichocarpa. New Phytol. 2012, 196, 713–725.
- Ma, T.; Wang, J.; Zhou, G.; Yue, Z.; Hu, Q.; Chen, Y.; Liu, B.; Qiu, Q.; Wang, Z.; Zhang, J. Genomic insights into salt adaptation in a desert poplar. Nat. Commun. 2013, 4, 2797.
- Zhang, Q.J.; Gao, L.Z. The complete chloroplast genome sequence of desert poplar (Populus euphratica). Mitochondrial DNA Part A 2016, 27, 721–723.
- Lowe, T.M.; Eddy, S.R. tRNAscan-SE: A program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997, 25, 955–964.
- Yu, L.; Ma, J.; Niu, Z.; Bai, X.; Lei, W.; Shao, X.; Chen, N.; Zhou, F.; Wan, D. Tissue-specific transcriptome analysis reveals multiple responses to salt stress in Populus euphratica seedlings. Genes 2017, 8, 372.
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963.
- Brini, F.; Masmoudi, K. Ion Transporters and Abiotic Stress Tolerance in Plants. ISRN Mol. Biol. 2012, 2012, 927436.
- Wu, Y.; Ding, N.; Zhao, X.; Zhao, M.; Chang, Z.; Liu, J.; Zhang, L. Molecular characterization of PeSOS1: The putative Na+/H+ antiporter of Populus euphratica. Plant Mol. Biol. 2007, 65, 1–11.
- Zhang, X.; Liu, L.; Chen, B.; Qin, Z.; Xiao, Y.; Zhang, Y.; Yao, R.; Liu, H.; Yang, H. Progress in understanding the physiological and molecular responses of Populus to salt stress. Int. J. Mol. Sci. 2019, 20, 1312.
- Ye, C.Y.; Zhang, H.C.; Chen, J.H.; Xia, X.L.; Yin, W.L. Molecular characterization of putative vacuolar NHX-type Na+/H+ exchanger genes from the salt-resistant tree Populus euphratica. Physiol. Plant. 2009, 137, 166–174.
- Xie, Q.; Zhou, Y.; Jiang, X. Structure, Function, and Regulation of the Plasma Membrane Na+/H+ Antiporter Salt Overly Sensitive 1 in Plants. Front. Plant Sci. 2022, 13, 866265.
- Zeng, F.; Yan, H.; Arndt, S.K. Leaf and whole tree adaptations to mild salinity in field grown Populus euphratica. Tree Physiol. 2009, 29, 1237–1246.
- Sun, J.; Zhang, X.; Deng, S.; Zhang, C.; Wang, M.; Ding, M.; Zhao, R.; Shen, X.; Zhou, X.; Lu, C. Extracellular ATP signaling is mediated by H2O2 and cytosolic Ca2+ in the salt response of Populus euphratica cells. PLoS ONE 2012, 7, e53136.
- Hoang, X.L.T.; Nhi, D.N.H.; Thu, N.B.A.; Thao, N.P.; Tran, L.P. Transcription Factors and Their Roles in Signal Transduction in Plants under Abiotic Stresses. Curr. Genom. 2017, 18, 483–497.
- Khoso, M.A.; Hussain, A.; Ritonga, F.N.; Ali, Q.; Channa, M.M.; Alshegaihi, R.M.; Meng, Q.; Ali, M.; Zaman, W.; Brohi, R.D.; et al. WRKY transcription factors (TFs): Molecular switches to regulate drought, temperature, and salinity stresses in plants. Front. Plant Sci. 2022, 13, 39329.
- Jiang, Y.; Tong, S.; Chen, N.; Liu, B.; Bai, Q.; Chen, Y.; Bi, H.; Zhang, Z.; Lou, S.; Tang, H.; et al. The PalWRKY77 transcription factor negatively regulates salt tolerance and abscisic acid signaling in Populus. Plant Cell Biol. 2021, 105, 1258–1273.
- Yao, J.; Shen, Z.; Zhang, Y.; Wu, X.; Wang, J.; Sa, G.; Zhang, Y.; Zhang, H.; Deng, C.; Liu, J.; et al. Populus euphratica WRKY1 binds the promoter of H+-ATPase gene to enhance gene expression and salt tolerance. J. Exp. Bot. 2019, 71, 1527–1539.
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 2006, 58, 221–227.
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324.
- Ullah, A.; Manghwar, H.; Shaban, M.; Khan, A.H.; Akbar, A.; Ali, U.; Ali, E.; Fahad, S. Phytohormones enhanced drought tolerance in plants: A coping strategy. Environ. Sci. Pollut. Res. 2018, 25, 33103–33118.
- Iqbal, S.; Wang, X.; Mubeen, I.; Kamran, M.; Kanwal, I.; Díaz, G.A.; Abbas, A.; Parveen, A.; Atiq, M.N.; Alshaya, H. Phytohormones trigger drought tolerance in crop plants: Outlook and future perspectives. Front. Plant Sci. 2022, 12, 799318.
- Egamberdieva, D.; Wirth, S.J.; Alqarawi, A.A.; Abd Allah, E.F.; Hashem, A. Phytohormones and Beneficial Microbes: Essential Components for Plants to Balance Stress and Fitness. Front. Microbiol. 2017, 8, 2104.
- Wang, S.; Ouyang, L.; Ju, X.; Zhang, L.; Zhang, Q.; Li, Y. Survey of plant drought-resistance promoting bacteria from Populus euphratica tree living in arid area. Indian J. Microbiol. 2014, 54, 419–426.
- Mawar, R.; Ranawat, M.; Sharma, S.K.; Sayyed, R. Plant Growth Promoting Microorganisms of Arid Region; Springer: Singapore, 2023; pp. 1–25.
- Dimkpa, C.; Weinand, T.; Asch, F. Plant–rhizobacteria interactions alleviate abiotic stress conditions. Plant Cell Environ. 2009, 32, 1682–1694.
- Gyaneshwar, P.; Naresh Kumar, G.; Parekh, L.; Poole, P. Role of soil microorganisms in improving P nutrition of plants. Plant Soil 2002, 245, 83–93.
- Rodriguez, P.A.; Rothballer, M.; Chowdhury, S.P.; Nussbaumer, T.; Gutjahr, C.; Falter-Braun, P. Systems biology of plant-microbiome interactions. Mol. Plant 2019, 12, 804–821.
- Boro, M.; Sannyasi, S.; Chettri, D.; Verma, A.K. Microorganisms in biological control strategies to manage microbial plant pathogens: A review. Arch. Microbiol. 2022, 204, 666.
- Xia, Z.; He, Y.; Yu, L.; Li, Z.; Korpelainen, H.; Li, C. Revealing interactions between root phenolic metabolomes and rhizosphere bacterial communities in Populus euphratica plantations. Biol. Fertil. Soils 2021, 57, 421–434.
- Hultine, K.R.; Grady, K.C.; Wood, T.E.; Shuster, S.M.; Stella, J.C.; Whitham, T.G. Climate change perils for dioecious plant species. Nat. Plants 2016, 2, 16109.
- Bellabarba, A.; Fagorzi, C.; diCenzo, G.C.; Pini, F.; Viti, C.; Checcucci, A. Deciphering the symbiotic plant microbiome: Translating the most recent discoveries on rhizobia for the improvement of agricultural practices in metal-contaminated and high saline lands. Agronomy 2019, 9, 529.
- Gazolla Volpiano, C.; Lisboa, B.; Granada, C.; São José, J.; Oliveira, A.; Beneduzi, A.; Perevalova, Y.; Passaglia, L.; Vargas, L. Rhizobia for Biological Control of Plant Diseases; Springer: Singapore, 2019; pp. 315–336.
- Rozahon, M.; Ismayil, N.; Hamood, B.; Erkin, R.; Abdurahman, M.; Mamtimin, H.; Abdukerim, M.; Lal, R.; Rahman, E. Rhizobium populi sp. nov., an endophytic bacterium isolated from Populus euphratica. Int. J. Syst. Evol. Microbiol. 2014, 64, 3215–3221.
- Tuo, Y.; Dong, Z.; Wang, X.; Gao, B.; Zhu, C.; Tuo, F. Metagenomics reveal correlations between microbial organisms in soils and the health of Populus euphratica. Front. Microbiol. 2020, 11, 2095.
- Xia, Z.; He, Y.; Xu, J.; Zhu, Z.; Korpelainen, H.; Li, C. Rhizosphere microbe populations but not root traits induced by drought in Populus euphratica males. Soil Ecol. Lett. 2023, 5, 220152.
- Juvany, M.; Munné-Bosch, S. Sex-related differences in stress tolerance in dioecious plants: A critical appraisal in a physiological context. J. Exp. Bot. 2015, 66, 6083–6092.
- Hukin, D.; Cochard, H.; Dreyer, E.; Thiec, D.L.; Bogeat-Triboulot, M.B. Cavitation vulnerability in roots and shoots: Does Populus euphratica Oliv., a poplar from arid areas of Central Asia, differ from other poplar species? J. Exp. Bot. 2005, 56, 2003–2010.
- Bogeat-Triboulot, M.B.A.; Brosché, M.; Renaut, J.; Jouve, L.; Le Thiec, D.; Fayyaz, P.; Vinocur, B.; Witters, E.; Laukens, K.; Teichmann, T.; et al. Gradual Soil Water Depletion Results in Reversible Changes of Gene Expression, Protein Profiles, Ecophysiology, and Growth Performance in Populus euphratica, a Poplar Growing in Arid Regions. Plant Physiol. 2006, 143, 876–892.
- He, Y.; Lin, X.; Wang, L.; Ma, X.; Fang, L.; Xia, Z. Effects of long-term irrigation on soil phosphorus fractions and microbial communities in Populus euphratica plantations. For. Res. 2023, 3, 17.
- Fan, Y.; Lv, G.; Chen, Y.; Chang, Y.; Li, Z. Differential effects of cow dung and its biochar on Populus euphratica soil phosphorus effectiveness, bacterial community diversity and functional genes for phosphorus conversion. Front. Plant Sci. 2023, 14, 1242469.
- Bahadur, A.; Batool, A.; Nasir, F.; Jiang, S.; Mingsen, Q.; Zhang, Q.; Pan, J.; Liu, Y.; Feng, H. Mechanistic Insights into Arbuscular Mycorrhizal Fungi-Mediated Drought Stress Tolerance in Plants. Int. J. Mol. Sci. 2019, 20, 4199.
- Zou, Y.N.; Qin, Q.Y.; Ma, W.Y.; Zhou, L.J.; Wu, Q.S.; Xu, Y.J.; Kuča, K.; Hashem, A.; Al-Arjani, A.B.F.; Almutairi, K.F.; et al. Metabolomics reveals arbuscular mycorrhizal fungi-mediated tolerance of walnut to soil drought. BMC Plant Biol. 2023, 23, 118.
- Cheng, S.; Zou, Y.N.; Kuča, K.; Hashem, A.; Abd Allah, E.F.; Wu, Q.S. Elucidating the Mechanisms Underlying Enhanced Drought Tolerance in Plants Mediated by Arbuscular Mycorrhizal Fungi. Front. Microbiol. 2021, 12, 809473.
- Madouh, T.A.; Quoreshi, A.M. The Function of Arbuscular Mycorrhizal Fungi Associated with Drought Stress Resistance in Native Plants of Arid Desert Ecosystems: A Review. Diversity 2023, 15, 391.
- Yang, Y.; Chen, Y.; Cai, B.; Jie, W.; Lv, D. The arbuscular mycorrhizal symbiotic status of Populus euphratica, a drought resistant tree species from arid lands. Ecohydrology 2013, 6, 1001–1008.
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2008, 103, 551–560.
- Meng, H.; Zhao, J.; Yang, Y.; Diao, K.; Zheng, G.; Li, T.; Dai, X.; Li, J. PeGSTU58, a Glutathione S-Transferase from Populus euphratica, Enhances Salt and Drought Stress Tolerance in Transgenic Arabidopsis. Int. J. Mol. Sci. 2023, 24, 9354.
More
