You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Peter Tang and Version 1 by Abhiram Gunaratnam.
Nano-nitrogen fertilizers (NNFs) have emerged as a promising technology in the field of agriculture, offering potential solutions to improve nutrient uptake efficiency, enhance crop productivity, and reduce environmental impacts. NNFs showed superior characteristics and performance on crops and, therefore, became a potential alternative to conventional nitrogen (N) fertilizers. These fertilizers enhance plant uptake while simultaneously reducing environmental losses. For example, a hydroxy appetite-based urea NNF extended the N release for 112 days, which could cover the N demand of many perennial crops, thus reducing losses.
- crop responses
- environmental impacts
- nanoparticles
- smart fertilizers
- synergist
1. Introduction
The ever-growing population has a substantial impact on food demand and necessitates higher agricultural production [1]. With limited arable land, increasing the agricultural input rates, including nitrogen (N) fertilizers, to obtain higher productivity is seen as a successful way to address the growing food demand [2]. Nitrogen is a major nutrient for plants that primarily influences vegetative growth and crop yield [3]. Although higher levels of N fertilizer are applied with the goal of achieving higher yields, only a small fraction is actually taken up by the plants, while a significant portion is lost through processes such as leaching, runoff, and gaseous emissions, namely N2O, NO, and NH3 [4,5][4][5]. With the main focus on increasing productivity, the negative environmental consequences of increasing the application rate or excessively applying N fertilizers have been often overlooked in the past [6]. However, after several studies showed the detrimental effects of higher N application rates on soil health, water quality, and ecosystem sustainability, a paradigm shift has taken place toward the sustainable use of N fertilizers in agricultural systems.
The current and future agriculture practices should align with the Sustainable Development Goals (SDGs) established by the United Nations to effectively tackle global challenges such as poverty, hunger, climate change, and environmental degradation. Sustainable agriculture plays a crucial role in achieving the SDGs. Achieving SDGs can be facilitated through the precise application of input resources, particularly N fertilizers. Toward this, several approaches are being used, namely split applications, integrated nutrient management, application closer to the root zone, foliar application, the application of fertilizer after substantial development of crops, and the use of smart fertilizers [6,7][6][7]. Of these, smart fertilizers are the latest technology used to minimize nitrogen losses to the environment and increase nitrogen utilization efficiency (NUE).
Smart fertilizers, also known as slow- (SRFs) or controlled-release fertilizers (CRFs), have the ability to release nutrients in a slower manner than conventional fertilizer, and their release pattern matches the crop nutrient demand [8,9][8][9]. Several materials have been used in the formulation of SRFs, and nanomaterials have emerged as a recent addition to this repertoire. These materials are within the range of 1–100 nm. Nanomaterials offer unique properties to improve nutrient releases, such as high surface area, high reactivity, and high porosity, thus advancing the field of fertilizer technology [10,11][10][11]. Owing to the high surface area to volume ratio, nanomaterials adsorb nutrients higher than bulk materials. Hence, the loading capacity of nanomaterials is very high [12]. Nanofertlizers showed higher absorbance than conventional fertilizers when they were used as foliar applications [13]. Furthermore, nanoparticles are applied as synergists along with fertilizers to increase crop performance [10,14][10][14].
2. Materials Used in Nanoparticle Preparation
2.1. Clay Minerals
Clay minerals are used in nanofertilizers due to their unique properties such as high cation exchange capacity (CEC), porous structure, high surface-to-volume ratio, colloidal property, and ease of modification. Additionally, they are readily available and cheap materials compared to their counterparts. Bentonite [15], attapulgite [16], kaoline [17], and glauconite [18] are a few of the clay minerals used for the formulation of NNFs. Bentonite is an aluminum phyllosilicate clay that is primarily composed of montmorillonite, a member of the smectite group of minerals [19]. It is derived from volcanic ash deposits that have undergone weathering and transformation over time. The lamellar structure of bentonite consists of two silica tetrahedral sheets with an alumina octahedral sheet sandwiched in between. The sheet structure of attapulgite contains interlayer spaces that are occupied by various cations. These cations have the ability to be replaced by other cations [20]. Therefore, this provides the opportunity to incorporate several macro and micronutrients with this clay. The CEC of this clay ranged between 60 and 150 meq 100 g−1 of soil [21]. Several NNFs were developed using bentonite as a raw material [11,15][11][15]. In a study, Umar et al. [11,15][11][15] developed an SRF by coating urea with Zn-fortified nano-bentonite. To enhance the presence of Zn2+ on the active sites of bentonite, nano-bentonite was treated with varying concentrations of a ZnSO4 solution. Subsequently, urea was coated using two different methods: firstly, by employing vegetable oil and nano-bentonite (referred to as ZnBenVegU), and secondly, using stearic acid, paraffin oil, Ca(OH)2, and nano-bentonite (referred to as ZnBenParU). The results of a soil incubation study indicated that the release of urea was effectively controlled for up to 10 days with ZnBenVegU and up to 15 days with ZnBenParU. The authors propose that the network structure of bentonite potentially increases the distance water must traverse, thereby reducing urea dissolution. Liu et al. [11,15][11][15] developed an SRF by reacting biochar together with bentonite and polyvinyl alcohol (PVA) and impregnated the urea into it. Through a reaction between the -OH group of bentonites and biochar along with polyvinyl alcohol (PVA), new bonds were formed. The findings of this restudyearch demonstrated that bentonite and urea were successfully incorporated within the cavities and channels of biochar and underwent polymerization with PVA. This impregnation and polymerization process effectively slowed down the dissolution of urea in water for a period of up to 42 days. In another study, urea was intercalated with quaternary ammonium lignin (QAL) modified nano-bentonite and then mixed with sodium alginate to form NNFs [22]. Attapulgite, also known as palygorskite, is a hydrated aluminum–magnesium silicate (Mg,Al)4(Si)8(O,OH,H2O)26·nH2O). This is a naturally occurring nano-clay with a nanorod structure, and the average length and width of this rod are 800–1000 and 30–40 nm, respectively [16]. Attapulgite exhibits a ribbon-layer structure with a 2:1 arrangement in which ribbons are interconnected through the inversion of SiO4 tetrahedra (Figure 1). This arrangement allows for the formation of channels and tunnels [34][23], which could accommodate foreign materials or other minerals. The cation exchange capacity of attapulgite ranged between 11 and 33 meq 100 g−1 of soil [35][24].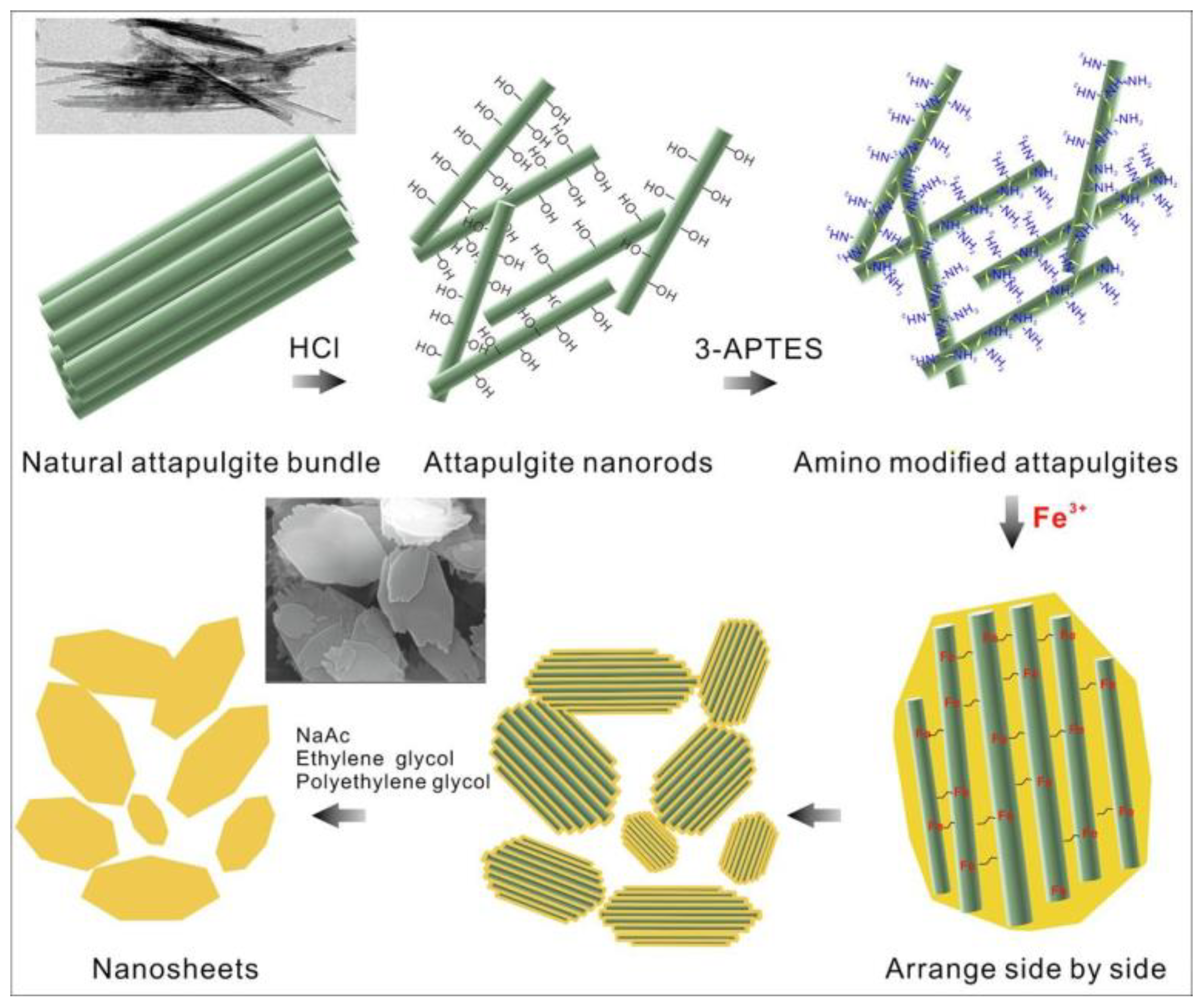
2.2. Minerals
Glauconite is a green-colored mineral belonging to the mica group. It is a hydrous potassium, iron, and aluminum silicate mineral ((K,Na)(Fe,Al,Mg)2(Si,Al)4O10(OH)2). It is commonly found in sedimentary rocks, such as sandstones and shales. The structure of glauconite consists of a 2:1 layered arrangement, with an octahedral layer sandwiched between two tetrahedral layers [37][28]. In preparing NNF, Rudmin et al. [28][27] found that glauconite was activated using chemical methods, mechanical methods, or a combination of both. Ammonium dihydrogen phosphate (ADP) was then loaded into the activated glauconite. Various analyses confirmed that ADP molecules were adsorbed by the surface and meso and macro pores, as well as intercalated between layers of glauconite. In a soil leaching column test, this formulation demonstrated an extended release of nutrients for more than 56 days. Zeolites are characterized as three-dimensional, microporous, crystalline solids that possess distinct structures composed of aluminum, silicon, and oxygen within their framework. Additionally, the pores of zeolites contain cations and water molecules [32][29]. Zeolite’s high pore density and anion exchange capacity allow for the incorporation of a significant number of anions within its structure. Two different zeolites, synthesized zeolite clinoptilonite (SZC) and synthesized zeolite montmorillonite (SZM), were prepared using silica and aluminum nitrate and loaded with ammonium nitrate (AN) [30]. The nitrogen release of this NNF was 35% lower than AN. Lateef et al. [28][27] prepared another nano-zeolite with sodium silicate and ethylene glycol, and then sodium nitrate (SN), urea, and other macro and micronutrients were doped into it. The water incubation and soil leaching study exhibited that these fertilizers could extend the N release for more than 7 and 16 days, respectively. Nano ZnO is extensively utilized as a nanomaterial in various applications, including the formulation of NNFs. Due to its commercial availability, nano-ZnO is easily accessible and can be conveniently used in various applications within the agricultural sector. Milani et al. [31] developed nao-ZnO-coated and regular-ZnO-coated urea/mono ammonium phosphate (MAP) NNFs and compared their characteristics. Results revealed that coated urea slightly dissolved and dispersed in the soil compared to the coated MAP, possibly due to the high ionic strength of the urea solution and high pH [38][32]. This study provides evidence that the solubility of nano-ZnO-coated fertilizers is influenced by the acidity generated by the main nutrient used in the fertilizer. Further, this study revealed that ZnO in coated MAP underwent different speciation like ZnSO4, Zn(NH4)PO4, (CaZn2(PO4)2·2H2O), and Zn(OH)2. Interestingly, the speciation of ZnO differs between nano- and bulk-coated ZnO, suggesting that the speciation of ZnO is dependent on particle size. Indeed, the interactions among ZnO, fertilizers, and soil have a significant impact on the formation of various soluble constituents, which, in turn, affect the controlled release ability of the coatings. These reactions play a crucial role in determining the release kinetics and availability of nutrients from the coated fertilizers in the soil environment. In a previous study by these researchers, it was found that Zn solubility was not significantly influenced by the size of the ZnO particle used for coating urea or MAP [33].2.3. Nano-Biochar
Nano-biochar has gained significant attention in the field of agriculture due to its diverse applications. It has been recognized for its potential in various areas such as wastewater treatments, soil amendment, environmental remediation, pesticide formulation, and nutrient delivery [39][34]. Khan et al. [23][35] produced biochar from wheat straw and prepared the nano-biochar by mechanical grinding. An NNF was prepared by impregnating sodium nitrate and other macro- and micronutrients into nano-biochar. The XRD and FTIR analysis confirmed that nutrients impregnated well into the nano-biochar. It controlled the nitrate release for more than 10 days. In a similar method, corn-based nano-biochar was prepared, and nutrients (N, Ca, P, K, Mg) were impregnated [25][36]. The nutrient release from this NNF extended for more than 14 days.2.4. Other Nano-Materials
In addition to the materials discussed in this section, other substances, such as nanocellulose [24][37], and hydroxyapatite [29][38], were also used for preparing NNFs. Nanocellulose, derived from cellulose fibers, offers unique properties, including high surface area, biodegradability, and stability, which make it a promising material for NNF formulations [40][39]. Nanocellulose was prepared from eucalyptus pulp and it was mixed with sodium alginate, FeCl3.6H2O (Ferric Chloride; FC), and urea to form a hydrogel as a pH-sensitive NNF [24][37]. Under microscopic examination, the gel without FC (only nanocellulose) displayed a smooth structure, whereas the introduction of FC resulted in an increase in surface coarseness and roughness. The optimum level of FC is important for the correct level of cross-linking. Increasing the FC content above optimum level led to a weakening of the bonding within the gel matrix. This was evident in the nutrient release in water and soil as well. The NNF with 5%, 10%, and 20% FC extended the 80% of urea release by 3.5, 25, and 5 h, respectively. Therefore, this study concluded that 10% of FC and pH 11 was conducive for longer urea retention. Hydroxyapatite (HA) is a naturally occurring mineral form of calcium apatite, and its chemical formula is Ca10(PO4)6(OH)2. Hydroxyapatite finds extensive use in the medical field, specifically in diverse dental applications such as toothpaste, dental fillings, and coatings for dental implants. However, there has been a recent increase in its application within the agricultural sector, especially in developing NNFs [29][38]. The first nano-HA urea was developed with a ratio of urea:HA of 1:1 using the oven drying method [41][40].3. Methods of Nanoparticle Formulations and Modifications for Preparing NNFs
There are several nanoparticle formulation and modification methods used in the preparation of NNFs, which are summarized in Figure 3.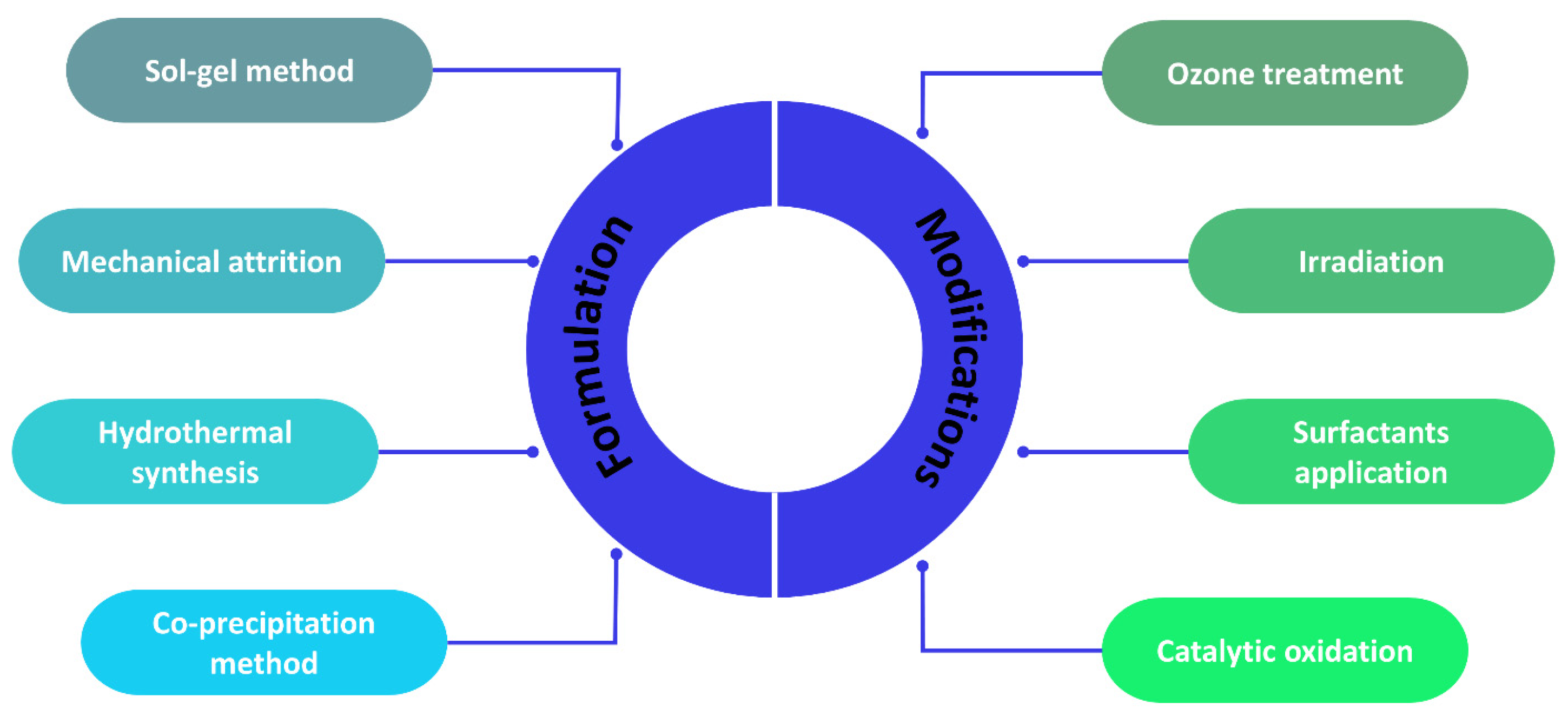
Figure 3.
Nanoparticle formulation and modification methods.
3.1. Nanoparticle Formulation Methods
There are several methods available for formulating nanoparticles. They are mainly categorized into two methods: top-down and bottom-up approaches. The top-down approach involves breaking down larger particles or bulk materials physically or mechanically to obtain nanoparticles. This approach involves breaking down the starting material into smaller particles through processes such as milling, grinding, or lithography. In the bottom-up approach, nanoparticles are formulated from smaller building blocks or molecular precursors. This approach involves the controlled growth or self-assembly of these building blocks to form nanoparticles with desired properties. The sol–gel method, self-assembly, template-assisted synthesis, microfluidic synthesis, and biomimetic synthesis are a few examples of bottom-up approaches.3.2. Nanoparticle Modification Methods
The surface properties, composition, or structural changes to increase its desired characteristics are achieved through the modification of nanoparticles. This can be achieved through various methods including surface modification, chemical functionalization, and coating techniques. Several modification techniques, namely high-energy electron beam irradiation, ozone treatment, applying surfactants, and catalytic oxidation, are a few methods employed in the formulation of NNFs. Although the nanonature of attapulgite is beneficial in the formulation of NNFs, the rod structures tend to aggregate with each other due to the high surface area and nano-effect [16,26][16][25]. This necessitates modifications of attapulgite to improve the dispersion and retain its nanocarrier property. Toward this, Zhou, et al. [16] applied high-energy electron beam (HEEB) irradiation to natural attapulgite, which separated the rods from each other and increased the effective surface area (Figure 4). However, Cai, et al. [26][25] applied ozone (O3) oxidation and hydrothermal processes to increase the dispersion of nanorods and increase –OH active sites on the surface. The authors reported that increased active sites might help to form a micro-nano network with urea.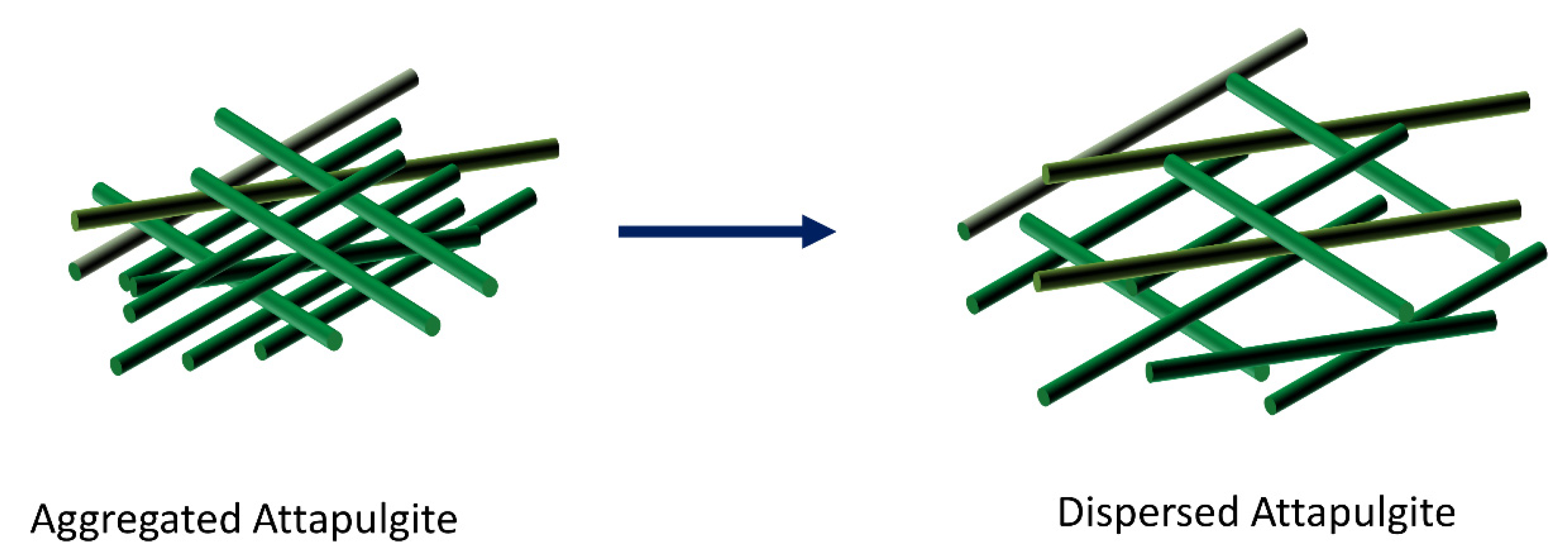
Figure 4.
A schematic diagram of aggregated attapulgite dispersed after irradiation.
4. Crop Responses for Nano-Nitrogen Fertilizers
Several studies have been conducted to investigate the responses of NNFs on different crops, considering different climate and soil conditions. These studies primarily examined agronomic performance, yield response, nitrogen uptake, and physiological changes.4.1. Yield Responses
Ever growing population, limited arable land, and declining land productivity pose significant challenges to the agriculture sector. Henceforward, it is crucial to focus on increasing crop yield per unit application of nutrients [1]. Several studies showed that NNFs significantly increased crop yield compared to conventional N fertilizers. For example, Rudmin et al. [18] formulated new nano-ADP-glauconite fertilizers and tested them on oats. It was found that these fertilizers significantly (p < 0.05) increased the yield by 4.6% compared to non-fertilized treatment. The application rate of NNF influences the yield of crops. A greenhouse experiment conducted by Rop et al. [44][42] showed that a lower application rate (266 kg ha−1) of NNFs significantly (p < 0.05) decreased maize yield by 91–191% compared to urea (the application rate was 532 and 1064 kg ha−1). However, maize yield significantly (p < 0.05) increased the yield by 11% for NNFs compared to urea when a higher application rate (1064 kg ha−1) was employed. This observation was consistent with the yield of capsicum and kale in the same study [44][42]. A higher application rate of 1064 kg ha−1 NNFs increased capsicum and kale yield by 14% and 18.6%, respectively, for NNFs compared to conventional fertilizers. In a study, nano-Zno and vegetable oil-coated urea significantly (p < 0.05) increased the grain yield of wheat than non-fertilized wheat [45][43]. Karoline–urea NNFs applied to rice increased the yield by 80% compared to single urea application [46][44].4.2. Crop Nitrogen Uptake
The uptake of nitrogen by crops is vital for promoting healthy plant growth, protein synthesis, and photosynthesis, ultimately leading to increased crop productivity. Efficient nitrogen uptake also contributes to environmental sustainability by minimizing nutrient waste and reducing the environmental impact of agricultural practices. Studies showed mixed effects of NNFs on crop nitrogen uptake. For instance, 15N labeled urea in an attapulgite sodium polyacrylate polyacrylamide complex was tested against urea alone on corn in a pot experiment [16]. This NNF showed significantly (p < 0.05) higher plant total 15N compared to urea. Rop et al. [44][42] developed a cellulose-graft-poly(acrylamide)/nanohydroxyapatite NNF and tested it on maize, kale, and capsicum. At a 1064 kg ha−1 application rate, NNF significantly (p < 0.05) lowered capsicum herbage N compared to urea. However, maize and kale herbage N were not significantly different between NNF and urea treatment.4.3. Nitrogen Utilization Efficiency (NUE)
Nitrogen utilization efficiency (NUE) refers to the ability of crops to convert the applied nitrogen to a useful component (i.e., yield). Improving NUE is essential for sustainable agriculture, as it helps minimize N losses, thus reducing environmental pollution and optimizing crop productivity. Only a limited number of studies have reported the NUE of NNFs. For example, a study reported that the NUE of winter wheat increased by 5–22% by applying nano-carbon and nano-CaCO3 as synergists [10]. An HA-urea nanohybrid applied to tea in Sri Lanka significantly (p < 0.05) improved NUE [48][45]. In a field study, an NNF was applied to lettuce as a foliar application at different levels, with ammonium nitrate (AN) as a compensator for AN [52][46]. NUE was significantly (p < 0.05) three times higher for a 100% NNF application than a 100% AN application in both years. Some other studies suggested that this could be due to the regulation of important nitrogen metabolism-related unigenes [56][47].4.4. Germination of Seeds
Few studies have reported that NNFs contribute to an increase in germination percentage in many plant species. A study reported that a urea/carboxylated nanocellulose NNF increased the germination percentage of wheat by 20–27% compared to urea [24][37]. Nano-ADP-glauconite, an NNF, significantly (p < 0.05) increased the germination percentage of oats by 6% for the best treatment [18]. Badran et al. [51][48] conducted a comprehensive study to examine the influence of urea, ammonium sulfate, and NNFs (urea surface-modified hydroxy appetite nanoparticles) on the germination of almond seeds under varying levels of saline conditions. The study found that the germination rate was significantly (p < 0.05) higher for NNFs compared to other fertilizers at all application rates and under all levels of saline conditions (1, 3, and 5 ds m−1). However, germination percentage significantly (p < 0.05) improved only at higher application levels of NNFs under all saline conditions. Therefore, this study shows that NNFs can be used to alleviate the effect of saline water on seed germination. The higher germination exhibited by NNFs could potentially be attributed to the intake of nanomaterials by seeds, leading to an increase in the micropores for water intrusion that could increase germination [57][49].4.5. Chlorophyll Content
Chlorophyll is a color pigment that is responsible for capturing light energy during photosynthesis, and its content directly influences energy production and essential metabolic processes. Studies have shown that the application of NNFs enhances chlorophyll content in plants compared to conventional nitrogen fertilizers. For example, a 25% AN and 50% NNF application significantly (p < 0.05) increased the chlorophyll content of lettuce by 10 SPAD compared to AN application alone in two years [52][46]. This observation is directly correlated with the leaf N content. Quaternary ammonium lignin (QAL)-modified nano-bentonite-coated urea applied to tomatoes significantly (p < 0.05) increased the leaf chlorophyll content compared to urea at higher application rates [22]. However, at lower-level applications, the chlorophyll content is only comparable to urea.4.6. Gene Expression
Several studies reported the positive influence of NPs on gene expression [58,59,60,61][50][51][52][53]. However, only a few studies reported that NNFs induce gene expression, which is beneficial for crops. Glutamine synthase (GS) genes are responsible for N assimilation in crops, which induce nitrate transportation family genes. Yang et al. [10] found that nanocalcium carbonate (NCa) and nano-carbon (NC) synergists applied to wheat with N fertilizers significantly (p < 0.05) increased the expression of GS, such as TaGS1, TaGS2, TaNRT2.2, and TaNRT2.3, compared to N fertilizer alone. Therefore, these synergists increased the N transportation and accumulation in wheat. Chew et al. [62][54] reported that biochar-based nano-iron-fortified compound fertilizer significantly (p < 0.05) downregulated the expression of ammonium transportation genes such as OsAMT1.1, OsAMT1.2, and OsAMT1.3 in roots compared to the control, whereas this NNF significantly (p < 0.05) increased nitrate transporter genes, such as OsNAR2.1 and OsNRT2.3, in roots compared to the control. It is evident that an NNF promotes the transportation of nitrates over ammonium ions.References
- Abhiram, G.; Grafton, M.; Jeyakumar, P.; Bishop, P.; Davies, C.E.; McCurdy, M. Iron-rich sand promoted nitrate reduction in a study for testing of lignite based new slow-release fertilisers. Sci. Total Environ. 2023, 864, 160949.
- Spiertz, J. Nitrogen, sustainable agriculture and food security: A review. In Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2009; pp. 635–651.
- Gnaratnam, A.; McCurdy, M.; Grafton, M.; Jeyakumar, P.; Bishop, P.; Davies, C. Assessment of Nitrogen Fertilizers under Controlled Environment–A Lysimeter Design; Massey University: Palmerston North, New Zealand, 2019.
- Mahmud, K.; Panday, D.; Mergoum, A.; Missaoui, A. Nitrogen losses and potential mitigation strategies for a sustainable agroecosystem. Sustainability 2021, 13, 2400.
- Abhiram, G.; Grafton, M.; Jeyakumar, P.; Bishop, P.; Davies, C.E.; McCurdy, M. The nitrogen dynamics of newly developed lignite-based controlled-release fertilisers in the soil-plant cycle. Plants 2022, 11, 3288.
- Gunaratnam, A. Design and Fabrication of a Climate-Controlled Lysimeter and Testing of New Controlled-Release Fertilisers: A Thesis Presented in Partial Fulfilment of the Requirements for the Degree of Doctor of Philosophy (PHD) in Agricultural Engineering and Environmental Sciences at Massey University, Palmerston North, New Zealand; Massey University: Palmerston North, New Zealand, 2021.
- Abhiram, G.; McCurdy, M.; Davies, C.E.; Grafton, M.; Jeyakumar, P.; Bishop, P. An innovative lysimeter system for controlled climate studies. Biosyst. Eng. 2023, 228, 105–119.
- Abhiram, G.; Bishop, P.; Jeyakumar, P.; Grafton, M.; Davies, C.E.; McCurdy, M. Formulation and characterization of polyester-lignite composite coated slow-release fertilizers. J. Coat. Technol. Res. 2023, 20, 307–320.
- Trenkel, M. Slow-and Controlled-Release and Stabilized Fertilizers: An Option for Enhancing Nutrient Use Effiiency in Agriculture; International Fertilizer Industry Association (IFA): Paris, France, 2010.
- Yang, M.; Dong, C.; Shi, Y. Nano fertilizer synergist effects on nitrogen utilization and related gene expression in wheat. BMC Plant Biol. 2023, 23, 26.
- Liu, X.; Liao, J.; Song, H.; Yang, Y.; Guan, C.; Zhang, Z. A biochar-based route for environmentally friendly controlled release of nitrogen: Urea-loaded biochar and bentonite composite. Sci. Rep. 2019, 9, 9548.
- Thakor, A.S.; Gambhir, S.S. Nanooncology: The future of cancer diagnosis and therapy. CA A Cancer J. Clin. 2013, 63, 395–418.
- Shweta; Sood, S.; Sharma, A.; Chadha, S.; Guleria, V. Nanotechnology: A cutting-edge technology in vegetable production. J. Hortic. Sci. Biotechnol. 2021, 96, 682–695.
- Xiumei, L.; Fudao, Z.; Shuqing, Z.; Xusheng, H.; Rufang, W.; Zhaobin, F.; Yujun, W. Responses of peanut to nano-calcium carbonate. Plant Nutr. Fertitizer Sci. 2005, 11, 385–389.
- Umar, W.; Czinkota, I.; Gulyás, M.; Aziz, T.; Hameed, M.K. Development and characterization of slow release N and Zn fertilizer by coating urea with Zn fortified nano-bentonite and ZnO NPs using various binders. Environ. Technol. Innov. 2022, 26, 102250.
- Zhou, L.; Cai, D.; He, L.; Zhong, N.; Yu, M.; Zhang, X.; Wu, Z. Fabrication of a high-performance fertilizer to control the loss of water and nutrient using micro/nano networks. ACS Sustain. Chem. Eng. 2015, 3, 645–653.
- Liu, X.-M.; Feng, Z.-B.; Zhang, F.-D.; Zhang, S.-Q.; He, X.-S. Preparation and testing of cementing and coating nano-subnanocomposites of slow/controlled-release fertilizer. Agric. Sci. China 2006, 5, 700–706.
- Rudmin, M.; Makarov, B.; López-Quirós, A.; Maximov, P.; Lokteva, V.; Ibraeva, K.; Kurovsky, A.; Gummer, Y.; Ruban, A. Preparation, Features, and Efficiency of Nanocomposite Fertilisers Based on Glauconite and Ammonium Dihydrogen Phosphate. Materials 2023, 16, 6080.
- Moosavi, M. Bentonite clay as a natural remedy: A brief review. Iran. J. Public Health 2017, 46, 1176.
- Gandhi, D.; Bandyopadhyay, R.; Soni, B. Naturally occurring bentonite clay: Structural augmentation, characterization and application as catalyst. Mater. Today Proc. 2022, 57, 194–201.
- Shahid, S.A.; Abdelfattah, M.A.; Taha, F.K. Developments in Soil Salinity Assessment and Reclamation: Innovative Thinking and Use of Marginal Soil and Water Resources in Irrigated Agriculture; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013.
- Helal, M.I.; El-Mogy, M.M.; Khater, H.A.; Fathy, M.A.; Ibrahim, F.E.; Li, Y.C.; Tong, Z.; Abdelgawad, K.F. A Controlled-Release Nanofertilizer Improves Tomato Growth and Minimizes Nitrogen Consumption. Plants 2023, 12, 1978.
- Liu, J.; Zhong, J.; Chen, Z.; Mao, J.; Liu, J.; Zhang, Z.; Li, X.; Ren, S. Preparation, characterization, application and structure evolution of attapulgite: From nanorods to nanosheets. Appl. Surf. Sci. 2021, 565, 150398.
- Pushpaletha, P.; Lalithambika, M. Modified attapulgite: An efficient solid acid catalyst for acetylation of alcohols using acetic acid. Appl. Clay Sci. 2011, 51, 424–430.
- Cai, D.; Wu, Z.; Jiang, J.; Wu, Y.; Feng, H.; Brown, I.G.; Chu, P.K.; Yu, Z. Controlling nitrogen migration through micro-nano networks. Sci. Rep. 2014, 4, 3665.
- Panda, A.K.; Mishra, B.G.; Mishra, D.K.; Singh, R.K. Effect of sulphuric acid treatment on the physico-chemical characteristics of kaolin clay. Colloids Surf. A Physicochem. Eng. Asp. 2010, 363, 98–104.
- AlShamaileh, E.M.; Al-Rawajfeh, A.E.; Alrbaihat, M.R. Solid-state mechanochemical synthesis of Kaolinite-Urea complexes for application as slow release fertilizer. J. Ecol. Eng. 2019, 20, 267–276.
- Hassan, M.S.; Baioumy, H.M. Structural and chemical alteration of glauconite under progressive acid treatment. Clays Clay Miner. 2006, 54, 491–499.
- Lateef, A.; Nazir, R.; Jamil, N.; Alam, S.; Shah, R.; Khan, M.N.; Saleem, M. Synthesis and characterization of zeolite based nano–composite: An environment friendly slow release fertilizer. Microporous Mesoporous Mater. 2016, 232, 174–183.
- Bhardwaj, D.; Tomar, R. Use of surface modified inorganic nano materials as slow release nitrogen fertilizer. In Sustainable Agricultural Development: Recent Approaches in Resources Management and Environmentally-Balanced Production Enhancement; Springer: Berlin/Heidelberg, Germany, 2011; pp. 171–184.
- Milani, N.; Hettiarachchi, G.M.; Kirby, J.K.; Beak, D.G.; Stacey, S.P.; McLaughlin, M.J. Fate of zinc oxide nanoparticles coated onto macronutrient fertilizers in an alkaline calcareous soil. PLoS ONE 2015, 10, e0126275.
- Cornelis, G.; Hund-Rinke, K.; Kuhlbusch, T.; Van den Brink, N.; Nickel, C. Fate and bioavailability of engineered nanoparticles in soils: A review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2720–2764.
- Milani, N.; McLaughlin, M.J.; Stacey, S.P.; Kirby, J.K.; Hettiarachchi, G.M.; Beak, D.G.; Cornelis, G. Dissolution kinetics of macronutrient fertilizers coated with manufactured zinc oxide nanoparticles. J. Agric. Food Chem. 2012, 60, 3991–3998.
- Sashidhar, P.; Kochar, M.; Singh, B.; Gupta, M.; Cahill, D.; Adholeya, A.; Dubey, M. Biochar for delivery of agri-inputs: Current status and future perspectives. Sci. Total Environ. 2020, 703, 134892.
- Khan, H.A.; Naqvi, S.R.; Mehran, M.T.; Khoja, A.H.; Niazi, M.B.K.; Juchelková, D.; Atabani, A. A performance evaluation study of nano-biochar as a potential slow-release nano-fertilizer from wheat straw residue for sustainable agriculture. Chemosphere 2021, 285, 131382.
- Lateef, A.; Nazir, R.; Jamil, N.; Alam, S.; Shah, R.; Khan, M.N.; Saleem, M. Synthesis and characterization of environmental friendly corncob biochar based nano-composite–A potential slow release nano-fertilizer for sustainable agriculture. Environ. Nanotechnol. Monit. Manag. 2019, 11, 100212.
- Wang, Y.; Shaghaleh, H.; Hamoud, Y.A.; Zhang, S.; Li, P.; Xu, X.; Liu, H. Synthesis of a pH-responsive nano-cellulose/sodium alginate/MOFs hydrogel and its application in the regulation of water and N-fertilizer. Int. J. Biol. Macromol. 2021, 187, 262–271.
- Rop, K.; Karuku, G.N.; Mbui, D.; Michira, I.; Njomo, N. Formulation of slow release NPK fertilizer (cellulose-graft-poly (acrylamide)/nano-hydroxyapatite/soluble fertilizer) composite and evaluating its N mineralization potential. Ann. Agric. Sci. 2018, 63, 163–172.
- Mateo, S.; Peinado, S.; Morillas-Gutiérrez, F.; La Rubia, M.D.; Moya, A.J. Nanocellulose from agricultural wastes: Products and applications—A review. Processes 2021, 9, 1594.
- Gunaratne, G.P.; Kottegoda, N.; Madusanka, N.; Munaweera, I.; Sandaruwan, C.; Priyadarshana, W.M.G.I.; Siriwardhana, A.; Madhushanka, B.A.D.; Rathnayake, U.A.; Karunaratne, V. Two New Plant Nutrient Nanocomposites Based on Urea Coated Hydroxyapatite: Efficacy and Plant Uptake. Indian J. Agr. Sci. 2016, 86, 494–499.
- Kottegoda, N.; Sandaruwan, C.; Priyadarshana, G.; Siriwardhana, A.; Rathnayake, U.A.; Berugoda Arachchige, D.M.; Kumarasinghe, A.R.; Dahanayake, D.; Karunaratne, V.; Amaratunga, G.A. Urea-hydroxyapatite nanohybrids for slow release of nitrogen. ACS Nano 2017, 11, 1214–1221.
- Rop, K.; Karuku, G.N.; Mbui, D.; Njomo, N.; Michira, I. Evaluating the effects of formulated nano-NPK slow release fertilizer composite on the performance and yield of maize, kale and capsicum. Ann. Agric. Sci. 2019, 64, 9–19.
- Dimkpa, C.O.; Andrews, J.; Fugice, J.; Singh, U.; Bindraban, P.S.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Facile coating of urea with low-dose ZnO nanoparticles promotes wheat performance and enhances Zn uptake under drought stress. Front. Plant Sci. 2020, 11, 168.
- Roshanravan, B.; Mahmoud Soltani, S.; Mahdavi, F.; Abdul Rashid, S.; Khanif Yusop, M. Preparation of encapsulated urea-kaolinite controlled release fertiliser and their effect on rice productivity. Chem. Speciat. Bioavailab. 2014, 26, 249–256.
- Raguraj, S.; Wijayathunga, W.M.S.; Gunaratne, G.P.; Amali, R.K.A.; Priyadarshana, G.; Sandaruwan, C.; Karunaratne, V.; Hettiarachchi, L.S.K.; Kottegoda, N. Urea–hydroxyapatite nanohybrid as an efficient nutrient source in Camellia sinensis (L.) Kuntze (tea). J. Plant Nutr. 2020, 43, 2383–2394.
- Sharaf-Eldin, M.A.; Elsawy, M.B.; Eisa, M.Y.; El-Ramady, H.; Usman, M.; Zia-ur-Rehman, M. Application of nano-nitrogen fertilizers to enhance nitrogen efficiency for lettuce growth under different irrigation regimes. Pak. J. Agric. Sci. 2022, 59, 367–379.
- Wang, Y.; Chen, R.; Hao, Y.; Liu, H.; Song, S.; Sun, G. Transcriptome analysis reveals differentially expressed genes (DEGs) related to lettuce (Lactuca sativa) treated by TiO2/ZnO nanoparticles. Plant Growth Regul. 2017, 83, 13–25.
- Badran, A.; Savin, I. Effect of nano-fertilizer on seed germination and first stages of bitter almond seedlings’ growth under saline conditions. BioNanoScience 2018, 8, 742–751.
- Khodakovskaya, M.; Dervishi, E.; Mahmood, M.; Xu, Y.; Li, Z.; Watanabe, F.; Biris, A.S. Carbon nanotubes are able to penetrate plant seed coat and dramatically affect seed germination and plant growth. ACS Nano 2009, 3, 3221–3227.
- Chun, S.-C.; Chandrasekaran, M. Chitosan and chitosan nanoparticles induced expression of pathogenesis-related proteins genes enhances biotic stress tolerance in tomato. Int. J. Biol. Macromol. 2019, 125, 948–954.
- Syu, Y.-Y.; Hung, J.-H.; Chen, J.-C.; Chuang, H.-W. Impacts of size and shape of silver nanoparticles on Arabidopsis plant growth and gene expression. Plant Physiol. Biochem. 2014, 83, 57–64.
- Thakur, S.; Saini, R.V.; Singh, P.; Raizada, P.; Thakur, V.K.; Saini, A.K. Nanoparticles as an emerging tool to alter the gene expression: Preparation and conjugation methods. Mater. Today Chem. 2020, 17, 100295.
- Bethanis, J.; Golia, E.E. Micro-and nano-plastics in agricultural soils: A critical meta-analysis of their impact on plant growth, nutrition, metal accumulation in plant tissues and crop yield. Appl. Soil Ecol. 2023, 194, 105202.
- Chew, J.; Joseph, S.; Chen, G.; Zhang, Y.; Zhu, L.; Liu, M.; Taherymoosavi, S.; Munroe, P.; Mitchell, D.R.; Pan, G. Biochar-based fertiliser enhances nutrient uptake and transport in rice seedlings. Sci. Total Environ. 2022, 826, 154174.
More
