Hyaluronic acid (HA) naturally occurs as a biopolymer in the human body, primarily in connective tissues like joints and skin. Functioning as a vital element of synovial fluid, it lubricates joints, facilitating fluid movement and diminishing bone friction to protect articular well-being. Its distinctive attributes encompass notable viscosity and water retention capacities, ensuring flexibility and absorbing shock during motion. Furthermore, HA has gained significant attention for its potential benefits in various medical applications, including rehabilitation. Ongoing research explores its properties and functions, especially its biomedical applications in several clinical trials, with a focus on its role in improving rehabilitation outcomes. But the clinical and biochemical implications of HA in musculoskeletal rehabilitation have yet to be fully explored.
- hyaluronic acid
- active biomolecule
- biomaterial
- rehabilitation
- HA injection
- physical therapy
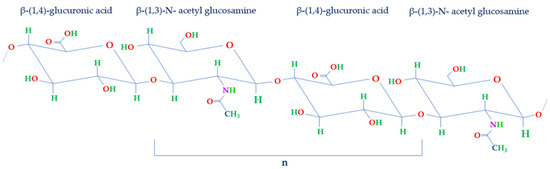
1. Hyaluronic Acid
2. HA in the Rehabilitation of Musculoskeletal Diseases
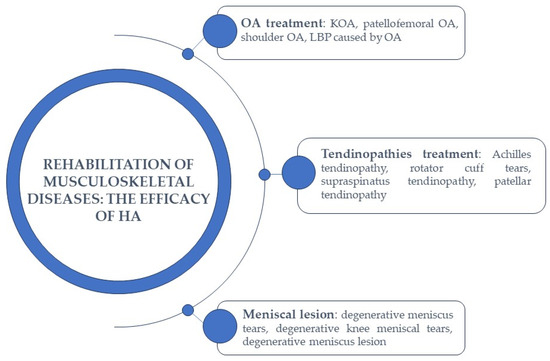
2.1. HA-Based Therapy in Osteoarthritis Treatment
HA-Based Therapy in Low Back Pain
2.2. HA-Based Therapy in Tendinopathies Treatment
2.3. HA-Based Therapy in Meniscal Lesions
References
- Gallo, N.; Nasser, H.; Salvatore, L.; Natali, M.L.; Campa, L.; Mahmoud, M.; Capobianco, L.; Madaghiele, M. Hyaluronic acid for advanced therapies: Promises and challenges. Eur. Polym. J. 2019, 117, 134–147.
- Iaconisi, G.N.; Lunetti, P.; Gallo, N.; Cappello, A.R.; Fiermonte, G.; Dolce, V.; Capobianco, L. Hyaluronic Acid: A Powerful Biomolecule with Wide-Ranging Applications—A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 10296.
- Farì, G.; de Sire, A.; Fallea, C.; Albano, M.; Grossi, G.; Bettoni, E.; Di Paolo, S.; Agostini, F.; Bernetti, A.; Puntillo, F.; et al. Efficacy of Radiofrequency as Therapy and Diagnostic Support in the Management of Musculoskeletal Pain: A Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 600.
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic Acid: Molecular Mechanisms and Therapeutic Trajectory. Front. Vet. Sci. 2019, 6, 192.
- Kosiński, J.; Jarecki, J.; Przepiorka-Kosińska, J.; Ratajczak, M. Hyaluronic acid in orthopedics. Wiad. Lek. 2020, 73, 1878–1881.
- Onu, I.; Matei, D.; Galaction, A. Efficacy of intra-articular hyaluronic acid injections in the rehabilitation programme of Knee Osteoarthritis. BALNEO 2019, 225–230.
- Monticone, M.; Frizziero, A.; Rovere, G.; Vittadini, F.; Uliano, D.; LA Bruna, S.; Gatto, R.; Nava, C.; Leggero, V.; Masiero, S. Hyaluronic acid intra-articular injection and exercise therapy: Effects on pain and disability in subjects affected by lower limb joints osteoarthritis. A systematic review by the Italian Society of Physical and Rehabilitation Medicine (SIMFER). Eur. J. Phys. Rehabil. Med. 2016, 52, 389–399.
- Kobayashi, T.; Chanmee, T.; Itano, N. Hyaluronan: Metabolism and Function. Biomolecules 2020, 10, 1525.
- Li, C.; Cao, Z.; Li, W.; Liu, R.; Chen, Y.; Song, Y.; Liu, G.; Song, Z.; Liu, Z.; Lu, C.; et al. A review on the wide range applications of hyaluronic acid as a promising rejuvenating biomacromolecule in the treatments of bone related diseases. Int. J. Biol. Macromol. 2020, 165, 1264–1275.
- Gomis, A.; Pawlak, M.; Balazs, E.A.; Schmidt, R.F.; Belmonte, C. Effects of different molecular weight elastoviscous hyaluronan solutions on articular nociceptive afferents. Arthritis Rheum. 2004, 50, 314–326.
- Gomis, A.; Miralles, A.; Schmidt, R.F.; Belmonte, C. Nociceptive nerve activity in an experimental model of knee joint osteoarthritis of the guinea pig: Effect of intra-articular hyaluronan application. Pain 2007, 130, 126–136.
- Yang, X.; Liang, W.; Li, J.; Liu, P. A meta-analysis and systematic review of the therapeutic effects of arthroscopy combined with intra-articular injection of sodium hyaluronate in the treatment of knee osteoarthritis. Ann. Palliat. Med. 2021, 10, 9859–9869.
- Ingram, K.R.; Wann, A.K.; Angel, C.K.; Coleman, P.J.; Levick, J.R. Cyclic movement stimulates hyaluronan secretion into the synovial cavity of rabbit joints. J. Physiol. 2008, 586, 1715–1729.
- Castrogiovanni, P.; Di Rosa, M.; Ravalli, S.; Castorina, A.; Guglielmino, C.; Imbesi, R.; Vecchio, M.; Drago, F.; Szychlinska, M.A.; Musumeci, G. Moderate Physical Activity as a Prevention Method for Knee Osteoarthritis and the Role of Synoviocytes as Biological Key. Int. J. Mol. Sci. 2019, 20, 511.
- Clark, R.B.; Schmidt, T.A.; Sachse, F.B.; Boyle, D.; Firestein, G.S.; Giles, W.R. Cellular electrophysiological principles that modulate secretion from synovial fibroblasts. J. Physiol. 2017, 595, 635–645.
- Saccomanno, M.F.; Donati, F.; Careri, S.; Bartoli, M.; Severini, G.; Milano, G. Efficacy of intra-articular hyaluronic acid injections and exercise-based rehabilitation programme, administered as isolated or integrated therapeutic regimens for the treatment of knee osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 1686–1694.
- Arden, N.; Nevitt, M.C. Osteoarthritis: Epidemiology. Best. Pract. Res. Clin. Rheumatol. 2006, 20, 3–25.
- Murphy, L.; Schwartz, T.A.; Helmick, C.G.; Renner, J.B.; Tudor, G.; Koch, G.; Dragomir, A.; Kalsbeek, W.D.; Luta, G.; Jordan, J.M. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008, 59, 1207–1213.
- Christensen, R.; Bartels, E.M.; Astrup, A.; Bliddal, H. Effect of weight reduction in obese patients diagnosed with knee osteoarthritis: A systematic review and meta-analysis. Ann. Rheum. Dis. 2007, 66, 433–439.
- Roos, E.M.; Arden, N.K. Strategies for the prevention of knee osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 92–101.
- Palazzo, C.; Nguyen, C.; Lefevre-Colau, M.M.; Rannou, F.; Poiraudeau, S. Risk factors and burden of osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 134–138.
- Farì, G.; Santagati, D.; Pignatelli, G.; Scacco, V.; Renna, D.; Cascarano, G.; Vendola, F.; Bianchi, F.P.; Fiore, P.; Ranieri, M.; et al. Collagen Peptides, in Association with Vitamin C, Sodium Hyaluronate, Manganese and Copper, as Part of the Rehabilitation Project in the Treatment of Chronic Low Back Pain. Endocr. Metab. Immune Disord. Drug Targets 2022, 22, 108–115.
- Jang, S.; Lee, K.; Ju, J.H. Recent Updates of Diagnosis, Pathophysiology, and Treatment on Osteoarthritis of the Knee. Int. J. Mol. Sci. 2021, 22, 2619.
- Notarnicola, A.; Maccagnano, G.; Farì, G.; Bianchi, F.P.; Moretti, L.; Covelli, I.; Ribatti, P.; Mennuni, C.; Tafuri, S.; Pesce, V.; et al. Extracorporeal shockwave therapy for plantar fasciitis and gastrocnemius muscle: Effectiveness of a combined treatment. J. Biol. Regul. Homeost. Agents 2020, 34, 285–290.
- Farì, G.; Megna, M.; Scacco, S.; Ranieri, M.; Raele, M.V.; Noya, E.C.; Macchiarola, D.; Bianchi, F.P.; Carati, D.; Gnoni, A.; et al. Effects of Terpenes on the Osteoarthritis Cytokine Profile by Modulation of IL-6: Double Face versus Dark Knight? Biology 2023, 12, 1061.
- Farì, G.; Megna, M.; Scacco, S.; Ranieri, M.; Raele, M.V.; Chiaia Noya, E.; Macchiarola, D.; Bianchi, F.P.; Carati, D.; Panico, S.; et al. Hemp Seed Oil in Association with β-Caryophyllene, Myrcene and Ginger Extract as a Nutraceutical Integration in Knee Osteoarthritis: A Double-Blind Prospective Case-Control Study. Medicina 2023, 59, 191.
- Ayhan, E.; Kesmezacar, H.; Akgun, I. Intraarticular injections (corticosteroid, hyaluronic acid, platelet rich plasma) for the knee osteoarthritis. World J. Orthop. 2014, 5, 351–361.
- Jones, I.A.; Togashi, R.; Wilson, M.L.; Heckmann, N.; Vangsness, C.T., Jr. Intra-articular treatment options for knee osteoarthritis. Nat. Rev. Rheumatol. 2019, 15, 77–90.
- Bruyère, O.; Honvo, G.; Veronese, N.; Arden, N.K.; Branco, J.; Curtis, E.M.; Al-Daghri, N.M.; Herrero-Beaumont, G.; Martel-Pelletier, J.; Pelletier, J.P.; et al. An updated algorithm recommendation for the management of knee osteoarthritis from the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Semin. Arthritis. Rheum. 2019, 49, 337–350.
- Kon, E.; Di Matteo, B.; Delgado, D.; Cole, B.J.; Dorotei, A.; Dragoo, J.L.; Filardo, G.; Fortier, L.A.; Giuffrida, A.; Jo, C.H.; et al. Platelet-rich plasma for the treatment of knee osteoarthritis: An expert opinion and proposal for a novel classification and coding system. Expert. Opin. Biol. Ther. 2020, 20, 1447–1460.
- Fransen, M.; McConnell, S.; Harmer, A.R.; Van der Esch, M.; Simic, M.; Bennell, K.L. Exercise for osteoarthritis of the knee: A Cochrane systematic review. Br. J. Sports. Med. 2015, 49, 1554–1557.
- Abramoff, B.; Caldera, F.E. Osteoarthritis: Pathology, Diagnosis, and Treatment Options. Med. Clin. N. Am. 2020, 104, 293–311.
- Latourte, A.; Kloppenburg, M.; Richette, P. Emerging pharmaceutical therapies for osteoarthritis. Nat. Rev. Rheumatol. 2020, 16, 673–688.
- Laudy, A.B.; Bakker, E.W.; Rekers, M.; Moen, M.H. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: A systematic review and meta-analysis. Br. J. Sports. Med. 2015, 49, 657–672.
- Meheux, C.J.; McCulloch, P.C.; Lintner, D.M.; Varner, K.E.; Harris, J.D. Efficacy of Intra-articular Platelet-Rich Plasma Injections in Knee Osteoarthritis: A Systematic Review. Arthroscopy 2016, 32, 495–505.
- Hermann, W.; Lambova, S.; Muller-Ladner, U. Current Treatment Options for Osteoarthritis. Curr. Rheumatol. Rev. 2018, 14, 108–116.
- Baselga García-Escudero, J.; Miguel Hernández Trillos, P. Treatment of Osteoarthritis of the Knee with a Combination of Autologous Conditioned Serum and Physiotherapy: A Two-Year Observational Study. PLoS ONE 2015, 10, 0145551.
- Bowman, S.; Awad, M.E.; Hamrick, M.W.; Hunter, M.; Fulzele, S. Recent advances in hyaluronic acid based therapy for osteoarthritis. Clin. Transl. Med. 2018, 7, 6.
- Ferreira, R.M.; Torres, R.T.; Duarte, J.A.; Gonçalves, R.S. Non-Pharmacological and Non-Surgical Interventions for Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Acta Reumatol. Port. 2019, 44, 173–217.
- Serban, O.; Porojan, M.; Deac, M.; Cozma, F.; Solomon, C.; Lenghel, M.; Micu, M.; Fodor, D. Pain in bilateral knee osteoarthritis-correlations between clinical examination, radiological, and ultrasonographical findings. Med. Ultrason. 2016, 18, 318–325.
- Liao, C.D.; Chen, H.C.; Huang, M.H.; Liou, T.H.; Lin, C.L.; Huang, S.W. Comparative Efficacy of Intra-Articular Injection, Physical Therapy, and Combined Treatments on Pain, Function, and Sarcopenia Indices in Knee Osteoarthritis: A Network Meta-Analysis of Randomized Controlled Trials. Int. J. Mol. Sci. 2023, 24, 6078.
- Onu, I.; Matei, D.; Sardaru, D.-P.; Cascaval, D.; Onu, A.; Gherghel, R.; Serban, I.L.; Mocanu, G.D.; Iordan, D.A.; Murariu, G.; et al. Rehabilitation of Patients with Moderate Knee Osteoarthritis Using Hyaluronic Acid Viscosupplementation and Physiotherapy. Appl. Sci. 2022, 12, 3165.
- Miller, L.E.; Block, J.E. An 8-Week Knee Osteoarthritis Treatment Program of Hyaluronic Acid Injection, Deliberate Physical Rehabilitation, and Patient Education is Cost Effective at 2 Years Follow-up: The OsteoArthritis Centers of America (SM) Experience. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2014, 7, 49–55.
- Foster, N.E.; Anema, J.R.; Cherkin, D.; Chou, R.; Cohen, S.P.; Gross, D.P.; Ferreira, P.H.; Fritz, J.M.; Koes, B.W.; Peul, W.; et al. Prevention and treatment of low back pain: Evidence, challenges, and promising directions. Lancet 2018, 391, 2368–2383.
- Hartvigsen, J.; Hancock, M.J.; Kongsted, A.; Louw, Q.; Ferreira, M.L.; Genevay, S.; Hoy, D.; Karppinen, J.; Pransky, G.; Sieper, J.; et al. What low back pain is and why we need to pay attention. Lancet 2018, 391, 2356–2367.
- Aicale, R.; Tarantino, D.; Maffulli, N. Overuse injuries in sport: A comprehensive overview. J. Orthop. Surg. Res. 2018, 13, 309.
- Oliva, F.; Marsilio, E.; Asparago, G.; Frizziero, A.; Berardi, A.C.; Maffulli, N. The Impact of Hyaluronic Acid on Tendon Physiology and Its Clinical Application in Tendinopathies. Cells 2021, 10, 3081.
- Minetto, M.A.; Giannini, A.; McConnell, R.; Busso, C.; Torre, G.; Massazza, G. Common Musculoskeletal Disorders in the Elderly: The Star Triad. J. Clin. Med. 2020, 9, 1216.
- Bisciotti, G.N.; Eirale, C.; Corsini, A.; Baudot, C.; Saillant, G.; Chalabi, H. Return to football training and competition after lockdown caused by the COVID-19 pandemic: Medical recommendations. Biol. Sport. 2020, 37, 313–319.
- Oliva, F.; Piccirilli, E.; Berardi, A.C.; Frizziero, A.; Tarantino, U.; Maffulli, N. Hormones and tendinopathies: The current evidence. Br. Med. Bull. 2016, 117, 39–58.
- Hollander, K.; Rahlf, A.L.; Wilke, J.; Edler, C.; Steib, S.; Junge, A.; Zech, A. Sex-specific differences in running injuries: A systematic review with meta-analysis and meta-regression. Sports Med. 2021, 51, 1011–1039.
- Del Buono, A.; Battery, L.; Denaro, V.; Maccauro, G.; Maffulli, N. Tendinopathy and inflammation: Some truths. Int. J. Immunopathol. Pharmacol. 2011, 24, 45–50.
- Loiacono, C.; Palermi, S.; Massa, B.; Belviso, I.; Romano, V.; Di Gregorio, A.; Sirico, F.; Sacco, A.M. Tendinopathy: Pathophysiology, Therapeutic Options, and Role of Nutraceutics. A Narrative Literature Review. Medicina 2019, 55, 447.
- Litwiniuk, M.; Krejner, A.; Speyrer, M.S.; Gauto, A.R.; Grzela, T. Hyaluronic Acid in Inflammation and Tissue Regeneration. Wounds 2016, 28, 78–88.
- Ciardulli, M.C.; Marino, L.; Lovecchio, J.; Giordano, E.; Forsyth, N.R.; Selleri, C.; Maffulli, N.; Porta, G.D. Tendon and Cytokine Marker Expression by Human Bone Marrow Mesenchymal Stem Cells in a Hyaluronate/Poly-Lactic-Co-Glycolic Acid (PLGA)/Fibrin Three-Dimensional (3D) Scaffold. Cells 2020, 9, 1268.
- Arshad, Z.; Lau, E.J.S.; Leow, S.H.; Bhatia, M. Management of chronic Achilles ruptures: A scoping review. Int. Orthop. 2021, 45, 2543–2559.
- Fitzpatrick, J.; Bulsara, M.; Zheng, M.H. The Effectiveness of Platelet-Rich Plasma in the Treatment of Tendinopathy: A Meta-analysis of Randomized Controlled Clinical Trials. Am. J. Sports Med. 2017, 45, 226–233.
- Frizziero, A.; Oliva, F.; Vittadini, F.; Vetrano, M.; Bernetti, A.; Giordan, N.; Vulpiani, V.; Santilli, S.; Masiero, N.; Maffulli, N. Efficacy of ultrasound-guided hyaluronic acid injections in achilles and patellar tendinopathies: A prospective multicentric clinical trial. Muscles Ligaments Tendons J. 2019, 9, 305–313.
- Huang, S.H.; Hsu, P.C.; Wang, K.A.; Chou, C.L.; Wang, J.C. Comparison of single platelet-rich plasma injection with hyaluronic acid injection for partial-thickness rotator cuff tears. J. Chin. Med. Assoc. 2022, 85, 723–729.
- Flores, C.; Balius, R.; Álvarez, G.; Buil, M.A.; Varela, L.; Cano, C.; Casariego, J. Efficacy and tolerability of peritendinous hyaluronic acid in patients with supraspinatus tendinopathy: A multicenter, randomized, controlled trial. Sports Med. Open. 2017, 3, 1–8.
- Kumai, T.; Muneta, T.; Tsuchiya, A.; Shiraishi, M.; Ishizaki, Y.; Sugimoto, K.; Samoto, N.; Isomoto, S.; Tanaka, Y.; Takakura, Y. The short-term effect after a single injection of high-molecular-weight hyaluronic acid in patients with enthesopathies (lateral epicondylitis, patellar tendinopathy, insertional Achilles tendinopathy, and plantar fasciitis): A preliminary study. J. Orthop. Sci. 2014, 19, 603–611.
- Markes, A.R.; Hodax, J.D.; Ma, C.B. Meniscus Form and Function. Clin. Sports Med. 2020, 39, 1–12.
- Stocco, E.; Porzionato, A.; De Rose, E.; Barbon, S.; De Caro, R.; Macchi, V. Meniscus regeneration by 3D printing technologies: Current advances and future perspectives. J. Tissue Eng. 2022, 13, 20417314211065860.
- Logerstedt, D.S.; Ebert, J.R.; MacLeod, T.D.; Heiderscheit, B.C.; Gabbett, T.J.; Eckenrode, B.J. Effects of and Response to Mechanical Loading on the Knee. Sports Med. 2022, 52, 201–235.
- Kopf, S.; Beaufils, P.; Hirschmann, M.T.; Rotigliano, N.; Ollivier, M.; Pereira, H.; Verdonk, R.; Darabos, N.; Ntagiopoulos, P.; Dejour, D.; et al. Management of traumatic meniscus tears: The 2019 ESSKA meniscus consensus. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 1177–1194.
- Machado, G.C.; Maher, C.G.; Ferreira, P.H.; Pinheiro, M.B.; Lin, C.W.; Day, R.O.; McLachlan, A.J.; Ferreira, M.L. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: Systematic review and meta-analysis of randomised placebo controlled trials. BMJ 2015, 350, 1225.
- Schaffer, D.; Florin, T.; Eagle, C.; Marschner, I.; Singh, G.; Grobler, M.; Fenn, C.; Schou, M.; Curnow, K.M. Risk of serious NSAID-related gastrointestinal events during long-term exposure: A systematic review. Med. J. Aust. 2006, 185, 501–506.
- Arai, T.; Suzuki-Narita, M.; Takeuchi, J.; Tajiri, I.; Inage, K.; Kawarai, Y.; Eguchi, Y.; Shiga, Y.; Hozumi, T.; Kim, G.; et al. Analgesic effects and arthritic changes following intra-articular injection of diclofenac etalhyaluronate in a rat knee osteoarthritis model. BMC Musculoskelet. Disord. 2022, 23, 960.
- Zhang, W.; Nuki, G.; Moskowitz, R.W.; Abramson, S.; Altman, R.D.; Arden, N.K.; Bierma-Zeinstra, S.; Brandt, K.D.; Croft, P.; Doherty, M.; et al. OARSI recommendations for the management of hip and knee osteoarthritis: Part III: Changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthr. Cartil. 2010, 18, 476–499.
- Wang, Z.; Zhu, P.; Liao, B.; You, H.; Cai, Y. Effects and action mechanisms of individual cytokines contained in PRP on osteoarthritis. J. Orthop. Surg. Res. 2023, 18, 713.
- Balius, R.; Guillermo, A.R.; Fernando, B.P.; Domínguez, M.; Gastaldi, E.; Gómez, A.; Ibáñez, L.; Lara, F.; Leal, A.; León, A.; et al. Evaluation of quality-of-life following treatment with Hymovis in patients with knee osteoarthritis and/or meniscal tear. Clin. Exp. Rheumatol. 2023. posted.
- Başar, B.; Başar, G.; Büyükkuşçu, M.Ö.; Başar, H. Comparison of physical therapy and arthroscopic partial meniscectomy treatments in degenerative meniscus tears and the effect of combined hyaluronic acid injection with these treatments: A randomized clinical trial. J. Back. Musculoskelet. Rehabil. 2021, 34, 767–774.
