Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Lindsay Dong and Version 1 by Zhaosheng (Jims) Jin.
Postoperative delirium (POD) represents a perioperative neurocognitive disorder that has dreaded ramifications on a patient’s recovery from surgery. Dexmedetomidine displays multiple mechanisms of neuroprotection to assist in preventing POD as a part of a comprehensive anesthetic care plan.
- postoperative delirium
- dexmedetomidine
- perioperative neurocognitive disorder
- neuroprotection
1. Introduction
The perioperative setting is host to a variety of potential neurocognitive insults. Even when patients are optimized for elective surgery they are often famished, stressed, and in a state of discomfort. From the moment they enter the preoperative staging area till discharge, patients are in an unfamiliar and institutional environment that regularly interrupts them and imposes limitations on visitation. During the perioperative period, patients are exposed to various stressors, including surgical trauma, anesthesia, pain, disturbances to sleep, and changes in nutrition. These and other factors contribute to the prevalence of postoperative neurocognitive complications, especially in patients who are at high risk.
One of the responsibilities of an anesthesiologist is to minimize the stresses patients face before, during, and after surgery. Fortunately, modern advancements in the field of anesthesiology and perioperative medicine equip anesthesiologists with an armamentarium of techniques to help protect their patients. The Federal Drug Administration has approved dexmedetomidine for the sedation of patients in an intensive care setting who are intubated and mechanically ventilated.
2. Perioperative Neurocognitive Disorders
Emergence delirium, postoperative delirium (POD), and postoperative cognitive dysfunction (POCD) are the three major perioperative neurocognitive disorders. Each represents changes in mental status and cognition with varying timeframes. Emergence delirium occurs during or immediately after emergence from anesthesia, compared to postoperative delirium (POD) which has a delayed onset between postoperative days 1 and 5 [2,3][1][2]. POCD represents a more chronic disorder that is centered on the decline of a patient’s cognitive ability without criteria established by the most recent edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM) [4][3]. Some authors believe that POCD is detectable as early as 7 days after surgery with effects lasting 1 year or more [5,6][4][5].2.1. Postoperative Delirium Risk Factors
While the risk of POD is 2–5% of all patients undergoing surgery, the risk is multiplied ten-fold or more in patients with risk factors, including age greater than 60 years and a pre-existing diagnosis of dementia, cardiovascular disease, diabetes mellitus, or anemia [9,10,11,12,13,14][6][7][8][9][10][11]. Cognitive decline prior to surgery, defined as significant reductions relative to a patient’s baseline cognitive level, has also been shown as a risk factor in developing POD [15][12]. In addition to risk factors associated with patient comorbidities, the type of surgery can also play a role in increasing POD incidence. Emergency surgery and complex surgeries, including elective cardiac surgery, liver transplant, and pulmonary endarterectomy, carry an elevated risk of POD with incidence up to 56% [16,17,18,19,20][13][14][15][16][17].2.2. Postoperative Delirium Sequalae
POD drastically impacts a patient’s recovery and postoperative outcomes. A 2022 investigation that studied patients 70 years old and older undergoing non-emergent cardiac and orthopedic surgery revealed that the presence of POD, as evidenced by one of four validated assessment tools, between postoperative days 1 through 5 increased the total length of hospital stay by 8 days and increased time in the intensive care unit (ICU) by 6 days [24][18]. Extended hospital and ICU length of stay due to delirium has also been seen amongst patients as young as 50 years old and seen in other surgical disciplines, including gastrointestinal surgery [25][19]. Patients who experience POD are at high risk of functional decline after discharge and often require discharge to care facilities. POD following surgery for colorectal cancer was associated with loss of independence as measured through activities of daily living (ADL). A subpopulation analysis of patients 80 years old or older revealed an association between POD and loss of independence at 30 days post-discharge [26][20]. The prior literature on patients 70 years of age or older undergoing elective major orthopedic, vascular, or abdominal surgeries corroborates this reduction in independence with a 50% increased risk of discharge to a nursing home, subacute rehabilitation facility, or acute rehabilitation facility following POD [27][21]. These sequelae come with remarkable healthcare costs, making the mitigation of POD a prime target to curb Medicare spending. A study of patients 70 years of age or older undergoing elective surgery investigated the healthcare costs of patients who developed POD compared to those who did not. Major costs included readmissions and institutional rehabilitation stays. The incidence of delirium resulted in USD 44,291 in additional costs per patient per year with a direct increase in cost to USD 55,474 when the patient’s CAM score indicated severe delirium. The authors extrapolated their findings and found that USD 32.9 billion is spent in the United States on POD sequelae costs per year [29][22]. A single intervention should not be expected to eliminate POD’s nationwide economic impact, but a nationwide reduction in POD by 4% has the potential for over USD one billion in cost savings.3. Etiology of Postoperative Delirium
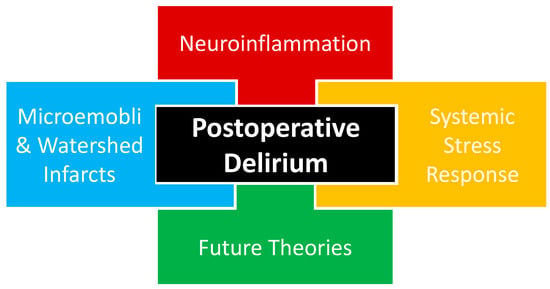
The complete pathophysiology of POD remains unknown with multiple theories proposed, including neuroinflammation, systemic stress response, and microemboli or watershed stroke. POD is likely multifactorial in nature, as a combination of proposed and yet-to-be-proposed etiologies that will be presented in future works (Figure 1).
Figure 1. Current proposed etiological theories of postoperative delirium likely interact in a multifactorial fashion, including neuroinflammation, systemic stress response, and cerebrovascular events. An additional etiologic component of postoperative delirium is the theories that will emerge based on future investigations that may help us to better understand the etiology of postoperative delirium.
3.1. Neuroinflammation
Evidence of inflammation has been associated with POD through studies correlating inflammatory biomarkers and POD assessment tools, implicating neuroinflammation in POD (Figure 1). A meta-analysis of a variety of surgeries from cardiac to abdominal to orthopedic found a relationship between POD and both non-specific inflammatory biomarkers, interleukin-6 (IL-6) and c-reactive protein (CRP), and neuronal injury biomarkers, neurofilament light chain (NfL) and S100 calcium-binding protein B (S-100B) [33][23].
Blood–brain barrier damage can be a sequela of neuroinflammation and has been associated with POD following non-intracranial surgery using the blood–brain barrier damage surrogates cerebrospinal fluid/plasma albumin ratio and plasma S100B. Elevated levels of these blood–brain barrier damage surrogates are also correlated with POD severity [35][24].
The neutrophil-to-lymphocyte ratio (NLR) is a white blood cell-derived inflammatory biomarker readily available in many patients perioperatively and is associated with POD. An investigation into lower limb fracture surgery found that a postoperative NLR of 10.2 or above was strongly associated with POD [36][25]. The same study found that a POD prediction model that took into consideration multiple factors, including age, perioperative benzodiazepine administration, change in CRP, and change in white blood cell-derived biomarkers, was able to better predict POD than a change in NLR alone. In addition to finding utility in a clinically ubiquitous inflammation marker, this finding supports POD having a multifactorial etiological basis.
3.2. Systemic Stress Response
Surgical trauma triggers a stress response that may also be implicated in the pathophysiology of POD. Surgery activates the hypothalamic–pituitary–adrenal (HPA) axis and causes substantial neuroendocrine changes designed to facilitate the body’s response to trauma. Like accidental trauma, surgical trauma is sensed by the hypothalamus through changes in hemodynamics and inflammatory markers. These changes initiate a potent HPA cascade with effects on multiple organ systems and hormones, including the posterior pituitary, anti-diuretic hormone, anterior pituitary, adrenocorticotropic hormone, growth hormone, adrenal cortex, cortisol, adrenal medulla, catecholamines, arteriolar smooth muscle, renal blood flow, renin, angiotensin, aldosterone, pancreas, glucagon, and insulin (Figure 1) [37][26].3.3. Microemboli or Watershed Stroke
Cerebrovascular incidents have also been proposed as a theory in the development of POD, with the disruption of reliable perfusion to the brain thought to contribute to a patient’s altered mental status after surgery. Clamping and manipulation of the aorta during off-pump coronary artery bypass grafting (CABG) surgery has been shown to cause multiple cerebrovascular embolic events seen on a transcranial Doppler [41][27]. The anaortic method of off-pump CABG features no aorta clamping or manipulation and has been associated with a three-fold reduction in POD [42][28]. These findings both support the microemboli theory of POD and provide a POD mitigation strategy for patients undergoing surgery involving aorta manipulation. Sections of the brain located between two arterial supplies are classified as border zones or watershed areas and rely on uninterrupted perfusion, especially in advanced cerebrovascular disease. During times of reduced cerebral blood flow, there is a risk for watershed infarction, resulting in damage to a considerable amount of the brain. Peripheral arterial disease (PAD) has been associated with underlying cerebrovascular disease [43][29]. Patients with PAD undergoing elective lower extremity bypass surgery were found to have a greater incidence of POD with more advanced PAD, measured by both lower ankle-brachial index values and higher Rutherford class [44][30]. The above vascular and cardiothoracic surgery investigations have both demonstrated that a disturbance in cerebrovascular perfusion has been associated with POD and lend credence to a microemboli or watershed stroke theory being involved with the etiology of POD (Figure 1).4. Dexmedetomidine
4.1. Alpha-Adrengeric Agonism
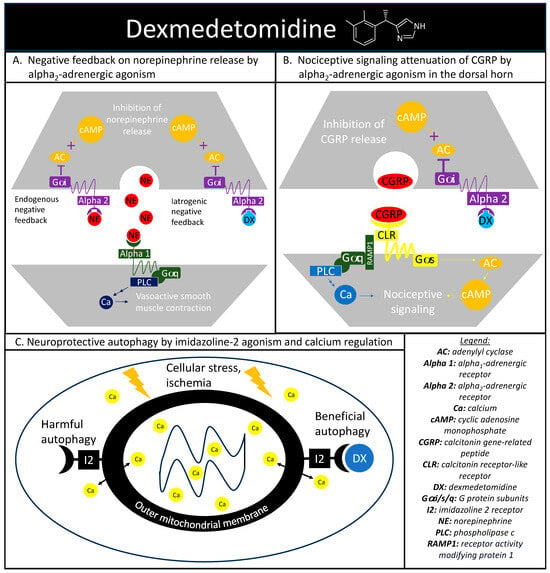
The alpha-adrenergic receptors play a vital role in maintaining proper hemodynamics and play a substantial role in the stress response previously discussed. Norepinephrine is a catecholamine released during a stress response with vasoconstrictive effects mediated through the alpha1-adrenergic receptor agonism, with alpha2-androgenic receptors functioning as a negative norepinephrine feedback loop on the presynaptic nerve terminal [45][31]. Dexmedetomidine is a sedative that exploits this intrinsic negative feedback loop through selective alpha2-adrenergic receptor agonisms (Figure 2A). Activated alpha2-andregneic receptors attenuate noradrenergic pathways through the decrease in norepinephrine release from the locus ceruleus through an inhibitory G-protein coupled receptor action causing a reduction in adenylyl cyclase activity and cyclic adenosine monophosphate levels. The alpha2-adrenergic receptors also explain the analgesic effect seen with dexmedetomidine. Within the dorsal horn of the spinal column, there are also alpha2-adrenergic receptors and the agonism of these receptors reduces the release of substance P and calcitonin gene-related peptide (CGRP) and mitigates nociceptive signaling (Figure 2B) [46][32].
Figure 2. Dexmedetomidine’s highlighted mechanisms of action. (A) depicts the cellular signaling pathways present during the binding of norepinephrine to alpha1- and alpha2-adrenergic receptors. Alpha2-adrenergic receptors play a role in an endogenous negative feedback loop to reduce norepinephrine release, which is the mechanism that dexmedetomidine is designed to mimic. (B) depicts dexmedetomidine’s cellular signaling pathway behind the therapeutic agent’s antinociception through alpha2-adrenergic activation in reducing the release of calcitonin gene-related peptide and subsequent nociceptive signaling. (C) depicts the basic interaction between dexmedetomidine and imidazoline-2 receptors on the outer mitochondrial membrane in regulating intracellular calcium levels and producing beneficial autophagy in the setting of cellular stress.
4.2. Imidazoline Agonism
Dexmedetomidine also has an affinity to receptors outside of the alpha-adrenergic system, including imidazoline type 2 (I2) receptors. While there is a paucity of research surrounding dexmedetomidine and I2 receptors, evidence suggests that dexmedetomidine-I2 binding can regulate calcium levels within chromaffin cells and may be protective against neuronal damage [48][33]. Additional evidence has implicated the I2 receptor agonism in the regulation of cytosolic calcium concentrations at the level of the outer mitochondria membrane and in the mitigation of endoplasmic reticulum stress-induced apoptosis (Figure 2C) [49][34].
4.3. Neurotransmitter Alteration
Dexmedetomidine may exert its neuroprotection via the alteration of neurotransmitter pathways including catecholamines, glutamate, acetylcholine, and butyrylcholine. A pre-clinical trial explored the effects of dexmedetomidine on cerebral neurotransmitter concentrations in rats with induced cerebral ischemia. The animals were assigned to three groups including one control group, one experimental group receiving 100 mcg/kg of intraperitoneal dexmedetomidine 30 min prior to ischemia, and one group of sham-operated rats. Concentrations of cerebral catecholamines and glutamate, as well as plasma catecholamines, were measured, with a resulting 95% reduction in circulating plasma norepinephrine in rats pre-treated with dexmedetomidine
Although further research is needed to explore the exact mechanism of neuroprotection from dexmedetomidine, these studies demonstrated a link between dexmedetomidine and the alteration of catecholamine levels. Lower circulating plasma norepinephrine and a limited decrease in AChE and BChE after surgery may result in less inflammation and a less robust systemic response to surgical stress, resulting in neuroprotection.
4.4. Pharmacodynamics and Drug–Drug Interactions
When given as a loading dose intravenously, dexmedetomidine exhibits a rapid onset of action in 5 to 10 min with peak effect seen in 15 to 30 min. This onset of action is prolonged to 60 min when given as a continuous intravenous infusion. The highly lipophilic nature of dexmedetomidine facilitates rapid distribution with a 6 min distribution half-life, producing rapid onset. The duration of effect is dependent on administration, given the notable context-sensitive half-time, over 4 h after an 8 h infusion. The potentiation of other pharmaceuticals has been observed with dexmedetomidine administration, including reducing benzodiazepine requirements and opioid-sparing effects [52,53][35][36]. Therefore, the effects of benzodiazepines and opioids may be greater in a patient undergoing treatment with dexmedetomidine than without co-administration of dexmedetomidine. Dexmedetomidine is metabolized by the liver via direct glucuronidation and CYP2A6 metabolism followed by clearance in the urine and secondarily in feces [54,55][37][38].4.5. Pre-Clinical Data
Immune cells within the central nervous system are at least partially responsible for neuroinflammation, especially microglia, the central nervous system-specific and primary immune cell. In a pre-clinical study of human microglial cells, cells were stress-stimulated through exposure to lipopolysaccharide (LPS), a component of gram-negative bacterial cell walls, leading to an increase in an inflammatory marker also associated with POD, IL-6. While the investigation was not able to significantly reduce levels of IL-6 when stressed by LPS, unstressed cells exposed to dexmedetomidine had significantly decreased production of IL-6 compared to unstressed cells not exposed to dexmedetomidine [56][39]. Again, there was visibly reduced nerve cell loss on hippocampal tissue sampling when they were pre-treated with dexmedetomidine [58][40]. This group of investigators had two groups pre-treated with dexmedetomidine, one that was exposed to 3-methyladenine, an autophagy inhibitor, and another that was able to undergo autophagy. The group that had autophagy inhibited did not have a reduction in nerve cell loss on tissue sampling, implying that dexmedetomidine’s hippocampal safeguarding effect may be due to beneficial autophagy (Figure 2C). W4.6. Clinical Data Risks, Benefits, and Alternatives
The above basic science investigations are supported by a meta-analysis of clinical trials investigating dexmedetomidine in the prevention of a perioperative neurocognitive disorder following a variety of surgical procedures, including liver transplant, prostatectomy, femoral head replacement, and CABG. Mirroring the pre-clinical data, the use of dexmedetomidine was associated with a significantly reduced level of the inflammatory marker IL-6 and a neuronal injury biomarker, neuron-specific enolase (NSE) [59][41]. In addition to the adverse effects of bradycardia and hypotension, delayed emergence has also been reported with dexmedetomidine. When dexmedetomidine was co-administered with propofol, a randomized prospective trial revealed a significantly longer time to eye opening by an average of 10 min following cessation of anesthesia compared to propofol alone [63][42]. Providing patients with alternatives to any intervention is fundamental to informed treatment. Thise article views dexmedetomidine as a complementary measure of a comprehensive, personalized anesthetic care plan with the aim of preventing POD.5. Clinical Evidence of Postoperative Delirium Prevention with Dexmedetomidine
5.1. Cardiac and Non-Cardiac Surgery
In the non-cardiac adult surgical population, several trials and meta-analyses have shown a decrease in perioperative neurocognitive disorders after administration of dexmedetomidine. In pooled data from thirteen studies, a 40% reduction in PCOD was estimated [73][43]. In another meta-analysis of 18 RCTs with 3309 patients, dexmedetomidine was associated with a significant reduction in POD (OR 0.35, p-value < 0.01). Subgroup analysis of cardiac (nine studies, 1301 patients) and non-cardiac (nine studies, 2008 patients) patients showed a significant difference in POD incidence in both groups favoring dexmedetomidine over control (OR 0.41, p-value < 0.01) for cardiac surgery and for non-cardiac surgery (OR 0.33, p-value < 0.01). Also demonstrated was a reduced POD incidence in both the elderly age group, defined as 65 years of age or older, as well as the younger group, defined as younger than 65 years of age [74][44].5.2. Intraoperative Dosing Regimen
Although the literature specifically pertaining to the efficacy of specific dosing regimens is currently limited, one recent randomized double-blind controlled trial studied 150 elderly patients undergoing hip replacement under general anesthesia and showed encouraging results in reducing rates of POD and emergence delirium. The patients were randomized to groups based on different dexmedetomidine loading dosages (0.25, 0.5, and 0.75 µg/kg for 15 min) followed by 0.5 µg/kg/h continuous infusion until the completion of the surgery. While all dosage groups were associated with significantly lower POD, the higher-dose groups experienced improved agitation scores compared to the lower-dose group. This significantly improved agitation scoring and was also accompanied by bradycardia and hypotension with higher infusion rates compared to the lower infusion rate group [80][45]. These results should encourage caution in high-dose perioperative dexmedetomidine among patients 65 years of age or older, especially those with low preoperative blood pressures or heart rates. Certain comorbidities and treatments, such as heart block and beta blockers for the treatment of coronary artery disease, may predispose patients to low preoperative hemodynamic measurements. Beyond hemodynamic considerations, surgical intervention must also be considered. If a perioperative neurologic exam is a surgical requirement, dexmedetomidine has been shown to delay the return to baseline neurologic and cognitive status up to 45 min post-transfusion and should be avoided [81][46].5.3. Postoperative Infusion in the Intensive Care Unit
Dexmedetomidine infusions are frequently used in ICU patients for sedation and the prevention of agitation and delirium. One meta-analysis focused on dexmedetomidine infusion compared to other pharmacological interventions in ICU patients, showed a reduction in the incidence of POD. Dexmedetomidine was compared to lorazepam, midazolam, and propofol, and showed decreased LOS in the ICU, the duration of mechanical ventilation, and the incidence of POD (RR = 0.812, p-value = 0.020). Dexmedetomidine was also shown to be associated with increased incidences of bradycardia (RR = 1.947, p = 0.001) and hypotension (RR = 1.264, p = 0.038) [83][47].6. Conclusions
Perioperative neurocognitive disorders, specifically POD, represent an area in need of neural protection and repair. The impact of POD and similar disorders on a patient needing surgery can be life-altering. Dexmedetomidine shows repetitive promise across pre-clinical and clinical trials as a sedative, analgesic, neuroprotectant, anxiolytic, and potentiator within a multimodal anesthetic care plan. The mechanisms of action seen in dexmedetomidine administration act as counterpoints to many of the proposed pathophysiologic components of POD and may be a welcome step toward improving a patient’s recovery from surgery. Given that the pathophysiology of POD is still not completely understood, it is anticipated to evolve with future findings and is likely a multifactorial disease; the prevention and treatment of POD require a multifaceted approach that warrants consideration of complementary interventions by anesthesiologists, including dexmedetomidine, close intraoperative monitoring, and discharge from a post-anesthesia care unit to a specialized recovery unit versed in POD. While the adverse effects observed with dexmedetomidine may or may not be clinically significant, anesthesiologists must balance the risks and benefits of each intervention within a personalized anesthetic care plan for a patient’s surgery. Patients at high risk of perioperative neurocognitive disorders often face multiple comorbidities and require special consideration in their perioperative care plan. Unlike other therapeutic agents used perioperatively, there is not yet a reversal agent for dexmedetomidine on the market for human use.References
- Jin, Z.; Hu, J.; Ma, D. Postoperative delirium: Perioperative assessment, risk reduction, and management. Br. J. Anaesth. 2020, 125, 492–504.
- Zhang, Y.; He, S.-T.; Nie, B.; Li, X.-Y.; Wang, D.-X. Emergence delirium is associated with increased postoperative delirium in elderly: A prospective observational study. J. Anesth. 2020, 34, 675–687.
- American Psychiatric Association, D. S. M. T. F.; American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5; American Psychiatric Association: Washington, DC, USA, 2013; Volume 5.
- Needham, M.; Webb, C.; Bryden, D. Postoperative cognitive dysfunction and dementia: What we need to know and do. BJA Br. J. Anaesth. 2017, 119, i115–i125.
- Brodier, E.; Cibelli, M. Postoperative cognitive dysfunction in clinical practice. BJA Educ. 2021, 21, 75.
- Drews, T.; Franck, M.; Radtke, F.M.; Weiss, B.; Krampe, H.; Brockhaus, W.R.; Winterer, G.; Spies, C.D. Postoperative delirium is an independent risk factor for posttraumatic stress disorder in the elderly patient A prospective observational study. Eur. J. Anaesthesiol. 2015, 32, 147–151.
- Iamaroon, A.; Wongviriyawong, T.; Sura-Arunsumrit, P.; Wiwatnodom, N.; Rewuri, N.; Chaiwat, O. Incidence of and risk factors for postoperative delirium in older adult patients undergoing noncardiac surgery: A prospective study. BMC Geriatr. 2020, 20, 1–8.
- de Groot, R.; Nijmeijer, W.S.; Folbert, E.C.; Vollenbroek-Hutten, M.M.R.; Hegeman, J.H. ‘Nonagenarians’ with a hip fracture: Is a different orthogeriatric treatment strategy necessary? Arch. Osteoporos. 2020, 15, 19.
- Krzych, L.J.; Wybraniec, M.T.; Krupka-Matuszczyk, I.; Skrzypek, M.; Bolkowska, A.; Wilczynski, M.; Bochenek, A.A. Detailed Insight Into the Impact of Postoperative Neuropsychiatric Complications on Mortality in a Cohort of Cardiac Surgery Subjects: A 23,000-Patient-Year Analysis. J. Cardiothorac. Vasc. Anesth. 2014, 28, 448–457.
- Winter, A.; Steurer, M.; Dullenkopf, A. Postoperative delirium assessed by post anesthesia care unit staff utilizing the Nursing Delirium Screening Scale: A prospective observational study of 1000 patients in a single Swiss institution. BMC Anesthesiol. 2015, 15, 1–6.
- Ha, A.; Krasnow, R.E.; Mossanen, M.; Nagle, R.; Hshieh, T.T.; Rudolph, J.L.; Chang, S.L. A contemporary population-based analysis of the incidence, cost, and outcomes of postoperative delirium following major urologic cancer surgeries. In Urologic Oncology: Seminars and Original Investigations; Elsevier: Amsterdam, The Netherlands, 2018; pp. 341.e315–341.e322.
- Lin, X.; Liu, F.; Wang, B.; Dong, R.; Sun, L.; Wang, M.; Bi, Y. Subjective cognitive decline may be associated with post-operative delirium in patients undergoing total hip replacement: The PNDABLE study. Front. Aging Neurosci. 2021, 13, 680672.
- Desrochers, R.M.; Lynch, L.J.; Gates, J.D.; Ricaurte, D.; Wade, J.T.; Dicks, R.S.; Keating, J.J. Outcomes in Post-operative Delirium Following Bowel Resection: A Single Center Retrospective Review. J. Surg. Res. 2022, 280, 163–168.
- Teller, J.; Gabriel, M.M.; Schimmelpfennig, S.-D.; Laser, H.; Lichtinghagen, R.; Schafer, A.; Fegbeutel, C.; Weissenborn, K.; Jung, C.; Hinken, L.; et al. Stroke, Seizures, Hallucinations and Postoperative Delirium as Neurological Complications after Cardiac Surgery and Percutaneous Valve Replacement. J. Cardiovasc. Dev. Dis. 2022, 9, 365.
- Zhou, J.; Xu, X.; Liang, Y.; Zhang, X.; Tu, H.; Chu, H. Risk factors of postoperative delirium after liver transplantation: A systematic review and meta-analysis. Minerva Anestesiol. 2021, 87, 684–694.
- Camous, J.; Decrombecque, T.; Louvain-Quintard, V.; Doubine, S.; Dartevelle, P.; Stephan, F. Outcomes of patients with antiphospholipid syndrome after pulmonary endarterectomy. Eur. J. Cardio-Thorac. Surg. 2014, 46, 116–120.
- Neumann, A.; Serna-Higuita, L.; Detzel, H.; Popov, A.-F.; Kruger, T.; Vohringer, L.; Schlensak, C. Off-pump coronary artery bypass grafting for patients with severely reduced ventricular function-A justified strategy? J. Card. Surg. 2022, 37, 7–17.
- Kirfel, A.; Guttenthaler, V.; Mayr, A.; Coburn, M.; Menzenbach, J.; Wittmann, M. Postoperative delirium is an independent factor influencing the length of stay of elderly patients in the intensive care unit and in hospital. J. Anesth. 2022, 36, 341–348.
- Scholz, A.F.M.; Oldroyd, C.; McCarthy, K.; Quinn, T.J.; Hewitt, J. Systematic review and meta-analysis of risk factors for postoperative delirium among older patients undergoing gastrointestinal surgery. Br. J. Surg. 2016, 103, E21–E28.
- Gearhart, S.L.; Do, E.M.; Owodunni, O.; Gabre-Kidan, A.A.; Magnuson, T. Loss of Independence in Older Patients after Operation for Colorectal Cancer. J. Am. Coll. Surg. 2020, 230, 573–582.
- Gleason, L.J.; Schmitt, E.M.; Kosar, C.M.; Tabloski, P.; Saczynski, J.S.; Robinson, T.; Cooper, Z.; Rogers, S.O.; Jones, R.N.; Marcantonio, E.R.; et al. Effect of Delirium and Other Major Complications on Outcomes After Elective Surgery in Older Adults. JAMA Surg. 2015, 150, 1134–1140.
- Gou, R.Y.; Hshieh, T.T.; Marcantonio, E.R.; Cooper, Z.; Jones, R.N.; Travison, T.G.; Fong, T.G.; Abdeen, A.; Lange, J.; Earp, B. One-year medicare costs associated with delirium in older patients undergoing major elective surgery. JAMA Surg. 2021, 156, 462–470.
- Wang, S.; Greene, R.; Song, Y.; Chan, C.; Lindroth, H.; Khan, S.; Rios, G.; Sanders, R.D.; Khan, B. Postoperative delirium and its relationship with biomarkers for dementia: A meta-analysis. Int. Psychogeriatr. 2022, 1–14.
- Taylor, J.; Parker, M.; Casey, C.P.; Tanabe, S.; Kunkel, D.; Rivera, C.; Zetterberg, H.; Blennow, K.; Pearce, R.A.; Lennertz, R.C.; et al. Postoperative delirium and changes in the blood–brain barrier, neuroinflammation, and cerebrospinal fluid lactate: A prospective cohort study. Br. J. Anaesth. 2022, 129, 219–230.
- Li, X.; Wang, G.; He, Y.; Wang, Z.; Zhang, M. White-cell derived inflammatory biomarkers in prediction of postoperative delirium in elderly patients undergoing surgery for lower limb fracture under non-general anaesthesia. Clin. Interv. Aging 2022, 383–392.
- Cusack, B.; Buggy, D. Anaesthesia, analgesia, and the surgical stress response. BJA Educ. 2020, 20, 321.
- Falk, V. Stay off-pump and do not touch the aorta! Eur. Heart J. 2010, 31, 278–280.
- Szwed, K.; Pawliszak, W.; Szwed, M.; Tomaszewska, M.; Anisimowicz, L.; Borkowska, A. Reducing delirium and cognitive dysfunction after off-pump coronary bypass: A randomized trial. J. Thorac. Cardiovasc. Surg. 2021, 161, 1275–1282.e4.
- Aday, A.W.; Matsushita, K. Epidemiology of peripheral artery disease and polyvascular disease. Circ. Res. 2021, 128, 1818–1832.
- Kang, J.; An, J.H.; Jeon, H.J.; Park, Y.J. Association between ankle brachial index and development of postoperative intensive care unit delirium in patients with peripheral arterial disease. Sci. Rep. 2021, 11, 12744.
- Giovannitti Jr, J.A.; Thoms, S.M.; Crawford, J.J. Alpha-2 adrenergic receptor agonists: A review of current clinical applications. Anesth. Prog. 2015, 62, 31–38.
- Li, R.; Qi, F.; Zhang, J.; Ji, Y.; Zhang, D.; Shen, Z.; Lei, W. Antinociceptive effects of dexmedetomidine via spinal substance P and CGRP. Transl. Neurosci. 2015, 6, 259–264.
- Liaquat, Z.; Xu, X.; Zilundu, P.L.M.; Fu, R.; Zhou, L. The current role of dexmedetomidine as neuroprotective agent: An updated review. Brain Sci. 2021, 11, 846.
- Inagaki, M.; Somei, M.; Oguchi, T.; Ono, R.; Fukutaka, S.; Matsuoka, I.; Tsuji, M.; Oguchi, K. Neuroprotective Effects of Dexmedetomidine against Thapsigargin-induced ER-stress via Activity of α 2-adrenoceptors and Imidazoline Receptors. AIMS Neurosci. 2016, 3, 237–252.
- Bielka, K.; Kuchyn, I.; Glumcher, F. Addition of dexmedetomidine to benzodiazepines for patients with alcohol withdrawal syndrome in the intensive care unit: A randomized controlled study. Ann. Intensive Care 2015, 5, 1–7.
- Zhang, B.; Wang, G.; Liu, X.; Wang, T.-L.; Chi, P. The opioid-sparing effect of perioperative dexmedetomidine combined with oxycodone infusion during open hepatectomy: A randomized controlled trial. Front. Pharmacol. 2018, 8, 940.
- Weerink, M.A.; Struys, M.M.; Hannivoort, L.N.; Barends, C.R.; Absalom, A.R.; Colin, P. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin. Pharmacokinet. 2017, 56, 893–913.
- Karol, M.D.; Maze, M. Pharmacokinetics and interaction pharmacodynamics of dexmedetomidine in humans. Best Pract. Res. Clin. Anaesthesiol. 2000, 14, 261–269.
- Yamazaki, S.; Yamaguchi, K.; Someya, A.; Nagaoka, I.; Hayashida, M. Anti-Inflammatory Action of Dexmedetomidine on Human Microglial Cells. Int. J. Mol. Sci. 2022, 23, 10096.
- Wang, J.; Xin, Y.; Chu, T.; Zhou, Y.; Liu, C.; Xu, A. Dexmedetomidine mitigates neuroinflammation and improves early postoperative neurocognitive dysfunction in rats by enhancing autophagy. J. Neurophysiol. 2023, 129, 1145–1156.
- Lei, D.; Sha, Y.; Wen, S.; Xie, S.; Liu, L.; Han, C. Dexmedetomidine may reduce IL-6 level and the risk of postoperative cognitive dysfunction in patients after surgery: A meta-analysis. Dose-Response 2020, 18, 1559325820902345.
- Ohtani, N.; Kida, K.; Shoji, K.; Yasui, Y.; Masaki, E. Recovery profiles from dexmedetomidine as a general anesthetic adjuvant in patients undergoing lower abdominal surgery. Anesth. Analg. 2008, 107, 1871–1874.
- Zhou, C.; Zhu, Y.; Liu, Z.; Ruan, L. Effect of dexmedetomidine on postoperative cognitive dysfunction in elderly patients after general anaesthesia: A meta-analysis. J. Int. Med. Res. 2016, 44, 1182–1190.
- Duan, X.; Coburn, M.; Rossaint, R.; Sanders, R.; Waesberghe, J.; Kowark, A. Efficacy of perioperative dexmedetomidine on postoperative delirium: Systematic review and meta-analysis with trial sequential analysis of randomised controlled trials. Br. J. Anaesth. 2018, 121, 384–397.
- Liu, H.; Gao, M.; Zheng, Y.; Sun, C.; Lu, Q.; Shao, D. Effects of dexmedetomidine at different dosages on perioperative haemodynamics and postoperative recovery quality in elderly patients undergoing hip replacement surgery under general anaesthesia: A randomized controlled trial. Trials 2023, 24, 1–14.
- Bustillo, M.A.; Lazar, R.M.; Finck, A.D.; Fitzsimmons, B.; Berman, M.F.; Pile-Spellman, J.; Heyer, E.J. Dexmedetomidine may impair cognitive testing during endovascular embolization of cerebral arteriovenous malformations: A retrospective case report series. J. Neurosurg. Anesthesiol. 2002, 14, 209.
- Constantin, J.-M.; Momon, A.; Mantz, J.; Payen, J.-F.; De Jonghe, B.; Perbet, S.; Cayot, S.; Chanques, G.; Perreira, B. Efficacy and safety of sedation with dexmedetomidine in critical care patients: A meta-analysis of randomized controlled trials. Anaesth. Crit. Care Pain Med. 2016, 35, 7–15.
More
