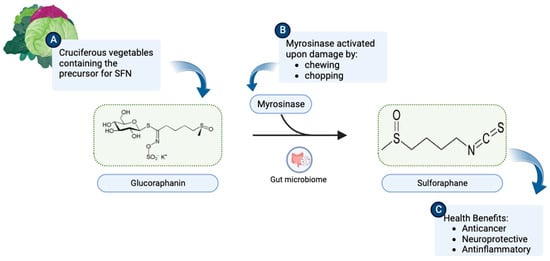
Sulforaphane (SFN) is a naturally occurring compound found in cruciferous vegetables such as broccoli and cauliflower. It has been widely studied for its potential as a neuroprotective and anticancer agent. SFN has been shown to exert neuroprotective effects through the activation of the Nrf2 pathway, the modulation of neuroinflammation, and epigenetic mechanisms. In cancer treatment, SFN has demonstrated the ability to selectively induce cell death in cancer cells, inhibit histone deacetylase, and sensitize cancer cells to chemotherapy. SFN has also shown chemoprotective properties through inhibiting phase I metabolizing enzymes, modulating phase II xenobiotic-metabolizing enzymes, and targeting cancer stem cells.
- sulforaphane
- antioxidant
- cancer
- chemotherapy
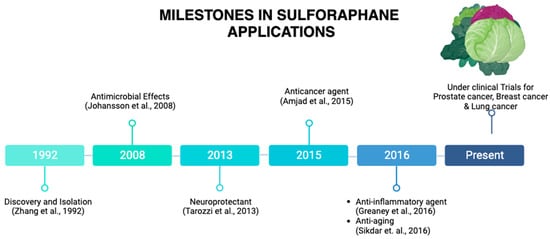
1. Origin and Discovery
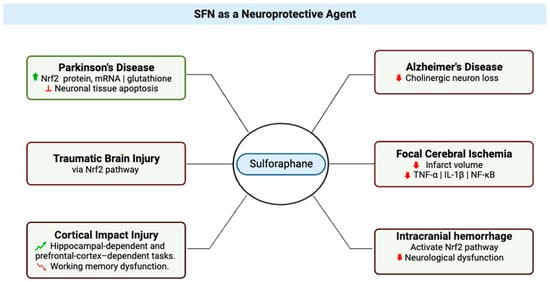
2. SFN as a Neuroprotective Agent
3. SFN as an Anticancer Agent
In recent years, SFN has gained attention for its potential use as an anticancer agent. In a study in 2015, Ullah proposed three major factors that enhance the plausibility of clinical applications and the translational value. First, normal cells are relatively resistant to SFN-induced cell death [60][41], an important feature for potential anticancer agents, and recent in vitro work demonstrated that SFN suppressed metastasis of triple-negative breast cancer cells by targeting the RAF/MEK/ERK pathway [61][42]. Second, SFN has good bioavailability; it can reach high intracellular and plasma concentrations, and it has been detected in breast tissues after a single oral administration [62,63][43][44]. Finally, a study by Myzak et al. in 2007 provided evidence that histone deacetylase was inhibited after human subjects ingested 68 g of broccoli sprouts, indicating that SFN provides anticancer pharmacological effects at levels that humans can readily ingest. Ullah proposed a mechanism by which SFN exerts its chemopreventive effects. In this model, low to moderate levels of reactive oxygen species (ROS) are active participants in cellular functions and act as signaling molecules that sustain cellular proliferation and differentiation and that activate responses to oxidative stress [64][45]. Under normal, unstressed conditions, the cellular NRF2 level is very low [65][46], but it significantly increases upon exposure to electrophilic chemicals or ROS [66][47]. In response to oxidative or electrophilic stress, Nrf2 stimulates antistress signaling and consequently inhibits carcinogenesis [67][48]. Under conditions of stress, Kelch-like ECH-Associated Protein 1 (KEAP1), which is a cytosolic inhibitor of Nrf2, is oxidized, leading to stabilization and translocation of Nrf2 into the nucleus, where the transcription factor activates expression of genes crucial to antioxidant defense [68][49]. In bladder cancer (Figure 4), an in vitro study highlighted SFN’s dose-dependent effects on cell growth, presenting opportunities for targeted therapeutic strategies [71][50]. In this study, at higher concentrations, ranging from 10–160 μM and after 24 to 48 h of treatment, SFN demonstrated a significant inhibitory effect on T24 cell growth. However, it is important to consider that at lower doses, specifically 2.5 μM, SFN resulted in a slight increase in cell proliferation by 5.18–11.84% within a 6 to 48 h treatment window [71][50]. These results suggest that SFN’s effects on cell growth are dose-dependent, with potential implications for further research and development of targeted therapeutic strategies in the context of bladder cancer.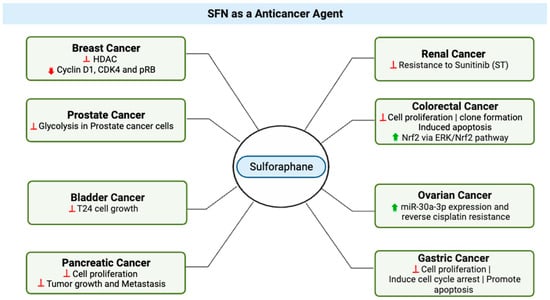
4. Chemoprotectant Properties of SFN
5. Effects of SFN on Tumors, Chemotherapy, Radiation Therapy, and Cardiotoxicity
Laboratory and animal studies have provided evidence that SFN has anticancer properties, but more research is needed to understand its effects on humans. Studies have proposed that SFN may prevent the growth of certain types of tumors, such as breast and prostate tumors, by causing cell death and stopping the formation of new blood vessels, which are required for tumor growth. Research supports the proposition that SFN may improve the effectiveness of chemotherapy by increasing cancer cell sensitivity to the drugs used to treat them [87][65], which is known as “chemo-sensitization”. One way in which SFN may sensitize cancer cells to chemotherapy is by inhibiting the Nrf2 pathway [87][65]. Some studies have suggested that SFN may have beneficial effects on the body after radiation therapy, although more research is needed to confirm these findings. Radiation therapy is a common treatment for cancer that uses high-energy radiation to kill cancer cells, but it can also damage healthy cells and tissues in the area being treated, leading to side effects such as skin irritation, fatigue, and a risk of developing secondary tumors. Preliminary studies have suggested that SFN may help protect healthy cells and tissues from the harmful effects of radiation [97][66]. Cardiotoxicity is the occurrence of heart dysfunction as electric or muscle damage, resulting in heart toxicity [92][67]. Several studies have demonstrated the protective role of SFN in cardiotoxicity. For example, in a study by Bose et al. [99][68] with a breast cancer model in rats, they found that SFN reduced cardiac oxidative stress (a contributing factor to cardiotoxicity) induced by doxorubicin (DOX). When DOX was administered alone, only an 11% survival rate was observed as compared to a 62% survival rate observed when DOX was combined with SFN6. SFN on Metastasis
SFN exhibits anticancer properties at various stages of carcinogenesis in prostate, lung, colon, and breast cancers [104,105,106][69][70][71]. Early research has focused on the ability of SFN to activate nuclear factor (erythroid-derived 2)-like 2 (Nrf2). SFN was shown to be effective in preventing breast cancer at different stages of carcinogenesis by increasing the levels of antioxidants and phase II detoxifying enzymes via the activation of the nuclear factor erythroid 2-related factor 2 [107][72]. SFN has been shown to alter key mechanisms in vivo and in vitro which impact induction of cell cycle arrest and apoptosis and inhibition of histone deacetylase. SFN inhibits transforming growth factor-β1 (TGF-β1)-induced migration and invasion in human triple negative breast cancer (TNBC) cells [61][42]. Sulforaphane (SFE), an SFN derivative, has been shown to reduce TNBC proliferation by mediating ERG1/PTEN axis [107][72]. Sulforaphane exerted its anti-metastatic effects on non-small cell lung cancer through down-regulation of miR-616-5p, which was identified as a marker associated with risk of relapse and metastasis in patients [108][73]. SFN has been reported to inhibit histone deacetylase (HDAC) enzymes, alter histone acetylation, and affect gene regulation. Natural inhibitors of HDAC have received considerable interest as anticancer agents because of their ability to induce p21Cip1/Waf1, leading to cell cycle arrest and apoptosis [62][43]. SFN inhibits HDAC activity in prostate cancer cells, in mouse xenografts, and in human peripheral blood mononuclear cells [109][74]. In colon cancers, SFN blocks cells’ progression and angiogenesis by inhibiting HIF-1α and VEGF expression [110][75].7. SFN Bioavailability and Pharmacokinetics
In mammals, SFN is metabolized rapidly via a conjugation reaction with glutathione. SFN is metabolized through the mercapturic acid pathway, starting with GSH conjugation by glutathione S-transferase and subsequently generating SFN-cysteine followed by SFN-N-acetylcysteine [111][76]. Pharmacokinetic studies in rodents have focused on either free SFN or its metabolite SFN-glutathione. Following consumption of broccoli, sulforaphane is excreted in urine predominantly as a conjugate with N-acetyl cysteine. In plasma, it has been found that approximately 50% of sulforaphane is found unconjugated with other thiols [104][69]. In rats, after an oral dose of 50 μmol of SFN, the plasma concentration of SFN can peak at 20 μM at 4 h and decline with a half-life of about 2.2 h [112][77]. SFN is well absorbed in the intestine, with an absolute bioavailability of approximately 82%. Elimination of was characterized by a long terminal phase; no major difference was evident in plasma concentrations between 6 and 24 h following intravenous administration or oral administration [113][78].8. Ongoing and Completed Clinical Trials on SFN
Clinical trials are an important part of the research process, and drug development benefits from both favorable and unfavorable results. Even when studies do not yield the predicted outcomes, trial results can help point scientists in the correct direction [72][53]. (Clinical trials of SFN that were withdrawn are not included in the data presented here.) The conditions with the greatest number of ongoing or previous clinical trials on SFN, including breast cancer and prostate cancer, are shown in Figure 5a; the general phases of clinical trials on SFN (both past and current) are presented in Figure 5b.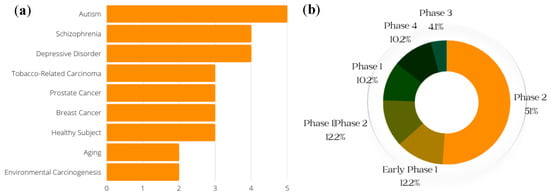
References
- Fahey, J.W.; Talalay, P. Antioxidant functions of sulforaphane: A potent inducer of Phase II detoxication enzymes. Food Chem. Toxicol. 1999, 37, 973–979.
- Zhang, Y.; Talalay, P.; Cho, C.G.; Posner, G.H. A major inducer of anticarcinogenic protective enzymes from broccoli: Isolation and elucidation of structure. Proc. Natl. Acad. Sci. USA 1992, 89, 2399–2403.
- Parker, J.K.; Elmore, S.; Methven, L. Flavour Development, Analysis and Perception in Food and Beverages; Elsevier: Amsterdam, The Netherlands, 2014.
- Zhang, Y.; Tang, L. Discovery and development of sulforaphane as a cancer chemopreventive phytochemical. Acta Pharmacol. Sin. 2007, 28, 1343–1354.
- Kushad, M.M.; Brown, A.F.; Kurilich, A.C.; Juvik, J.A.; Klein, B.P.; Wallig, M.A.; Jeffery, E.H. Variation of glucosinolates in vegetable crops of Brassica oleracea. J. Agric. Food Chem. 1999, 47, 1541–1548.
- Shapiro, T.A.; Fahey, J.W.; Wade, K.L.; Stephenson, K.K.; Talalay, P. Chemoprotective glucosinolates and isothiocyanates of broccoli sprouts: Metabolism and excretion in humans. Cancer Epidemiol. Biomark. Prev. 2001, 10, 501–508.
- Houghton, C.A.; Fassett, R.G.; Coombes, J.S. Sulforaphane: Translational research from laboratory bench to clinic. Nutr. Rev. 2013, 71, 709–726.
- Johansson, N.L.; Pavia, C.S.; Chiao, J.W. Growth inhibition of a spectrum of bacterial and fungal pathogens by sulforaphane, an isothiocyanate product found in broccoli and other cruciferous vegetables. Planta Med. 2008, 74, 747–750.
- Tarozzi, A.; Angeloni, C.; Malaguti, M.; Morroni, F.; Hrelia, S.; Hrelia, P. Sulforaphane as a Potential Protective Phytochemical against Neurodegenerative Diseases. Oxidative Med. Cell. Longev. 2013, 415078.
- Amjad, A.I.; Parikh, R.A.; Appleman, L.J.; Hahm, E.R.; Singh, K.; Singh, S.V. Broccoli-Derived Sulforaphane and Chemoprevention of Prostate Cancer: From Bench to Bedside. Curr. Pharmacol. Rep. 2015, 1, 382–390.
- Greaney, A.J.; Maier, N.K.; Leppla, S.H.; Moayeri, M. Sulforaphane inhibits multiple inflammasomes through an Nrf2-independent mechanism. J. Leukoc. Biol. 2016, 99, 189–199.
- Sikdar, S.; Papadopoulou, M.; Dubois, J. What Do We Know About Sulforaphane Protection against Photoaging? J. Cosmet. Dermatol. 2016, 15, 72–77.
- Bhat, S.A.; Kamal, M.A.; Yarla, N.S.; Ashraf, G.M. Synopsis on Managment Strategies for Neurodegenerative Disorders: Challenges from Bench to Bedside in Successful Drug Discovery and Development. Curr. Top. Med. Chem. 2017, 17, 1371–1378.
- Ladak, Z.; Garcia, E.; Yoon, J.; Landry, T.; Armstrong, E.A.; Yager, J.Y.; Persad, S. Sulforaphane (SFA) protects neuronal cells from oxygen & glucose deprivation (OGD). PLoS ONE 2021, 16, e0248777.
- Kraft, A.D.; Johnson, D.A.; Johnson, J.A. Nuclear factor E2-related factor 2-dependent antioxidant response element activation by tert-butylhydroquinone and sulforaphane occurring preferentially in astrocytes conditions neurons against oxidative insult. J. Neurosci. 2004, 24, 1101–1112.
- Sandouka, S.; Shekh-Ahmad, T. Induction of the Nrf2 Pathway by Sulforaphane Is Neuroprotective in a Rat Temporal Lobe Epilepsy Model. Antioxidants 2021, 10, 1702.
- Zhou, Q.; Chen, B.; Wang, X.; Wu, L.; Yang, Y.; Cheng, X.; Hu, Z.; Cai, X.; Yang, J.; Sun, X.; et al. Sulforaphane protects against rotenone-induced neurotoxicity in vivo: Involvement of the mTOR, Nrf2, and autophagy pathways. Sci. Rep. 2016, 6, 32206.
- Zhao, J.; Liu, L.; Li, X.; Zhang, L.; Lv, J.; Guo, X.; Chen, H.; Zhao, T. Neuroprotective effects of an Nrf2 agonist on high glucose-induced damage in HT22 cells. Biol. Res. 2019, 52, 53.
- Mizuno, K.; Kume, T.; Muto, C.; Takada-Takatori, Y.; Izumi, Y.; Sugimoto, H.; Akaike, A. Glutathione biosynthesis via activation of the nuclear factor E2-related factor 2 (Nrf2)—Antioxidant-response element (ARE) pathway is essential for neuroprotective effects of sulforaphane and 6-(methylsulfinyl) hexyl isothiocyanate. J. Pharmacol. Sci. 2011, 115, 320–328.
- Zhao, F.; Zhang, J.; Chang, N. Epigenetic modification of Nrf2 by sulforaphane increases the antioxidative and anti-inflammatory capacity in a cellular model of Alzheimer’s disease. Eur. J. Pharmacol. 2018, 824, 1–10.
- Zhao, X.; Wen, L.; Dong, M.; Lu, X. Sulforaphane activates the cerebral vascular Nrf2-ARE pathway and suppresses inflammation to attenuate cerebral vasospasm in rat with subarachnoid hemorrhage. Brain Res. 2016, 1653, 1–7.
- Jazwa, A.; Rojo, A.I.; Innamorato, N.G.; Hesse, M.; Fernández-Ruiz, J.; Cuadrado, A. Pharmacological targeting of the transcription factor Nrf2 at the basal ganglia provides disease modifying therapy for experimental parkinsonism. Antioxid. Redox Signal. 2011, 14, 2347–2360.
- Hong, Y.; Yan, W.; Chen, S.; Sun, C.R.; Zhang, J.M. The role of Nrf2 signaling in the regulation of antioxidants and detoxifying enzymes after traumatic brain injury in rats and mice. Acta Pharmacol. Sin. 2010, 31, 1421–1430.
- Morroni, F.; Sita, G.; Djemil, A.; D’Amico, M.; Pruccoli, L.; Cantelli-Forti, G.; Hrelia, P.; Tarozzi, A. Comparison of Adaptive Neuroprotective Mechanisms of Sulforaphane and its Interconversion Product Erucin in in Vitro and in Vivo Models of Parkinson’s Disease. J. Agric. Food Chem. 2018, 66, 856–865.
- Benedict, A.L.; Mountney, A.; Hurtado, A.; Bryan, K.E.; Schnaar, R.L.; Dinkova-Kostova, A.T.; Talalay, P. Neuroprotective effects of sulforaphane after contusive spinal cord injury. J. Neurotrauma 2012, 29, 2576–2586.
- Dash, P.K.; Zhao, J.; Orsi, S.A.; Zhang, M.; Moore, A.N. Sulforaphane improves cognitive function administered following traumatic brain injury. Neurosci. Lett. 2009, 460, 103–107.
- Trio, P.Z.; Fujisaki, S.; Tanigawa, S.; Hisanaga, A.; Sakao, K.; Hou, D.X. DNA Microarray Highlights Nrf2-Mediated Neuron Protection Targeted by Wasabi-Derived Isothiocyanates in IMR-32 Cells. Gene Regul. Syst. Biol. 2016, 10, 73–83.
- Zhao, J.; Kobori, N.; Aronowski, J.; Dash, P.K. Sulforaphane reduces infarct volume following focal cerebral ischemia in rodents. Neurosci. Lett. 2006, 393, 108–112.
- Ma, L.L.; Xing, G.P.; Yu, Y.; Liang, H.; Yu, T.X.; Zheng, W.H.; Lai, T.B. Sulforaphane exerts neuroprotective effects via suppression of the inflammatory response in a rat model of focal cerebral ischemia. Int. J. Clin. Exp. Med. 2015, 8, 17811–17817.
- Imai, T.; Matsubara, H.; Hara, H. Potential therapeutic effects of Nrf2 activators on intracranial hemorrhage. J. Cereb. Blood Flow. Metab. 2021, 41, 1483–1500.
- Pan, J.; Wang, R.; Pei, Y.; Wang, D.; Wu, N.; Ji, Y.; Tang, Q.; Liu, L.; Cheng, K.; Liu, Q.; et al. Sulforaphane alleviated vascular remodeling in hypoxic pulmonary hypertension via inhibiting inflammation and oxidative stress. J. Nutr. Biochem. 2023, 111, 109182.
- Cao, H.; Wang, L.; Chen, B.; Zheng, P.; He, Y.; Ding, Y.; Deng, Y.; Lu, X.; Guo, X.; Zhang, Y.; et al. DNA Demethylation Upregulated Nrf2 Expression in Alzheimer’s Disease Cellular Model. Front. Aging Neurosci. 2015, 7, 244.
- Schachtele, S.J.; Hu, S.; Lokensgard, J.R. Modulation of experimental herpes encephalitis-associated neurotoxicity through sulforaphane treatment. PLoS ONE 2012, 7, e36216.
- Hernandez-Rabaza, V.; Cabrera-Pastor, A.; Taoro-Gonzalez, L.; Gonzalez-Usano, A.; Agusti, A.; Balzano, T.; Llansola, M.; Felipo, V. Neuroinflammation increases GABAergic tone and impairs cognitive and motor function in hyperammonemia by increasing GAT-3 membrane expression. Reversal by sulforaphane by promoting M2 polarization of microglia. J. Neuroinflammation 2016, 13, 83.
- Kwon, H.S.; Koh, S.H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42.
- Subedi, L.; Lee, J.H.; Yumnam, S.; Ji, E.; Kim, S.Y. Anti-Inflammatory Effect of Sulforaphane on LPS-Activated Microglia Potentially through JNK/AP-1/NF-κB Inhibition and Nrf2/HO-1 Activation. Cells 2019, 8, 194.
- Subedi, L.; Lee, J.H.; Gaire, B.P.; Kim, S.Y. Sulforaphane Inhibits MGO-AGE-Mediated Neuroinflammation by Suppressing NF-κB, MAPK, and AGE-RAGE Signaling Pathways in Microglial Cells. Antioxidants 2020, 9, 792.
- Wang, Z.C.; Chen, Q.; Wang, J.; Yu, L.S.; Chen, L.W. Sulforaphane mitigates LPS-induced neuroinflammation through modulation of Cezanne/NF-κB signalling. Life Sci. 2020, 262, 118519.
- Kapoor, K.; Bhandare, A.M.; Farnham, M.M.; Pilowsky, P.M. Alerted microglia and the sympathetic nervous system: A novel form of microglia in the development of hypertension. Respir. Physiol. Neurobiol. 2016, 226, 51–62.
- von Bernhardi, R.; Heredia, F.; Salgado, N.; Muñoz, P. Microglia Function in the Normal Brain. Adv. Exp. Med. Biol. 2016, 949, 67–92.
- Zeng, H.; Trujillo, O.N.; Moyer, M.P.; Botnen, J.H. Prolonged sulforaphane treatment activates survival signaling in nontumorigenic NCM460 colon cells but apoptotic signaling in tumorigenic HCT116 colon cells. Nutr. Cancer 2011, 63, 248–255.
- Zhang, Y.; Lu, Q.; Li, N.; Xu, M.; Miyamoto, T.; Liu, J. Sulforaphane suppresses metastasis of triple-negative breast cancer cells by targeting the RAF/MEK/ERK pathway. NPJ Breast Cancer 2022, 8, 40.
- Myzak, M.C.; Karplus, P.A.; Chung, F.L.; Dashwood, R.H. A novel mechanism of chemoprotection by sulforaphane: Inhibition of histone deacetylase. Cancer Res. 2004, 64, 5767–5774.
- Cornblatt, B.S.; Ye, L.; Dinkova-Kostova, A.T.; Erb, M.; Fahey, J.W.; Singh, N.K.; Chen, M.S.; Stierer, T.; Garrett-Mayer, E.; Argani, P.; et al. Preclinical and clinical evaluation of sulforaphane for chemoprevention in the breast. Carcinogenesis 2007, 28, 1485–1490.
- Janssen-Heininger, Y.M.; Mossman, B.T.; Heintz, N.H.; Forman, H.J.; Kalyanaraman, B.; Finkel, T.; Stamler, J.S.; Rhee, S.G.; van der Vliet, A. Redox-based regulation of signal transduction: Principles, pitfalls, and promises. Free Radic. Biol. Med. 2008, 45, 1–17.
- Mimura, J.; Ema, M.; Sogawa, K.; Fujii-Kuriyama, Y. Identification of a novel mechanism of regulation of Ah (dioxin) receptor function. Genes. Dev. 1999, 13, 20–25.
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I.; et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322.
- Zhang, R.; Miao, Q.W.; Zhu, C.X.; Zhao, Y.; Liu, L.; Yang, J.; An, L. Sulforaphane ameliorates neurobehavioral deficits and protects the brain from amyloid β deposits and peroxidation in mice with Alzheimer-like lesions. Am. J. Alzheimers Dis. Other Demen 2015, 30, 183–191.
- Lee, S.; Choi, B.R.; Kim, J.; LaFerla, F.M.; Park, J.H.Y.; Han, J.S.; Lee, K.W.; Kim, J. Sulforaphane Upregulates the Heat Shock Protein Co-Chaperone CHIP and Clears Amyloid-β and Tau in a Mouse Model. of Alzheimer’s Disease. Mol. Nutr. Food Res. 2018, 62, e1800240.
- He, C.; Buongiorno, L.P.; Wang, W.; Tang, J.C.Y.; Miceli, N.; Taviano, M.F.; Shan, Y.; Bao, Y. The Inhibitory Effect of Sulforaphane on Bladder Cancer Cell Depends on GSH Depletion-Induced by Nrf2 Translocation. Molecules 2021, 26, 4919.
- Cao, C. HDAC5-LSD1 axis regulates antineoplastic effect of natural HDAC inhibitor sulforaphane in human breast cancer cells. Int. J. Cancer 2018, 143, 1388–1401.
- Singh, K.B.; Hahm, E.R.; Alumkal, J.J.; Foley, L.M.; Hitchens, T.K.; Shiva, S.S.; Parikh, R.A.; Jacobs, B.L.; Singh, S.V. Reversal of the Warburg phenomenon in chemoprevention of prostate cancer by sulforaphane. Carcinogenesis 2019, 40, 1545–1556.
- Royston, K.J.; Paul, B.; Nozell, S.; Rajbhandari, R.; Tollefsbol, T.O. Withaferin A and sulforaphane regulate breast cancer cell cycle progression through epigenetic mechanisms. Exp. Cell Res. 2018, 368, 67–74.
- Chen, X.; Jiang, Z.; Zhou, C.; Chen, K.; Li, X.; Wang, Z.; Wu, Z.; Ma, J.; Ma, Q.; Duan, W. Activation of Nrf2 by Sulforaphane Inhibits High Glucose-Induced Progression of Pancreatic Cancer via AMPK Dependent Signaling. Cell Physiol. Biochem. 2018, 50, 1201–1215.
- Tsaur, I.; Thomas, A.; Taskiran, E.; Rutz, J.; Chun, F.K.; Haferkamp, A.; Juengel, E.; Blaheta, R.A. Concomitant Use of Sulforaphane Enhances Antitumor Efficacy of Sunitinib in Renal Cell Carcinoma In Vitro. Cancers 2022, 14, 4643.
- Gong, T.T.; Liu, X.D.; Zhan, Z.P.; Wu, Q.J. Sulforaphane enhances the cisplatin sensitivity through regulating DNA repair and accumulation of intracellular cisplatin in ovarian cancer cells. Exp. Cell Res. 2020, 393, 112061.
- Hao, Q.; Wang, M.; Sun, N.X.; Zhu, C.; Lin, Y.M.; Li, C.; Liu, F.; Zhu, W.W. Sulforaphane suppresses carcinogenesis of colorectal cancer through the ERK/Nrf2-UDP glucuronosyltransferase 1A metabolic axis activation. Oncol. Rep. 2020, 43, 1067–1080.
- Wang, Y.; Wu, H.; Dong, N.; Su, X.; Duan, M.; Wei, Y.; Wei, J.; Liu, G.; Peng, Q.; Zhao, Y. Sulforaphane induces S-phase arrest and apoptosis via p53-dependent manner in gastric cancer cells. Sci. Rep. 2021, 11, 2504.
- Jin, J.; Xie, Y.; Zhang, J.S.; Wang, J.Q.; Dai, S.J.; He, W.F.; Li, S.Y.; Ashby, C.R., Jr.; Chen, Z.S.; He, Q. Sunitinib resistance in renal cell carcinoma: From molecular mechanisms to predictive biomarkers. Drug Resist. Updat. 2023, 67, 100929.
- Linsalata, M.; Orlando, A.; Russo, F. Pharmacological and dietary agents for colorectal cancer chemoprevention: Effects on polyamine metabolism (review). Int. J. Oncol. 2014, 45, 1802–1812.
- Chain, N.G. N-and O-Glycosylation; Springer: Berlin/Heidelberg, Germany, 2011.
- Tsai, J.Y.; Tsai, S.H.; Wu, C.C. The chemopreventive isothiocyanate sulforaphane reduces anoikis resistance and anchorage-independent growth in non-small cell human lung cancer cells. Toxicol. Appl. Pharmacol. 2019, 362, 116–124.
- Tsai, T.F.; Chen, P.C.; Lin, Y.C.; Chou, K.Y.; Chen, H.E.; Ho, C.Y.; Lin, J.F.; Hwang, T.I. Miconazole Contributes to NRF2 Activation by Noncanonical P62-KEAP1 Pathway in Bladder Cancer Cells. Drug Des. Devel Ther. 2020, 14, 1209–1218.
- Jiang, X.; Liu, Y.; Ma, L.; Ji, R.; Qu, Y.; Xin, Y.; Lv, G. Chemopreventive activity of sulforaphane. Drug Des. Devel Ther. 2018, 12, 2905–2913.
- Račkauskas, R.; Zhou, D.; Ūselis, S.; Strupas, K.; Herr, I.; Schemmer, P. Sulforaphane sensitizes human cholangiocarcinoma to cisplatin via the downregulation of anti-apoptotic proteins. Oncol. Rep. 2017, 37, 3660–3666.
- Wei, J.; Zhao, Q.; Zhang, Y.; Shi, W.; Wang, H.; Zheng, Z.; Meng, L.; Xin, Y.; Jiang, X. Sulforaphane-Mediated Nrf2 Activation Prevents Radiation-Induced Skin Injury through Inhibiting the Oxidative-Stress-Activated DNA Damage and NLRP3 Inflammasome. Antioxidants 2021, 10, 1850.
- Sishi, B.J.N. Autophagy Upregulation Reduces Doxorubicin-Induced Cardiotoxicity. In Autophagy: Cancer, Other Pathologies, Inflammation, Immunity, Infection, and Aging; Academic Press: Cambridge, MA, USA, 2015; pp. 157–173.
- Bose, C.; Awasthi, S.; Sharma, R.; Beneš, H.; Hauer-Jensen, M.; Boerma, M.; Singh, S.P. Sulforaphane potentiates anticancer effects of doxorubicin and attenuates its cardiotoxicity in a breast cancer model. PLoS ONE 2018, 13, e0193918.
- Traka, M.H.; Melchini, A.; Mithen, R.F. Sulforaphane and prostate cancer interception. Drug Discov. Today 2014, 19, 1488–1492.
- Jiang, L.L.; Zhou, S.J.; Zhang, X.M.; Chen, H.Q.; Liu, W. Sulforaphane suppresses in vitro and in vivo lung tumorigenesis through downregulation of HDAC activity. Biomed. Pharmacother. 2016, 78, 74–80.
- Atwell, L.L.; Beaver, L.M.; Shannon, J.; Williams, D.E.; Dashwood, R.H.; Ho, E. Epigenetic Regulation by Sulforaphane: Opportunities for Breast and Prostate Cancer Chemoprevention. Curr. Pharmacol. Rep. 2015, 1, 102–111.
- Jabbarzadeh Kaboli, P.; Afzalipour Khoshkbejari, M.; Mohammadi, M.; Abiri, A.; Mokhtarian, R.; Vazifemand, R.; Amanollahi, S.; Yazdi Sani, S.; Li, M.; Zhao, Y.; et al. Targets and mechanisms of sulforaphane derivatives obtained from cruciferous plants with special focus on breast cancer—Contradictory effects and future perspectives. Biomed. Pharmacother. 2020, 121, 109635.
- Wang, D.X.; Zou, Y.J.; Zhuang, X.B.; Chen, S.X.; Lin, Y.; Li, W.L.; Lin, J.J.; Lin, Z.Q. Sulforaphane suppresses EMT and metastasis in human lung cancer through miR-616-5p-mediated GSK3beta/beta-catenin signaling pathways. Acta Pharmacol. Sin. 2017, 38, 241–251.
- Ho, E.; Clarke, J.D.; Dashwood, R.H. Dietary sulforaphane, a histone deacetylase inhibitor for cancer prevention. J. Nutr. 2009, 139, 2393–2396.
- Kim, D.H.; Sung, B.; Kang, Y.J.; Hwang, S.Y.; Kim, M.J.; Yoon, J.H.; Im, E.; Kim, N.D. Sulforaphane inhibits hypoxia-induced HIF-1alpha and VEGF expression and migration of human colon cancer cells. Int. J. Oncol. 2015, 47, 2226–2232.
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51.
- Hu, R.; Hebbar, V.; Kim, B.R.; Chen, C.; Winnik, B.; Buckley, B.; Soteropoulos, P.; Tolias, P.; Hart, R.P.; Kong, A.N. In vivo pharmacokinetics and regulation of gene expression profiles by isothiocyanate sulforaphane in the rat. J. Pharmacol. Exp. Ther. 2004, 310, 263–271.
- Hanlon, N.; Coldham, N.; Gielbert, A.; Kuhnert, N.; Sauer, M.J.; King, L.J.; Ioannides, C. Absolute bioavailability and dose-dependent pharmacokinetic behaviour of dietary doses of the chemopreventive isothiocyanate sulforaphane in rat. Br. J. Nutr. 2008, 99, 559–564.
