Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Piyush Baindara and Version 2 by Jason Zhu.
Through the promotion of phagolysosome formation, autophagy has emerged as a crucial mechanism to eradicate intracellular Mycobacterium tuberculosis (Mtb). A cell-autonomous host defense mechanism called lysosome biogenesis and autophagy transports cytoplasmic cargos and bacterial phagosomes to lysosomes for destruction during infection. Similar occurrences occurred in stressful or starvation circumstances and led to autophagy, which is harmful to the cell. It is interesting to note that under both hunger and infection states, the transcription factor EB (TFEB) acts as a master regulator of lysosomal activities and autophagy.
- autophagy
- Mycobacterium tuberculosis
- TFEB
1. Introduction
Mtb is an intracellular bacterium that may avoid fusion with phagolysosomes to live and stay within macrophages [1][2][1,2]. According to the World Health Organization’s global tuberculosis report, 2022, 10.6 million people contracted the disease, and 1.6 million people are predicted to have died from it in just the year 2021 (https://www.who.int/publications/i/item/9789240061729, accessed on 6 October 2023). Numerous methods are employed by host macrophages to eliminate pathogenic mycobacteria [1]. As a result of multiple extracellular and intracellular challenges, including food or growth factor deficiency, oxidative stress, an accumulation of damaged organelles or misfolded proteins, and viral or microbial infection, Mtb is removed from infected macrophages through the onset of autophagy. Autophagy controls the intracellular loads of Mtb in macrophages as a result; however, Mtb evolved several immunological escape strategies to promote infection [3].
One of the transcription factors involved in the induction of autophagy is TFEB, which is a crucial regulator of autophagic activation [4][5][4,5]. The TFEB belongs to the family of proteins known as the microphthalmia/transcription factor E (MiT/TFE) and is a basic helix-loop-helix-leucine-zipper (bHLH-Zip) protein [5]. The TFEB, transcription factor E3 (TFE3), transcription factor EC (TFEC), and melanocyte inducing transcription factor (MITF) are the four members of the MiT-TFE family. The same basic domain that binds particular DNA sequences, a helix-loop-helix (HLH), and a leucine-zipper (Zip) region that is crucial for protein-protein interactions with other MiTF/TFE family members is shared by all four of the members [5][6][5,6]. A conserved activation region is also present in TFEB, MITF, and TFE3 and is crucial for the transcriptional activation of these genes [7]. The most divergent member of the family, TFEC, lacks the activation domain and appears to inhibit rather than trigger transcription [6]. Several genes, including those involved in substrate targeting, degradation, and autophagosomal and lysosomal biogenesis, are transcriptionally regulated by the TFEB. By attaching to a 10-base E-box-like motif at the promoter region known as coordinated lysosomal expression and regulation (CLEAR), the TFEB also stimulates the transcription of several lysosomal genes. Through post-translational changes, protein-protein interactions, and spatial organization, the TFEB function is tightly controlled. In resting cells and nutrient-rich environments, the TFEB is primarily cytosolic and inactive [8][9][8,9]. However, the TFEB quickly moves to the nucleus in response to hunger or lysosomal dysfunction and triggers the transcription of its target genes. The degree of phosphorylation of the TFEB is a major determinant of both its cellular location and activity. Ser142 and Ser211, two specific serine residues in the TFEB protein, are critical regulators of the protein’s subcellular distribution [10]. The TFEB is kept in the cytoplasm when both of these two serine residues are phosphorylated. It has been demonstrated that phosphorylation of Ser211 in particular acts as a docking site for the chaperone 14-3-3, which locks the TFEB in the cytoplasm and inhibits it from moving to the nucleus, most likely by obscuring its nuclear localization signal (NLS) [11]. Additionally, the TFEB positively controls the expression of genes in the CLEAR network, including ATG9, LC3, SQSTM1, and LAMP1, which are engaged in multiple sequential phases of autophagy in response to famine, stress, or lysosomal dysfunction [10][12][13][14][10,12,13,14]. It is discovered through starvation tests that the TFEB controls its transcription by interacting with the CLEAR sites in the first intron [15]. Other transcriptional regulators of the TFEB include the fasting transcriptional activator cAMP response element-binding protein (CREB) and the fed state sensing nuclear receptor farnesoid X receptor (FXR), which respectively inhibit or induce the TFEB expression in the liver when in a fed or fasted state. The FXR-CREB axis performs as a crucial physiological switch that controls the TFEB-mediated autophagy, leading to continuous autophagic regulation of nutrients during feeding and fasting cycles [16]. Then, in nutrient-rich conditions, the mTORC1 phosphorylates TFEB on lysosomes, where it remains in the cytoplasm. The TFEB is dephosphorylated and translocates from the cytoplasm to the nucleus in response to starvation, where it upregulates genes related to autophagy and lysosomal biogenesis [17][18][17,18].
2. TFEB Is Differentially Regulated by Host Factors to Restrict Mtb Growth
Several host factors, including PPAR (nuclear receptor peroxisome proliferator-activated receptor), NR1D1 (nuclear receptor subfamily 1, group D, member 1), IFNγ, IRGM (Immunity related GTPase M), TRIMs (tripartite motif family proteins), and NCoR1 (Nuclear receptor corepressor) mediate the nuclear translocation of TFEB, a significant transcription factor that connects autophagy and lysosome formation, as a result of Mtb infection. PPAR: Kim et al. have demonstrated that the PPAR is essential for eliciting a host response during Mtb infection. The PPAR controls inflammation, mitochondrial and peroxisomal activity, and energy metabolism. Additionally, the PPAR activates the TFEB and prevents the development of lipid bodies. During Mtb infection, the TFEB silencing decreases phagosomal maturation and antibacterial responses while increasing macrophage inflammatory responses. TFEB is necessary for Mtb clearance and the PPAR-mediated activation of the autophagy-lysosomal pathway [19]. Later in 2019, Kim et al. demonstrated that the mitochondrial deacetylase sirtuin 3 (SIRT3) can lessen oxidative stress and pathological inflammation following Mtb or Bacillus Calmette-Guerin (BCG) infection. The SIRT3’s anti-mycobacterial activity is carried out via PPAR and TFEB. It is proven that mice lacking Sirt3 had higher bacterial loads and more lung inflammation than mice with Sirt3, although macrophages lacking Sirt3 were able to regain their anti-mycobacterial activity by overexpressing either TFEB or PPAR. Overall, it can be said that the SIRT3-PPAR-TFEB axis is crucial in promoting host resistance against Mtb infection by antibacterial autophagy (Figure 1) [20].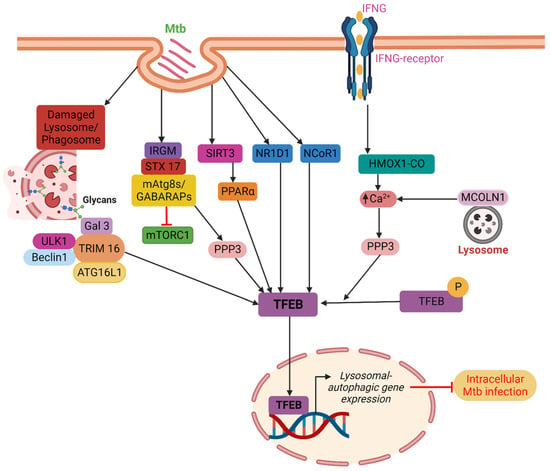
Figure 1. TFEB localization into the nucleus is indirectly influenced by PPARα, IFNγ, IRGM, and TRIMs. PPARα facilitates the disposal of Mtb in a TFEB-dependent manner through the SIRT3–PPARα–TFEB axis. IFNγ stimulates autophagy and Mtb clearance via the PPP3-TFEB signaling axis in a way that is reliant on HMOX1. IRGM blocks mTORC1 while encouraging PPP3 activity, which together results in efficient TFEB activation. TRIM16 binds ATG16L1 and is associated with the key autophagy regulators ULK1 and Beclin1. Furthermore, TRIM16 controls the activity of mTORC1 and the localization of TFEB in the nucleus and cytoplasm. Host factors such as NR1D1 and NCoR1 directly promote the nuclear localization of TFEB. Finally, TFEB causes lysosomal biogenesis and boosts autophagic flow in the nucleus that promotes the clearance of intracellular Mtb from infected macrophages.
3. Regulation of the mTORC1-TFEB Axis and Mtb Clearance under Starvation
Inducing autophagy through starvation can destroy pathogenic Mtb in macrophages by simulating stress or infection conditions [33][34]. Induced by nutritional deprivation, cellular injury, or stress, starvation-induced autophagy, also known as macroautophagy, involves the breakdown of damaged organelles, abnormally folded or folded proteins, and cytoplasmic foreign entities. By engulfing bacteria in autophagosomes, which fuse with lysosomes to produce autolysosomes, pathogens are destroyed in a process known as xenophagy [34][35][35,36]. To liberate nutrients for the de novo production of molecules during starvation, autophagy sequesters the cytosolic components of cells [36][37]. Unc-51-like kinase complex (ULK1) dissociates from adenosine monophosphate-activated protein kinase (AMPK) during stress situations such as nutrient deprivation or Mtb invasion. Beclin 1 is phosphorylated by the ULK1 complex, which then joins with phosphatidylinositol 3-kinase class 3 (PI3KC3) to create a complex. Phosphatidylinositol 3-phosphate (PI3P) is produced on endoplasmic reticulum membranes by beclin1-PI3KC3 complexes. The creation of an autophagic phagophore, which develops into a whole autophagosome, is then signaled by PI3P [37][38]. The WD-repeat protein interacting with phosphoinositide (WIPI) is recruited by the synthesis of PI3P to aid in the development of the phagophore. The ubiquitin-like conjugation mechanism, which is unique to autophagy, is recruited to the phagophore by WIPI2B. The lipidation of microtubule-associated protein 1A/1B light chain 3 (LC3) to phosphatidylethanolamine, also known as LC3 lipidation, is facilitated by WIPI2B-dependent recruitment of the ATG12-5/ATG16 complex. Additionally, LC3 lipidation causes the double membrane to self-fuse to form the autophagosome, which then merges with the lysosome to break down the ingested contents [38][39]. Soluble N-ethylmaleimide-sensitive factor attachment (SNARE) Syntaxin 17 (STX 17) promotes this fusion of the autophagosome and lysosome, and trapped Mtb or other cellular cargos are ultimately broken down in the resulting autolysosomes [37][38]. This is accomplished by increasing the expression of genes related to autophagy and lysosomal biogenesis by the AMPK-activated transcription factors Forkhead Box O3 (FoxO3) and TFEB (Figure 2) [39][40][40,41].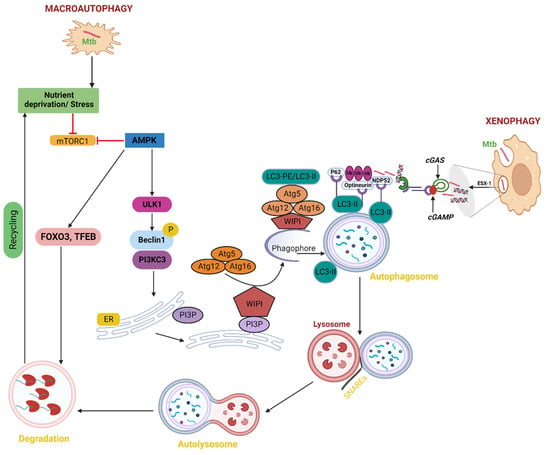
Figure 2. Macroautophagy and autophagy caused by starvation in Mtb. Starvation inhibits mTORC1, which in turn triggers ULK1 activation, the creation of autophagosomes, and TFEB activation, which further boosts the transcription of genes related to autophagy and lysosomes. Phagophores combine to generate autophagosomes. The phagophore enlarges by capturing cytoplasmic cargos, and Mtb contracts to create an autophagosome, a double-membrane vesicle. The Mtb and cytoplasmic cargos are then quickly broken down by lysosomal hydrolases and released into the cytosol for additional recycling after the lysosome and autophagosome combine to produce autolysosomes. Pathogens inside cells are targeted by xenophagy for lysosomal breakdown. The same processes that are involved in classical autophagy; initiation, elongation, substrate targeting, maturation/lysosomal fusion, which destroys cargo are also present in xenophagy. In the case of Mtb infection, substrate targeting entails the ubiquitination of bacteria or the colocalization of ubiquitin to bacteria. The recognition of the ubiquitin by autophagy receptors that interact with LC3 to attract the bacteria to autophagosomes, which then fuse to lysosomes to degrade the cargo, is then necessary for the recruitment of the bacteria to the autophagosomes.
4. Induction of Autophagy as a Host-Directed Therapeutic Approach against Mtb
Mtb can persist in a dormant, semi-replicating, or non-replicating form in the granuloma environment. It has been reported that granulomas help the host by preventing Mtb from spreading to other tissues or organs by enclosing the affected region with activating immune cells [52][53]. Also, the host-pathogen interactions in granulomas are extremely complicated, with the potential for bacterial death as well as survival. The host immunological response and the bacterial capacity to withstand or evade it are considered to have a cumulative influence on the intracellular survival of Mtb [53][54]. Thus, enhancing the immune repertoire to battle Mtb by the HDTs may be one strategy for efficient clearance and bacterial death [54][55]. To maximize the pro-inflammatory response or alter tissue physiology, host-directed treatment attempts to manipulate the metabolism and/or immune cell activity [55][56][57][56,57,58]. Due to the potential to repurpose medications that have already received approval for the treatment of chronic illnesses and the benefit that pathogenic bacteria, like Mtb, cannot become resistant to an HDT because it targets host cell functions, research on HDT as a potential therapeutic strategy for infectious diseases has recently gathered significant strength [58][59][59,60]. Thus, one emerging idea in the treatment of several chronic illnesses is to target the autophagy mechanism using small molecules and medications to enhance the host cell effector functions [60][61][61,62]. Here are the details of some known autophagy inducers that combat Mtb infection via upregulation of the TFEB. GSK4112: It has been shown that the synthetic small molecule GSK4112 increases lysosome biogenesis and induces autophagic flow by acting as an agonist to NR1D1 [22]. TFEB expression is modulated by the activation of the transcriptional protein NR1D1, which is important in infection and inflammation [62][63]. When Mtb strain H37Rv infected THP-1 cells, the overexpression of the TFEB increased the quantity of both autophagosomes and lysosomes [22]. GW7647: A synthetic small molecule agonist of the PPARα transcription factor, GW7647, has been shown by Kim et al. to increase autophagic flow in mice BMDMs against M. bovis BCG and Mtb strain H37Rv. The overexpression and translocation of TFEB, a crucial regulator of many genes involved in autophagic flux, is the outcome of GW7647’s activation of PPARα. Moreover, during mycobacterial infection, PPAR-α activation prevented the development of lipid bodies [19]. Lipid-lowering drugs (Simvastatin, Pravastatin, Rosuvastatin, Atorvastatin): These drugs decrease the Mtb load in human macrophages by lowering cholesterol levels and modulating the AMPK-mTORC1-TFEB axis in ways that encourage autophagy for Mtb clearance along with phagosome maturation and lysosome fusion [63][64][64,65]. Wy14643: It activates the PPARα receptor protein and subsequently enhances autophagic flux via upregulation of the TFEB signaling, along with increased lipid catabolism [19]. Compound 2062: An aminopyrimidine with the chemical name 2062 was the subject of a different investigation by Bryk et al. in 2020, which demonstrated enhanced control of intracellular Mtb in conjunction with rifampicin and linked with the activation of TFEB, which encourages lysosomal activation to accelerate Mtb clearance. Ambroxol: This mucoactive drug suppresses excessive mucus secretion by inhibiting NO-dependent activation of soluble guanylate cyclase that further induces autophagy via TFEB nuclear translocation stimulation [65][67]. Honokiol: Low molecular weight polyphenol honokiol has been shown to enhance the autophagic activities in human peripheral blood mononuclear cells (PBMCs), BMDMs, and HMDMs against strains of M. bovis BCG and Mtb H37Rv. It does this by activating SIRT3 deacetylase. The activation of SIRT3 by honokiol-mediated autophagy led to the subsequent induction of PPARα transcription factor expression, which in turn caused the overexpression and translocation of the TFEB, a crucial regulator of several genes involved in autophagic flux [20]. Bedaquiline: Bedaquiline sparks autophagy to promote clearance of Mtb. It induces autophagy by upregulating lysosomal activation via the TFEB and calcium signaling and potentiates the exertion of other anti-TB medicines [66][68]. Trehalose: Naturally occurring disaccharide trehalose has been shown to promote autophagy in many cell lines to combat Mtb and non-tuberculous mycobacterial (NTMs) [67][69]. Trehalose elevated phosphatidylinositol 3,5-bisphosphate, which functioned as an agonist for the mucolipin subfamily, member 1 (MCOLN1) channel and enhanced the release of Ca2+ from the lysosomal lumen, therefore inducing autophagic flow [68][70]. Released Ca2+ causes calcineurin, a serine-threonine phosphatase, to become active. This dephosphorylates TFEB and causes trehalose-mediated nuclear translocation of TFEB, which in turn triggers mTOR-independent autophagy in macrophages. Furthermore, trehalose competitively inhibited the GLUT transporters SLC2A3/GLUT3 and SLC2A8/GLUT8, inducing autophagy through AMPK activation and mTOR inhibition that resulted in a response that is similar to pseudo-starvation [67][69]. Autophagy targeting HDTs is an appealing adjunct to current medications. Importantly, autophagy inducers can restrict the Mtb growth via induction of TFEB-induced autophagy as well as can also combat the autophagy inhibition by Mtb itself. However, before being tested as a therapeutic intervention for tuberculosis in human clinical trials, considerable pre-clinical research employing suitable animal models of tuberculosis is necessary to determine the efficacy, toxicity, and other characteristics of these HDTs.5. Mtb Sulfolipid Controls the mTORC1-TFEB Axis and Prevents Infection
The most prevalent sulfated glycolipid in mycobacteria’s outer membrane and cell wall, sulfolipid-1 makes up to 1–2% of the dry weight of the cell wall but is only present in pathogenic mycobacteria [69][71]. Multiple mechanisms govern sulfolipid-1 synthesis, which has been shown to increase during infection in murine and human macrophages [70][71][72,73]. According to a recent study, pure Mtb sulfolipid-1 influences the kinetics of phagosome trafficking in macrophages and stimulates lysosomal biogenesis in host cells without regard to the cell type. Sulfolipid-1 works by preventing mTOR activity, which then causes TFEB to translocate into the nucleus and activate the expression of genes involved in lysosomal biogenesis (Figure 3). Human macrophages infected with Mtbpks2, a mutant that does not produce Sulfolipid-1 and lacks polyketide synthase 2, exhibit reduced lysosomal rewiring. Reduced bacterial interaction with lysosomes is caused by altered lysosomal activities, which is correlated with higher intracellular Mtb survival. In contrast, the mutant overproducing Sulfolipid-1 exhibits limited bacterial survival. The fact that mutants overproducing acetylated sulfated glycolipid (AC4SGL) are unable to stop phagosome maturation and are easily transported to lysosomes supports this as well [72][74]. To determine whether Mtb harbors a lipid that is beneficial to the host or whether targeting sulfolipid-1 could be a host-directed method to combat Mtb infection, more research will be needed [73][75].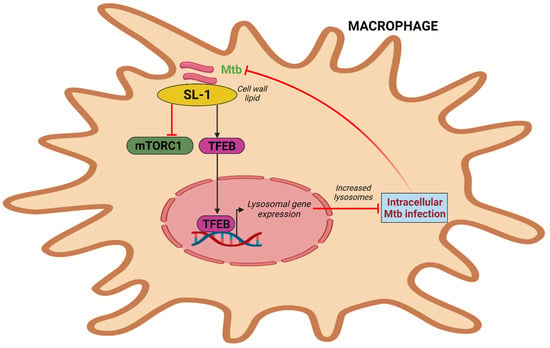
Figure 3. Intracellular Mtb simulates lysosome biogenesis via sulfolipid-1. Mtb sulfolipid-1 suppresses the activity of mTORC1 and causes the translocation of TFEB into the nucleus, which induces lysosomal gene expression and promotes lysosome biogenesis and subsequently Mtb clearance.
