Gestational diabetes mellitus (GDM) is one of the most common pregnancy complications. It is related to several gestational and fetal adverse outcomes. Moreover, women with GDM and their infants have a high risk of developing type 2 diabetes in the future. The pathogenesis of GDM is not completely understood; nevertheless, two factors that contribute to its development are oxidative stress and inflammation. Oxidative stress and inflammation are related; reactive oxygen species (ROS) production can activate inflammatory cells and enhance the production of inflammatory mediators. Inflammation, in turn, leads to an increased ROS release, causing a vicious circle to ensue. Inflammatory responses can be achieved via the activation of the NF-κB signaling pathway.
- oxidative stress
- inflammation
- gestational diabetes
1. Introduction
2. Reactive Oxygen Species
Mitochondria are the star organelles in all cell types, as they are the energy producers and suppliers. This process provides the ATP necessary for the vital functions of the cell. However, during the process of energy production, highly reactive molecules called free radicals are also synthesized. Mitochondria also play an important role in the control of cell proliferation, as they house several proteins that trigger the intrinsic pathway of apoptosis. Thus, mitochondria have a dual role in maintaining cellular homeostasis and the body itself; any alteration in its components or functions has implications for the development of various pathologies [15][17]. ATP synthesis is accomplished by the formation of an electrochemical proton gradient within the inner mitochondrial membrane resulting from the biochemical reactions that make up the respiratory chain. The arrival of nicotinamide and adenine dinucleotide coenzymes (NADH) and adenine-flavine dinucleotides (FADH2), products of pyruvate metabolism, amino acids and the oxidation of fatty acids, initiates the transfer of electrons through mitochondrial complexes (I, III and IV), which will eventually be transferred to oxygen. Additionally, the output of protons through the inner mitochondrial membrane generates the mitochondrial membrane potential necessary to produce ATP. When there is a proton overload, the electron transport in complex III is partially inhibited and promotes the return of electrons to coenzyme Q and its donation to molecular oxygen, thus facilitating the production of superoxide anion (O2•−). Hyperglycemia increases the activity of the sorbitol pathway and the accumulation of phosphate trioses (glyceraldehyde 3 phosphate and dihydroxyacetone phosphate) due to the inhibition of the glycolytic enzyme glyceraldehyde 3 phosphate dehydrogenase (G3PDH), which is capable of glycosylating proteins for the formation of advanced glycation products (AGEs), increasing protein kinase C (PKC) and activating the hexosamine pathway that generates uridine diphosphate-N-acetyl glucosamine (UDP-GlcNAc), which has the ability to bind to proteins and regulate their functions [16][17][18][19,20,21]. The activation of these mechanisms in addition to glucose autooxidation and mitochondrial dysfunction converges in a greater production of free radicals and oxidative stress [19][22]. ROS at physiological concentrations participate in various intracellular signaling pathways and thus contribute to proper cellular functioning. However, various stressors can increase ROS production and generate an oxidative stress state. In addition to mitochondrial respiration, in organisms, there are other sources of reactive species of biological importance, such as uncoupled nitric oxide synthase, peroxisomes, NADPH oxidase and the cytochrome P450 system. Reactive nitrogen species (RNS) are produced by uncoupled nitric oxide and, as their name says, contain nitrogen with different reactive capacities, such as nitric oxide (NO) and nitrogen dioxide (NO2). In peroxisomes, numerous oxidases, including xanthine oxidase (X/XO, for uric acid synthesis), together with cytochrome B5 and cytochrome P-450, also produce ROS [20][23]. NADPH oxidase is an enzymatic complex that is found in the plasma membrane. NADPH oxidase-generated ROS have an important role in cell signaling, the innate immune response and cell proliferation, including oncogenic transformation [21][24]. The body’s antioxidant defenses are classified into enzymatic and nonenzymatic defenses. The first group is composed of the enzymes superoxide dismutase (SOD) with three isoforms (SOD1, dependent on CuZn; SOD2, dependent upon Mn and SOD3, extracellular), catalase and glutathione peroxidase (GPx). SOD catalyzes the dismutation of O2•− into H2O2, which is transformed into water and oxygen by the action of catalase and GPx and thus prevents it from being integrated into the Fenton reaction and the production of •OH. This radical is one of the most reactive, as it quickly interacts with lipids, proteins and DNA, causing oxidative damage. The second group includes vitamins C and E, bilirubin, biliverdin, uric acid, glutathione (GSH) and flavonoids, of which GSH is the most abundant intracellular antioxidant [22][23][25,26].2.1. Oxidative Stress
The imbalance between ROS production and the activation of antioxidant systems leads to a state known as “oxidative stress”. This highlights the production of ROS and the oxidation of macromolecules that result in cellular and tissue damage. It is known that the body’s antioxidant defenses can decrease and/or be lost under certain circumstances, resulting in increased ROS and oxidative stress. Uncontrolled production of ROS alters metabolism and can induce cell death. ROS quickly react with biomolecules (DNA, proteins, lipids and carbohydrates), oxidize them and cause irreparable damage, disrupt homeostasis and lead to cell loss. The increase in the superoxide anion in the respiratory chain is an event that triggers the activation of glucotoxicity mechanisms. The •OH radical attacks DNA and generates important mutations and alterations at the transcriptional level. Oxidative damage to DNA can be measured by the presence of 8-hydroxy-2’-deoxyguanosine (8-OHdG). DNA attack by RNS causes purine nitration. Proteins experience oxidation and nitration in their amino acids by the action of ROS and RNS, with a subsequent loss of amino acids and biological functions. Oxidative damage can be evaluated by the concentration of carbonyl groups and tyrosine nitration. Glycosylation refers to the interaction between the lysine and arginine residues of proteins with glucose to form early-stage Amadori products or fructosamine. Oxidative stress has been identified as part of the physiopathology of various diseases, among which metabolic diseases (metabolic syndrome and diabetes) are highlighted, although it is also identified in obesity and gestation. In this context, increased glucose and lipids, which often accompany metabolic diseases, promote ROS production. Due to the increased electron supply in the mitochondrial respiratory chain, increased NADH and FADH2 and the activation of other metabolic pathways also produce ROS.2.2. Oxidative Stress and Its Interaction with Inflammation
Inflammation is a series of molecular and cellular responses that protect the body from infections and other insults. The inflammatory response is maintained until the stimulus is controlled or disappears and is known as acute inflammation; if this does not occur, then there are indications of chronic inflammation [24][32]. The inflammatory response involves the main cells of the immune system (neutrophils, basophils, mast cells, T cells, B cells, etc.). However, the presence of specific leukocytes in certain lesions has been demonstrated. The need for the strict regulation of cytokines, growth factors, eicosanoids (prostaglandin, leukotrienes, etc.), complement and peptides, as well as of the intracellular signaling needed, will depend on the type of instigator or aggressor (pathogenic, molecules) and/or involved tissues [25][33]. Alterations in metabolism can also induce chronic inflammation due to the presence of some endogenous molecules, such as AGEs, oxidized lipoproteins, saturated free fatty acids (palmitic), cytokines and hyperglycemia, in addition to molecules secreted by adipose tissue. This type of inflammation has been associated with the development of pathophysiological processes linked to a greater production of free radicals and oxidative stress [26][34], events present in metabolic diseases such as obesity, metabolic syndrome, type 2 diabetes (T2D) and GDM. The inflammation of adipose tissue can spread to pancreatic islets and other tissues, mainly in an insulin-dependent manner. This is evidenced by the presence of high levels of inflammatory markers in the serum of obese patients with insulin resistance [27][37]. TNF-α levels correlate with the body mass index and promote IL-6 expression, exacerbating hepatic triacylglycerol secretion and hypertriglyceridemia [28][38].3. Relationship between Oxidative Stress and GDM
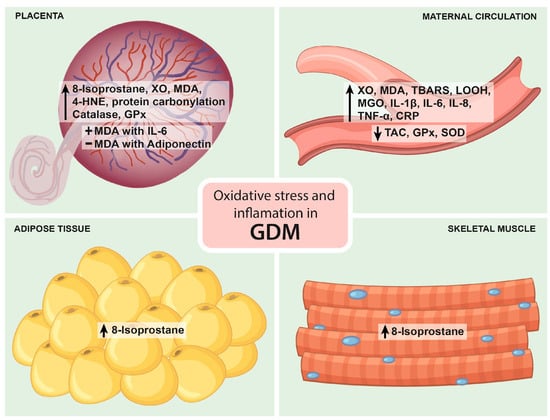
Physiological pregnancy increases oxidative stress, resulting in high levels of circulating ROS. The main source of ROS during pregnancy is the placenta. Increased oxidative stress is counterbalanced by increased antioxidant synthesis [29][41]. Placentas from women with GDM show a higher release of 8-isoprostane, a marker of lipid peroxidation, compared to placentas from normal pregnancies. Likewise, the GDM placenta also increases the expression of xanthine oxidase (XO), malondialdehyde (MDA), 4-hydroxynonenal (4-HNE) and protein carbonyl. When oxidative stress surpasses the antioxidant defense in the placenta, oxidative damage can spread to distal tissues. GDM is characterized by increased maternal circulating levels of oxidative stress markers (higher than in physiological pregnancy) and an altered antioxidant defense. The reactive oxygen species XO, MDA, thiobarbituric acid reactive substances (TBARS) and lipid hydroperoxide (LOOH) are significantly increased, and TAC is significantly decreased in GDM [12]. The increase in ROS and oxidative stress leads to impaired insulin secretion and insulin resistance [30][46]. Pancreatic β-cells are particularly sensitive to ROS because they have low levels of free radical quenching antioxidant enzymes such as catalase, glutathione peroxidase and superoxide dismutase [31][47]. Therefore, oxidative stress induces β-cell dysfunction via the induction of apoptotic events, impairing KATP channels and inhibiting transcription factors involved in β-cell neogenesis such as Pdx-1 and MafA and mitochondrial dysfunction, leading to decreased insulin production [32][48]. On the other hand, oxidative stress can impair insulin signaling, leading to lower insulin sensitivity in peripheral tissues.4. Relationship between Inflammation and GDM
Circulating inflammatory cells, such as monocytes, neutrophils and proinflammatory cytokines (e.g., IL-1β, IL-6 and TNF-α), are upregulated in GDM. Cytokines are produced by cells of the immune system, placenta and adipose tissue [13]. As the placenta and adiposity increase during pregnancy, there is an enhanced secretion of proinflammatory cytokines. Additionally, the activation of inflammatory pathways also occurs as a response to elevated glucose concentrations. The pro-inflammatory mediators induce the production of other pro-inflammatory cytokines and chemokines (CXCL1, CXCL5 CXCL8, CCL2). Proinflammatory cytokines inhibit insulin release from β-cells and impair insulin signaling, inducing Janus kinase pathways (JNKs), which in turn stimulate IRS-1 serine phosphorylation, leading to an impairment in insulin action [33][51]. Higher levels of TNF-α and high-sensitivity CRP (hs-CRP), an acute-phase inflammatory protein, have been studied as markers for GDM.5. Studies Evaluating the Relationship between Oxidative Stress and Inflammation in GDM
5.1. Studies in Maternal Serum Or Plasma
During the last 10 years, oxidative stress and inflammatory biomarkers have been detected simultaneously in the blood of women with GDM, primarily during the second and third trimesters. Notably, no difference in the total oxidant status between GDM and controls has been reported. However, the levels of MGO, an oxidizing substance, are significantly higher in GDM women than in normal pregnant women. In addition, the total antioxidant status and the enzymatic antioxidants GPX and SOD are decreased in women with GDM when compared to controls. On the other hand, women with GDM show increased levels of TNF-α, IL-8 and CRP at diagnosis [25][26][27][34][33,34,35,37]. TNF-α and CRP have been found to be elevated in the first trimester of women who subsequently develop GDM, suggesting a potential role in glucose metabolism regulation and a potential value as diagnostic biomarkers [35][36][52,53]. The interplay between oxidative stress and inflammation in the peripheral blood of GDM patients has been reported. Recent proteome studies have shown protein coregulation between oxidative stress and inflammation in GDM; in particular, Liu et al. (2020) identified protein coregulations among 52 inflammatory system proteins and 4 antioxidant proteins (extracellular superoxide dismutase, peroxiredoxin-1, serum haptoglobin and paraoxonase/arylesterase 1) [37][62]. Oxidative stress and inflammation biomarkers are also associated with metabolic features of GDM. Piuri et al. (2020) showed that PAF and MGO were positively associated with HOMA-IR and HbA1c at diagnosis [38][61]. Furthermore, the study by Ozler et al. (2019) illustrated that increased TNF-α levels and decreased TAS levels were independent predictors of the need for insulin treatment in GDM patients [39][57].5.2. Studies in Placenta
Different approaches have been used to evaluate markers of oxidative stress and inflammation in placentas from women with GDM at term. Their mRNA expression and release under basal conditions and in response to oxidative stress have been measured. Under basal conditions, the release of 8-isoprostane, MDA and the antioxidant gene expression from catalase and glutathione reductase are higher in placental samples from GDM women compared to controls. In contrast, the release of TNF-α, IL-6 and IL-8 was not different between the two groups in most studies.5.3. Studies in SAT and VAT Adipose Tissue
Under basal conditions, 8-isoprostane release is greater in GDM SAT, and stimulation with LPS and HX/XO increases its release in SAT and VAT as well as TNF-α, IL-6 and IL-8 release, while there is no change in SAT and VAT antioxidant gene expression under basal conditions and in response to HX/XO [40][41][55,56].5.4. Studies in Skeletal Muscle
Interestingly, a study investigated markers of oxidative stress and inflammation in skeletal muscle obtained from women with GDM. Under basal conditions, 8-isoprostane release was greater in GDM, and stimulation with LPS increased its release. On the other hand, there was no difference in the release of TNF-α, IL-6 and IL-8 under basal conditions, but stimulation with LPS resulted in greater release of IL-6 and IL-8 [40][55]. Figure 12 summarizes the main findings on oxidative stress and inflammation in GDM.