Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Akib Jabed and Version 2 by Lindsay Dong.
Metallic glasses can be a perfect alternative to conventional crystalline biomaterials (such as 316L stainless steel, Ti or Ti-based alloys, Zr or Zr-based alloys, Co-Cr alloys, etc.) when used as coatings for surgical devices and implants inside the human body. Owing to their outstanding electrocatalytic activity and durability, metallic glasses can be considered prominent candidates for energy-storage and -conversion devices, such as fuel and electrolysis cells, and batteries. Metallic-glass systems are gaining substantial momentum in the micro- and nano-imprinting of optoelectronic devices.
- metallic glass
- alloys
- glass
1. Biomedical Applications
1.1. Antibacterial Application
Nosocomial infections are often escalated due to bacterial infections from medical instruments or devices. Commonly known biomaterials have been found to be ineffective at preventing bacterial infections [1][2][3][4][58,336,337,338], whereas metallic-glass systems exhibit excellent antibacterial properties. This unique characteristic is attributed to the composition of their multicomponent amorphous systems, their lower surface roughness, and the presence of antibacterial species in the matrices [5][6][7][63,168,265]. Liu et al. studied the antibacterial capabilities of Zr-Cu-Al-Ag systems, and the observed antibacterial responses are shown in Figure 12 [6][168]. The Zr38Cu36Al18Ag8 system, which had a lower surface roughness, was found to have the highest antibacterial activity in this study [6][168]. Hydrophobic surfaces are well known for their better antibacterial response, and metallic-glass systems have been found to exhibit better wettability compared to crystalline materials due to their disordered microstructure [8][339]. The presence of certain species, such as silver (Ag) and copper (Cu), in a multicomponent system provides excellent resistance towards bacterial attack [9][10][11][59,169,340]. For example, a Zr39Cu39Ag22 metallic glass was found to be very efficient against S. aureus [1][58], whereas Zr61Al7.5Ni10Cu17.5Si4 was found to be efficient against S. aureus, E. coli, A. baumannii, P. aeruginosa, and C. albicans bacteria [12][341].
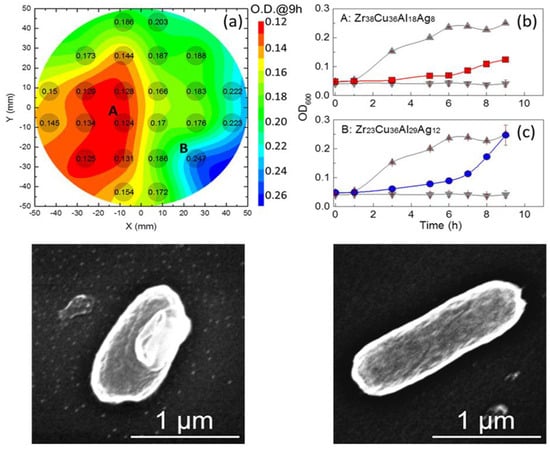
Figure 12. Antibacterial activity of Zr−Cu−Al−Ag metallic-glass systems at 9 h. (a) Contour plots of optical density (OD) of all the metallic-glass systems. (b,c) OD vs. time curves representing the highest (red) and lowest (blue) antibacterial activity of the Zr38Cu36Al18Ag8 and Zr23Cu36Al29Ag12 systems, respectively, where the controls are shown by the grey lines. The SEM morphologies of E. coli bacteria on the surface of Zr38Cu36Al18Ag8 (bottom left) and Zr23Cu36Al29Ag12 (bottom right) systems, respectively [6][168].
Commercially used surgical blades are usually made of stainless steel that contains micron-scale roughness on the edge tips and surfaces. The roughness hinders the smooth cut of the soft tissues, and the resulting wear and tear are often difficult to recover. Such limitations of surgical blades and scissors can be resolved by using metallic-glass systems, which exhibit exceptional surface characteristics [13][179]. A study conducted by Tsai et al. [13][179] reported lower roughness values, a higher blade sharpness index, and a lower depth of indentation when using a Zr-based metallic-glass system (Zr48Cu35.3Al8Ag8Si0.7). All of these characteristics make metallic-glass systems an optimum solution for surgical instruments without a compromise in their performance [13][179].
1.2. Bio-Implants
Durability and biosafety are two characteristics that are extremely desirable for implantable materials. Elastic modulus mismatch between an implant and a bone, and a lack of resistance towards localized corrosion have hindered the growth of conventional crystalline materials (such as 316L stainless steel, Co-Cr alloys, and Ti-based alloys) as premium choices for implantable materials. The lower corrosion resistance of crystalline materials is often attributed to the presence of grain boundaries and phase precipitates, which act as preferred regions for adverse electrochemical reactions [2][14][15][16][17][35,54,336,342,343]. Furthermore, the short interatomic distances of crystalline materials result in higher elastic modulus values, which may lead to failure of the implants due to a stress shielding effect [18][218]. Metallic-glass systems have the potential to mitigate this problem due to their homogenous microstructures, with longer interatomic distances and the absence of grain boundaries [14][35].
Due to their excellent electrochemical and mechanical properties, metallic-glass systems are being used as vascular stents, and dental and orthopedic implants. Zr- [19][20][20][21][22][23][212,273,273,284,344,345], Fe- [24][25][26][275,290,346], and Ti- based [27][28][29][347,348,349] metallic-glass systems have been widely explored in in vitro conditions for permanent implants. The biological responses of pre-osteoblast cells (MC3T3-E1) [30][31][32][33][34][180,350,351,352,353], fibroblast cells (L929 and NIH3T3) [22][26][35][36][302,344,346,354], human-osteoblast-like cells (SaOS2 and MG63) [28][37][38][348,355,356], and endothelial cells [39][297] reveal the outstanding bio-compatible characteristics of different multicomponent metallic-glass systems. Qiu et al. reported excellent mechanical (improved strength and plasticity), electrochemical (a low passive current density and high pitting potential), and biocompatible responses of a Zr-based system (Zr60Cu22.5Pd5Al7.5Nb5) in an embryonic-mouse-fibroblast cell line (NIH3T3 cell) [40][285]. The better biocompatibility of the metallic-glass system was attributed to the short/medium-range order and oxide-forming capability of the amorphous structure [40][285]. Fe-based metallic-glass systems, studied by Li et al. [25][290], exhibited better biocompatibility towards NIH3T3 cells and better electrochemical responses in artificial saliva compared to conventional biomaterials. Furthermore, Zr-based metallic glasses have been considered for cardiovascular stents, and studied for endothelial and muscle cells [39][297]. A cell-morphology and cell-metabolic-activity assessment, as shown in Figure 213, revealed the faster growth of endothelial (HAECs) cells on the Zr-based metallic glasses than on 316 L stainless steel, whereas the growth of smooth muscle (HASCMs) cells was relatively slower [39][297]. That study reported higher endothelial cell-adhesion capabilities on the Zr-based metallic glasses compared to their conventional crystalline counterpart [39][297].
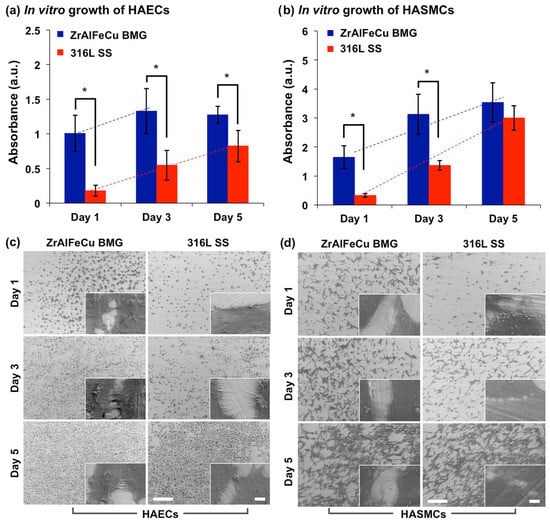

Figure 213. Metabolic activity of (a) HAECs and (b) HASMCs at day 1, 3 and 5, where (*) represents statistically a significant difference (p < 0.05; n = 3). SEM images depicting cell morphology at 1, 3, and 5 days for (c) HAECs and (d) HASCMs. (Scale bars = 500 μm and 5 μm for the larger and inset images, respectively) [39][297].
Research on biodegradable metallic glass is also gaining a lot of enthusiasm. The biocompatibility, mechanical properties, and electrochemical responses of Mg- [41][42][43][44][45][357,358,359,360,361], Ca- [46][47][362,363], Sr- [48][364], and Zn-based [49][365] degradable alloys have been studied extensively by several research groups. However, researchers investigating biodegradable metallic-glass systems used as fully functional bio-implants still face significant research challenges in their obtaining optimum mechanical and electrochemical properties. For example, bio-implants typically require a higher strength; however, degradation due to pitting corrosion creates surface defects that lead to a gradual loss of strength [14][35]. In addition, there is a possibility of tissue damage due to hydrogen evolution under a body-fluid environment [14][35]. Therefore, a proper understanding of this degradation mechanism and the relationship between its strength and degradation rate are required.
2. Electrochemical Devices
The lower efficiency and durability of catalysts are two primary obstacles that hinder the growth of electrochemical devices to meet the rising energy demand. Owing to their outstanding electrocatalytic activity and durability, metallic glasses can be considered prominent candidates for energy-storage and -conversion devices, such as fuel and electrolysis cells, and batteries. Nevertheless, some key components of electrochemical devices, such as the membrane, catalyst, and separator, still require further development to resolve issues associated with weight and cost [50][366]. For example, proton-exchange membrane (PEM) fuel cells are suitable candidates for power-generation applications [51][52][53][367,368,369]. PEM fuel cells consist of bipolar plates that are electrically conductive and essential to isolating the fuel and oxidant gases. Bipolar plates are conventionally made of carbon graphite; however, its brittleness and high production cost limit its use. To overcome this limitation, Kim et al. [51][367] investigated Ni65Cr15P16B4-metallic-glass-coated plates fabricated using HVOF spray coating and a subsequent hot-pressing technique. Enhanced corrosion resistance and durability suggest the potential of a multicomponent metallic-glass system to be used as bipolar plates in fuel cells [51][367]. Another prerequisite of a fuel cell is efficiency, and ultra-high-purity hydrogen was found to be crucial to enhancing its efficiency [54][370]. Crystalline materials are currently being used for hydrogen-storage applications [55][371]. But the embrittlement of crystalline materials limits its applications. A novel multicomponent metallic glass could be an alternate choice, as the glassy matrix of metallic glass possesses numerous sites for hydrogen absorption [55][371] and requires no modification to its microstructure [56][372]. A study conducted by Jayalakshmi et al. [54][370] explored Ni-Nb metallic-glass systems for hydrogen-related energy applications. The study determined the higher absorption-capacity and embrittlement-resistance characteristics of metallic-glass systems, which induce the better interaction of hydrogen with the metals [54][370]. Moreover, high permeability and a lower dissolution of metallic glasses make them ideal choices for hydrogen-permeable membranes and separators of fuel cells, respectively [54][370].
3. Optoelectronic Devices
Metallic-glass systems are gaining substantial momentum in the micro- and nano-imprinting of optoelectronic devices [57][373]. Smooth surface conditions and the negative enthalpy of mixing of metallic-glass systems facilitate optical transmittance and reflectivity, which are essential for different optoelectronic devices [58][304]. In the optoelectronic industry, indium-tin-oxide (ITO) is a prominent choice due to its excellent transparency and conductivity [59][374]. ITO films are also often used in solar cells and collectors, automobile windows, camera lenses, and lamps [59][60][374,375]. However, the cost of using ITO films is very high and the use of a metallic glass structure can be a prominent alternative [58][304]. Huang et al. studied a bi-layer ITO/ZrCu structure, which achieved good conductivity and transparency [61][376]. The study discovered that the lower resistivity and negative enthalpy between the atoms of the metallic-glass system can be beneficial by forming a continuous layer for transparent conductor design [61][376]. Wang et al. [62][377] investigated a Ag40Mg18Al42 metallic glass system and found that the lower surface roughness, atomic defects, free volumes, and electric resistivity improved its optical reflectivity. It is important to note that some of these features, such as surface morphology, atomic structure, and chemical composition, depend on fabrication routes and can be improved further by post-heat-treatment processes [61][63][376,378].
Chalcogenide glasses have found numerous applications in optical devices as well. These unique glasses are based on chalcogen elements such as S, Se, and Te, and are formed by the addition of Ge, As, Sb, and Ga and doped by rare-earth elements. A review article by Seddon [64][379] discusses their fabrication in bulk, fiber, and film form, their optical and thermal properties, and their applications.
4. Aerospace Application
The use of disordered-state solids in aerospace applications is gaining interest, although no concrete study has been conducted on any multi-component system until recently. Their high strength and lightweight attributes extend the demand of metallic-glass systems into lighter, smaller, and cost-effective aerospace applications [65][210]. Research conducted by Axinte [65][210] indicated that the aircraft and spacecraft fasteners can be produced from metallic glass. Another study by Burgess et al. [66][380] indicated that the higher strength and hardness of metallic-glass systems may be useful for coatings in aerospace applications. Aluminum alloys (Al-6061 and Al-7075) are widely used in automotive and aerospace industries due to their lighter weight and enhanced thermal conductivity [67][381]. Telford studied the possibility of using Al in a metallic-glass combination [68][211]. However, its limited glass-forming ability (GFA), higher affinity of oxide formation, and the need for an extreme experimental condition imposed a research challenge on the development of Al-based metallic-glass systems [67][381]. Vitreloy-1 (Zr41.2Ti13.8Cu12.5Ni10.0Be22.5) [69][11] is the only commercially available metallic glass that is being studied by the US Department of Energy and NASA for aerospace applications [68][211]. The primary limitation of using metallic-glass systems in industrial applications is their low plasticity, i.e., minimal plastic deformation before catastrophic failure. However, the combination of higher strength and better corrosion resistance may offset this limitation to foster industrial applications.
5. Memory Storage Devices
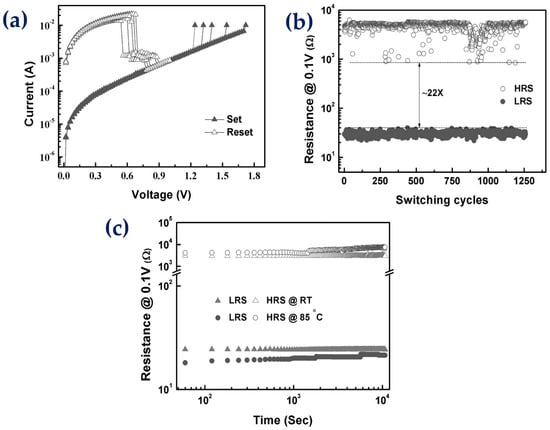
Non-volatile memory (NVM) is being used in computers, smartphones, flash memory devices, and other electronic devices. However, the requirement for a higher density and voltage hinders the writing capacity of NVM [70][382]. To find a suitable alternative, metal-oxide thin films are being used as storage devices or resistive random-access memory [71][72][73][383,384,385]. Tulu et al. investigated and optimized a thin-film metallic-glass oxide (TFMGO) for resistive switching and as a multicomponent oxide memory device [74][386]. A 15 nm thick oxide film of (ZrCuAlNi)Ox was fabricated on a Pt/Ti/Si substrate using magnetron sputtering in the presence of oxygen. The RS I-V curve, as shown in Figure 314a shows the current–voltage formation of a Pt/TFMGO/Pt device with a unipolar behavior of switching. Moreover, its endurance features, as shown in Figure 314b, indicate no reduction in resistance up to 1250 switching cycles [74][386]. The outstanding features are further confirmed by its retention characteristics, as shown in Figure 314c, which exhibit a good resistance ratio and the regeneration of resistive switching without the need of thermal forming [74][386]. These excellent behaviors are attributed to the amorphosity of the metallic-glass matrix, which has been confirmed through nano-scale characterization. These findings create an opportunity to use thin-sized metallic-glass systems for storage device applications.