Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Muhammad A.B. Siddik and Version 2 by Sirius Huang.
Seaweed, also known as macroalgae, represents a vast resource that can be categorized into three taxonomic groups: Rhodophyta (red), Chlorophyta (green), and Phaeophyceae (brown). They are a good source of essential nutrients such as proteins, minerals, vitamins, and omega-3 fatty acids. Seaweed also contains a wide range of functional metabolites, including polyphenols, polysaccharides, and pigments. The nutritional and functional properties of seaweed attest to their potential to be incorporated into aquafeed to safeguard fish growth and health as the global demand for fish and seafood products rapidly increases.
- bioactive compounds
- gut microbiota
- polyphenols
- polysaccharides
- pigments
- omega-3 fatty acids
- sustainable aquaculture
1. Introduction
Seaweed is a potential source of essential nutrients that can be used as a sustainable and cost-effective supplement to traditionally used aquafeed ingredients for fish. Incorporating seaweed into the diets of farmed fish can improve their growth, health, and resistance against invading pathogens, thereby improving disease resistance. Since there is a dearth of knowledge regarding the use of seaweed functional metabolites in aquafeed for fish, the studies discussed here are mostly on the use of seaweed and seaweed-based extracts in fish nutrition. An overview of some key effects on fish production when seaweed is included in aquafeed is presented below.
2. Growth Performance and Feed Utilization
2.1. Growth Performance
The effectiveness of seaweed as a feed additive varies greatly depending on the nutritional profile and the species-specific feeding nature of fish [1][2][78,79]. In general, low dietary inclusion of seaweed, up to 10%, has been shown to impart significant improvements in growth, feed utilization, and the assimilation of essential nutrients [3][4][80,81]. The dietary supplementation of Laminaria sp. with levels of 3 and 10% has been shown to significantly enhance the daily feed intake and weight gain in Atlantic salmon [5][82]. Likewise, Sony et al. [6][83] found that dietary supplementation of fucoidan, a polysaccharide derived from brown algae (Cladosiphon okamuranus), at a level of 0.4%, significantly improved the growth performance of juvenile red sea bream (Pagrus major). In contrast, the inclusion of red seaweed (Porphyra dioica) at up to 10% of the diet did not affect the growth, whilst a 15% inclusion caused a significant growth reduction in rainbow trout (Oncorhynchus mykiss) [7][12]. Similarly, a 6% dietary provision of Gracilaria pygmaea enhanced the growth performance of O. mykiss, while a 12% inclusion evoked negative impacts on growth [8][84]. Moreover, Soler-vila et al. [9][17] reported that 10% dietary red alga (Porphyra dioica) exhibited no negative impacts on the growth of rainbow trout, while 15% inclusion showed negative results compared to the control. Overall, these observations indicate that seaweed, when incorporated at an appropriate inclusion level, can either significantly improve or maintain growth performance at similar levels to non-seaweed diets, whereas higher inclusion levels can negatively impact the growth and health status of fish. Notably, the higher growth observed with seaweed-supplemented diets is likely attributable to elevated concentrations of bioactive compounds (phytonutrients, i.e., essential vitamins and minerals) [10][11][85,86] that play vital roles in the enhanced assimilation of dietary nutrients in fish [12][13][87,88]. Interestingly, it has also been speculated that seaweed contains a wide range of polysaccharides and oligosaccharides that act as prebiotics, which promote the activity of beneficial bacteria and thus enhance the digestion and absorption of essential nutrients, subsequently improving growth performance in fish [14][89]. On the other hand, reduced growth performance at higher inclusion levels (>10%) of seaweed in aquafeed may potentially be caused by the presence of substantial concentrations of antinutritional substances emanating from the seaweed that exerts various toxicity effects and restricts the absorption of essential nutrients [14][15][89,90]. For instance, protease inhibitors are found in many plant-based feeds, including seaweed. These are molecules that inhibit the activity of protease enzymes, which are responsible for breaking down proteins into smaller peptides and amino acids. When fish are fed a higher quantity of seaweed, they can bind to proteolytic enzymes and interfere with the normal digestive process by inhibiting the activity of digestive enzymes. This can lead to incomplete protein digestion, reduced nutrient absorption, and overall poor performance in fish [16][91]. Further, instances of growth reduction may also be attributable to the polysaccharide content in seaweed, which may influence the rapid transition of feed through the digestive tract, in turn causing enhanced feed uptake while lowering the absorption of nutrients [17][18][92,93]. Therefore, the removal or breakdown of these complex carbohydrates and antinutritional factors in seaweed via the incorporation of novel processing technologies may permit higher nutrient absorption efficiency and fish growth. The effects of seaweed supplementation on the growth performance of various fish species are presented in Table 1. A snapshot of some of the major effects of seaweed supplementation in aquafeed on fish performance is depicted in Figure 1.
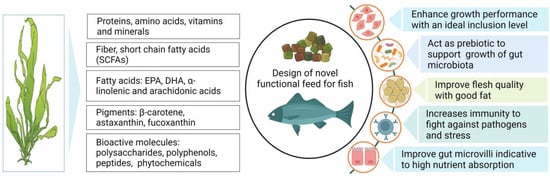
Figure 1.
Schematic diagram demonstrating the composition of seaweed and the potential impacts of its addition on growth and health performance of fish.
2.2. Feed Conversion Ratio (FCR)
FCR remains a fundamental metric to assess feed efficiency in fish, where a lower value represents an improved conversion of feed to fish biomass gain. The reliance on this metric stems from the fact that feed inputs are a major cost for intensive aquaculture operations. Several feed additives, including seaweed, have been incorporated into aquafeed to improve the FCR. Several studies reported that dietary seaweed inclusion resulted in a lower FCR in Nile tilapia [19][20][21,94], Salmo salar [5][82], Pagrus major [6][83], Acanthopagrus schlegelii [21][95], and Labeo rohita [22][96]. Improvements to FCR could be partly due to the presence of various bioactive compounds (carotenoids, polysaccharides, amino acids, and fatty acids) that significantly improve the palatability and, consequently, intake of feed, hence improving feed utilization [23][97]. Bioactive substances have been shown to stimulate the secretion of several enzymes (amylase, lipase, and protease) that are known to enhance the digestion of essential nutrients as well as their assimilation into fish tissues [24][98]. Likewise, improved FCR could result from the activities of the polysaccharides of seaweed that slow the passage of feed through the digestive tract, which ensures greater nutrient assimilation and bioavailability [8][25][84,99]. In addition, seaweed as a source of prebiotics may enhance the growth of beneficial bacteria in the gut, significantly improving digestibility and feed efficiency [26][100]. However, contrasting findings to those articulated above have been reported, with several studies indicating that dietary seaweed did not significantly affect feed utilization across a range of fish species, including seabass (Dicentrarchus labrax), Senegalese sole (Solea senegalensis), and gilthead seabream (Sparus aurata) [4][27][28][81,101,102]. Notably, high levels of seaweed in aquafeed may reduce the palatability of fish [29][103]. Moreover, these variations may be exhibited by the feed composition, physiology of fish species, size of the species, environmental quality, as well as the dietary inclusion level of the seaweed.
2.3. Feed Palatability
The palatability of aquafeed is one of the most crucial factors influencing the consumption of feeds by farmed species [30][104]. A reduction in palatability may lead to an increase in feed wastage, resulting in reduced fish production and negatively impacting the profitability of aquaculture operations. On the contrary, highly palatable feeds increase feed consumption and effectiveness, generally resulting in better fish growth, assuming the nutritional requirements of the species are being met (Table 1). However, the palatability of an aquafeed is largely influenced by the nutrient composition of its ingredients as well as feed processing techniques, nutrient digestibility, water stability, and species-specific nutritional requirements and physiology of fish [31][105]. The application of several plant-origin protein sources, including those emanating from algal species, to enhance the palatability of fish feed has attracted considerable research attention in recent times. Kamunde et al. [5][82] reported that Atlantic salmon consumed more feed when brown seaweed (Laminaria sp.) was included in the diet in comparison to a seaweed-free control feed. Similarly, greater consumption of an Ulva sp.-based diet was reported in seabream (S. aurata) [32][106] and sea urchin (Tripneustes gratilla) [33][107]. This higher feed response may be attributable to several bioactive compounds such as dimethyl-beta-propionthein, dimethyl sulfonyl propionate, amino acids, and peptides that enhance the attraction of a feed to the farmed fish species and, in turn, increase feed consumption [34][35][108,109]. In addition, the inclusion of seaweed can improve the overall physical structure of an aquafeed, including integrity, texture, and water stability, all of which are factors that may contribute to increased feed intake [36][110]. Furthermore, the volatile organic compounds emitted by seaweed [37][111] can contribute to the aroma and flavor of the feed, making it more attractive and palatable to fish. This could potentially lead to increased feed intake and improved growth rates. However, the application of seaweed in aquafeed should be carefully considered as high inclusion levels have been reported to reduce feed palatability and feed consumption, in turn negatively impacting the growth and health status of fish [15][90].
2.4. Feed Digestibility
The efficiency of an aquafeed is highly dependent on the digestibility of its constituent feed ingredients (Table 1). The incorporation of ingredients with a high digestible value will minimize feed wastage and maximize feed utilization, thus improving growth performance. It has been reported that apparent nutrient digestibility coefficients (ADC) of protein, lipid, and energy were not changed when up to 20% of Ulva sp. was included in diets for Nile tilapia [38][112]. Pereira et al. [39][113] revealed that the digestibility of Ulva meal in diets for Nile tilapia was higher in comparison to diets containing Gracilaria or Porphyra. Contrarily, Soler-vila et al. [7][12] found that up to 15% inclusion of Porphyra dioica did not result in significant alterations in comparison to a control diet in rainbow trout. However, Azaza et al. [17][92] reported that a 10% replacement of soybean meal with Ulva rigida decreased the ADC of protein in Nile tilapia from 87% to 82%. Notably, the effect of seaweed inclusion on nutrient ADC appears to be dependent on the type of seaweed itself, the nature of fish species, feed composition, and the degree of inclusion of the examined seaweed and, thus, the protein source being substituted. Different seaweed species exhibit varying effects on nutrient digestibility in different fish species, which can largely be explained by feeding habits and gut morphology, which determines the capacity for digestion and absorption of the nutrients contained in seaweed [40][114]. Most herbivorous and omnivorous fish species exhibit a higher level of amylase activity for the enhanced breakdown of the carbohydrates provided by dietary seaweed inclusion [3][41][80,115]. Importantly, carnivorous fish species have a reduced ability to break down complex seaweed polysaccharides due to a lack or limited amount of these enzymes [42][116]. As such, coupling digestive enzyme activity with the morphology of the gastrointestinal tract, the capacity for dietary seaweed incorporation into aquafeeds will likely be dictated by trophic level, where it stands to reason that herbivorous and omnivorous species will have a much higher tolerance to dietary seaweed than their carnivorous counterparts.
Table 1. Effects of seaweed and seaweed-based functional metabolites on growth, feed utilization, immunity, and disease resistance in farmed fish (studied parameters were compared to control—0% FM diet).
| Seaweed and Derivatives | Fish Species | Applied Levels | Effective Level | Trial Period (Day) | Response | Reference |
|---|---|---|---|---|---|---|
| Sargassum portieranum (Phaeophyceae) | Oreochromis niloticus | 5 and 10% | 10% | 84 | SW inclusion resulted in significant growth enhancement | [43][117] |
| Grateloupia acuminata and G. doryphora (Rhodophyta) | O. niloticus | 0.1, 0.25, 0.5, and 1.0% | 0.5 and 1.0% | 60 | Growth and digestibility increased compared to control | [44][118] |
| Polyphenols from Eisenia arborea (Phaeophyceae) | Haliotis fulgens | 13.9 and 33.3 mg/g | - | 12 | Polyphenol reduction in feed promoted feed attractiveness and consumption | [45][119] |
| Fucoidan from Fucus vesiculosus (Phaeophyceae) | Danio rerio | 100 μg/mL | - | 5 | Fucoidan reduced NO and ROS accumulation in D. rerio larvae, which indicated therapeutic role of fucodian against inflammatory disorder | [46][120] |
| Fucoidan from Saccharina japonica (Phaeophyceae) |
Clarias gariepinus | 0.04 and 0.06% | - | 21 | Dietary fucodian significantly enhanced the phagocytic activity, serum lysozyme, and bactericidal activity | [47][121] |
| Fucodian from Cladosiphon okamuranus (Phaeophyceae) |
Pagrus major | 0.4% | - | 56 | Fucoidan supplementation showed nonsignificant improvement in feed utilization. Catalase activity is significantly influenced by fucodian | [48][122] |
| Fucodian from Undaria pinnatifida (Phaeophyceae) | Marsupenaeus japonicus | 0.01, 0.05, and 0.10% | 0.05% | 56 | 0.05% fucodian supplementation remarkably increased the growth and immune performances | [49][123] |
| Fucodian from Undaria pinnatifida (Phaeophyceae) |
Lates calcarifer | 0.5 and 1.0% | 1.0% | 52 | 1% fucoidan inclusion diet exhibited enhanced growth | [50][124] |
Gracilaria pygmaea [8][84]. In addition, the dietary provision of either whole brown seaweed (Ascophyllum nodosum) or its extract significantly modulated the serum antioxidant profile, lowered lipid peroxidation, and enhanced the activity of SOD in ruminants [106][107][176,177]. All of these findings clearly highlight the potential role of seaweeds in modulating the antioxidant status of fishes either directly via an elevation of antioxidant substances or via an improvement to the functioning of antioxidant defense mechanisms.
4. Intestinal Morphology
The intestines of fish are important organs that play a vital role in fish’s immune statuses [108][178]. The morphological structure of the gastrointestinal tract (GIT) acts as an important indicator of the nutritional status and physiological state of fish [109][179]. Several studies have reported that dietary seaweeds, such as Sargassum dentifolium (S. ilicifolium) (Phaeophyceae), do not alter the normal intestinal tissue structure (e.g., enterocyte length and width and thickness of villi) [70][110][143,180], indicating its suitability as a feed ingredient. Dietary seaweed supplementation has also been shown to improve the intestinal epithelial mucosa, indicating an enhanced immune capacity of fish [16][91]. The immune activities of fish intestines are greatly dependent on the condition of the associated intestinal barriers that primarily consist of epithelial cells [111][181]. These epithelial cells assist in the production of IgA through the activation of T cells and B cells that play a defensive role against various antigens [111][181]. Dietary Laminaria digitata (Phaeophyceae) and Gracilaria gracilis (Rhodophyta) significantly boosted the intestinal acid goblet cells of mullet (Liza ramada) [112][182] and European seabass (D. labrax) [113][114][183,184], which perform key roles in intestinal immune activity. Goblet cells act as a protector of intestinal barriers through the production and secretion of mucus and antimicrobial proteins (chemokines and cytokines), enhancing the local immune response of the intestine [115][185]. Yu et al. [29][103] demonstrated that Gracilariopsis lemaneiformis provision in the diet of Litopenaeus vannamei increased the villi length of the intestine, which significantly improved the absorption capacity of several nutrients. Similarly, the provision of 1 to 3% Undaria pinnatifida in diets for shrimp (Penaeus monodon) significantly enhanced the length of intestinal fold when compared to the control [116][186]. On the contrary, 10% Gracilaria sp. supplementation caused lower villi length and diameter in Nile tilapia [117][187] and rainbow trout [9][17], which negatively affected the nutrient utilization and hence, growth of the associated species. These variabilities reported here could result from the presence of several antinutritional factors (phytic acid, saponin, and tannins) in seaweeds that alter the structure of the intestine and negatively affect the digestion process [17][118][92,188]. The effects of seaweed supplementation on the histo-morphological structures of various fish species are presented in Table 2.
Table 2. The effects of seaweed and seaweed-based functional metabolites on gut histo-morphometry and gut microbiota composition in farmed fish (studied parameters were compared to control—0% FM diet).
| Seaweed and Derivatives | Fish Species | Applied Levels | Effective Level | Response | Reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ulva sp. (Chlorophyta), Gracilaria gracilis (Rhodophyta) | D. labrax | 2 and 4% | - | SW-blend-supplemented diet enhanced anterior intestinal absorption area by up to 45% | [119][189] | ||||||||
| Fucoidan from Undaria pinnatifida (Phaeophyceae) | Carassius auratus gibelio | 0.1, 1.0 and 3.0% | 3.0% | Increased intestinal digestive enzyme activity, thereby enhancing intestinal microbial communities at a level of 3% dietary supplementation | [120][190] | ||||||||
| Fucoidan from Undaria pinnatifida (Phaeophyceae) |
Salmo salar | 1 and 3% | - | Fucoidan positively improved intestinal integrity and immune response | [121][191] | ||||||||
| Fucoidan from Saccharina japonica (Phaeophyceae) |
O. niloticus | 0.1, 0.2, 0.4, and 0.8% | - | Fucoidan in fish diets improved intestinal health and antioxidant status | [122][192] | ||||||||
| Sargassum dentifolium (Phaeophyceae) | O. mossambicus × O. niloticus | 1, 2, and 3% | - | No abnormal or histological changes were detected due to the dietary SW supplementation | [70][143] | ||||||||
| Sargassum ilicifolium (Phaeophyceae) | L. calcarfer | 3, 6, and 9% | 6% | No significant difference observed between enterocyte length, villi width, and muscle thickness in intestinal tissue between different treatments and the control group | [110][180] | ||||||||
| Spirulina platensis | L. calcarifer | 10, 20, and 40% | 20% | Decreased intestinal fold and microvilli height were observed in fish fed 40% of Spirulina sp. in the diet | [123][193] | ||||||||
| Pelvetia canaliculata (Phaeophyceae) | S. aurata | 1 and 10% | 10% | 10% SW supplementation led to greater thickness of the muscle layers and longer villi length | [124][194] | ||||||||
| Gracilaria persica (Rhodophyta) | Acipenser persicus | 0.25, 0.5, and 1.0% | 0.5 and 1.0% | 56 | |||||||||
| Gracilaria gracilis | No significant improvement in growth due to SW provision | (Rhodophyta) | D. labrax | 0.35, 2.5, and 5% | 2.5% | 2.5% SW inclusion boosted the intestinal acid goblet cells | [113][183[51][125] | ||||||
| ] | Mixture of Ulva lactuca (Chlorophyta), Jania rubens, and Pterocladia capillacea (Rhodophyta) | O. niloticus | 0.5, 1, 1.5, and 2.0% | 2.0% | 70 | Growth promoted at 2% dietary SW | |||||||
| G. gracilis (Rhodophyta) and the microalga Nannochloropsis oceanica (Eustigmatophyceae) |
D. labrax | 8% | - | All fish had well-preserved gut morphology; however, significant enhancement of goblet cells was observed in Nannochloropsis-based diet compared to Gracilaria-based feed | [ | 20][94] | |||||||
| [ | 114 | ] | [ | 184 | ] | Gracilaria sp. (Rhodophyta), Ulva sp. (Chlorophyta), or Fucus sp. (Phaeophyceae) | D. labrax | ||||||
| Ulva ohnoi (Chlorophyta) | 2.5 and 7.5% | 7.5% | 49 | S. senegalensis | 5% | Immunity and antioxidant status improved at 7.5% SW inclusion compared to control | - | SW significantly reduced damage to intestinal mucosa and enhanced the mucosal absorptive surface area | [16][4][81] | ||||
| Laminaria sp. (Phaeophyceae) | S. salar | 3, 6, and 10% | 10% | 30 | Growth and immune status developed at 10% SW inclusion | [5][82] | |||||||
| [ | 91 | ] | |||||||||||
| Laminaria sp. | S. salar | 3, 6, and 10% | - | Higher gut and intestinal weights and lengths were observed due to dietary SW provision. Lager surface area exhibited | [ | Gracilaria pygmaea (Rhodophyta) | O. mykiss | 3, 6, 9, and 12% | 9% | 56 | Growth improved at 9% SW, while it was reduced at 12% SW level | [8][84] | |
| 5 | ] | [ | 82 | ] | |||||||||
| Ulva lactuca (Chlorophyta), Chondrus crispus (Rhodophyta) | S. aurata | 2.5 and 5% | - | Dietary SW had no significant on distal intestine histomorphology | [125][195] | Fucodian from Cladosiphon okamuranus (Phaeophyceae) | P. major | 0.05, 0.1, 0.2, 0.4, and 0.8% | 0.4% | 60 | Growth promoted at 0.4% dietary SW. Enhanced immune response and disease resistance at 0.3–0.4% SW | [6][83] | |
| Gracilaria pygmaea (Rhodophyta) | O. mykiss | 3, 6, 9, and 12% | 9 and 12% | Normal histomorphology of anterior intestine and pyloric caeca was detected. Villi decreased due to 90 and 120 g/kg provision of SW | [8][84] | Ulva lactuca (Chlorophyta) Jania rubens and Pterocladia capillacea (Rhodophyta) |
Pangasianodon hypophthalmus | 1, 2, and 3% | 2% | 60 | SW at a level of 2% improved the growth and resistance against Aeromonous. hydrophila infection. | [52][126] | |
| Ulva rigida (Chlorophyta), Undaria pinnatifida (Phaeophyceae) | S. senegalensis | 10% | - | Dietary Undaria significantly lowered the width of intestine villi | [27][101] | Pelvetia canaliculata (Phaeophyceae) | Sparus aurata | 1, 5, and 10% | - | 56 | SW inclusion produced no changes in proximate composition and the fatty acid profile of fish when compared to control | ||
| Taonia atomaria (Phaeophyceae) | [ | 53 | ] | O. niloticus | [ | 127] | |||||||
| 5, 10, and 15% | - | No histopathological alterations were observed due to dietary SW provision | [ | 65 | ][138] | Gracilariopsis lemaneiformis (Rhodophyta) | Pagrosomus major | 3, 6, 9, 12, and 15% | 3% | 56 | Growth improved at 3% SW. Liver glycogen and hepatic AST were significantly higher in supplemented group | ||
| Asparagopsis taxiformis (Rhodophyta) | [ | S. salar | 54 | 1.8, 2.6, and 3% | ] | - | Increased bacteria diversity found in the hindgut | [126][23][128] | |||||
| Sargassum wightii (Phaeophyceae) | L. rohita | ||||||||||||
| Gracilaria cornea (Rhodophyta), | 2% | Ulva rigida (Chlorophyta) | - | 45 | Growth promoted by dietary SW without compromising its immune-modulating effects | S. aurata | 5, 15, and 25% | - | SW inclusion did not reveal any negative effects on gut structure | [18][93][22][96] | |||
| Ulva prolifera (formerly Enteromorpha prolifera) (Chlorophyta) | |||||||||||||
| Gracilaria vermiculophylla, Porphyra dioica (Rhodophyta), and | O. mossambicus × O. niloticus | 1, 2, 3, 4, and 5% | Ulva | 5% | 49 | spp. (Chlorophyta)Growth was enhanced by dietary U. prolifera. SOD, LYZ, acid phosphatase and alkaline phosphatase activities were enhanced | [55] | O. niloticus | 10% | - | Exhibited a significant reduction in villi length in Gracilaria- and Porphyra-based diets, while no significant reduction was observed in case of Ulva spp. | [129] | |
| [ | 117 | ] | [ | 187 | ] | Gracilaria arcuata (Rhodophyta) | O. niloticus | 20, 40, and 60% | 20% | ||||
| Ulva ohnoi | 84 | (Chlorophyta) | S. senegalensis | Growth and feed utilization improved at 20% SW | 5% | - | SW supplementation significantly enhanced the abundance of Vibrio while decreasing Stenotrophomonas abundance | [127][[56][130] | |||||
| 196 | ] | Gracilariopsis persica, Hypnea flagelliformis (Rhodophyta), and Sargassum boveanum (Phaeophyceae) | O. mykiss | 5 and 10% | - | ||||||||
| Gracilaria gracilis | 83 | (Rhodophyta) | Methylobacterium | Serum LYZ, SOD, and CAT activity increased by SW provision | [128] | [57 | D. labrax | [197][22] | |||||
| 8% | - | G. gracilis | ] | Gracilaria pulvinata (Rhodophyta) | Lates calcarifer | 3, 6, and 9% | 3% | 40 | No growth retardation up to 3% SW. Serum LYZ activitywas significantly enhanced at 3% supplementation, while ACH50 was lowered at 9% SW | [58] | |||
| Ulva ohnoi (Chlorophyta) | S. senegalensis | 5% | - | Pseudomonas and Mycopasmataceae were abundant in anterior and posterior GI tract, respectively | [ | 131] | |||||||
| [ | 129 | ] | [ | 198 | ] | Ulva rigida (Chlorophyta) and Undaria pinnatifida (Phaeophyceae) | Solea senegalensis | 10% | - | 150 | Growth retardation observed in growing stage for Undaria-based diet | [27][101] | |
| Gracilaria gracilis (Rhodophyta) | D. labrax | 2.5 and 5% | - | Gut microbiome diversity was not altered by SW supplementation. Abundance of Proteobacteria was reduced | [130][199] | Mixture of Gracilaria sp. (Rhodophyta), Ulva sp. (Chlorophyta), and Fucus sp. (Phaeophyceae) | D. labrax | 7.5% | - | 63 | Did not mitigate negative effects of environmental oscillations on growth and immunity by dietary SW | [59][132 | |
| Ulva rigida (Chlorophyta) | S. aurata | 25% | - | ] | |||||||||
| SW supplementation significantly modified intestinal microbiota | [ | 131 | ] | [ | 200] | Ulva sp. (Chlorophyta) | |||||||
| Sargassum angustifolium (Phaeophyceae), Gracilaria pulvinata (Rhodophyta) | Argyrosomus japonicus | 5, 10, and 20% | 5% | 63 | Growth and feed utility increased at 5% SW | O. mykiss | 0.025 and 0.05% | - | Supplementation of SW extracts did not affect total bacterial level; however, the abundance of Lactobacillus increased | [132][201][60][133] | |||
| Ulva lactuca (Chlorophyta) | S. aurata | 2.6 and 7.8%, 14.6 and 29.1% |
- | 140 | No growth retardation observed by dietary SW | [61][134] | |||||||
| Gracilaria sp. (Rhodophyta) | S. aurata | 2.5 and 5% | 5% | Abundance of Firmicutes phyla and Clostridium genera were enhanced with 5% SW | [133][202] | Gracilaria pygmaea (Rhodophyta) | O. mykiss | ||||||
| Ulva rigida (Chlorophyta), Ascophyllum nodosum (Rhodophyta) | 3, 6, 9, and 12% | 6% | 49 | Gadus morhua | 10% | - | Growth was enhanced at 6% SW | U. rigida did not significantly influence the microbial composition of hindgut, while A. nodosum altered the scenario | [134][203][62][135] | ||||
| Gracilaria sp. (Rhodophyta) and Alaria sp. (Phaeophyceae) | A. regius | 5% | - | 69 | No growth retardation by SW addition. Lipid peroxidation lowered | [63][136 | |||||||
| Mixture of red, brown, and green SW | ] | ||||||||||||
| Siganus fuscescens | - | - | Increased abundance of | Firmicutes and Proteobacteria while decreasing Bacteroides | [135][204] | Gracilariopsis lemaneiformis (Rhodophyta) and Sargassum horneri (Phaeophyceae) | Lutjanus stellatus | ||||||
| Laminaria | 5, 10, 15, and 20% | 15% | 60 | Growth retardation at 20% SW | sp. (Alginates) | S. salar | [ | 0.5 and 2.5% | 0.5% | Facilitated the abundance of several Proteobacteria such as Photobacterium phosphoreum, Aquabacterium parvum, and Achromobacter insolitus | 64 | [136][205][137] | |
| ] | Taonia atomaria (Phaeophyceae) | O. niloticus | 5, 10, and 15% | 5% | |||||||||
| Ulva australis (formerly Ulva pertusa | 84 | ) (Chlorophyta) | Significant growth improvement by SW inclusion | [ | S. canaliculatus | 10% | - | SW in the diets enhanced the diversity of Firmicutes, Bacteroidetes, and Proteobacteria | [137][206]65][138] | ||||
| Palmaria palmata (Rhodophyta) | S. salar | 5, 10, and 15% | - | 98 | ALT activity significantly decreased with no effects on LYZ or ACH50 activity | [66][139] | |||||||
| Ulva lactuca (Chlorophyta) | Lutjanus stellatus | 5, 10, 15, and 20% | 5% | 60 | Growth promoted at 5% SW | [67][140] | |||||||
| Sargassum angustifolium (Phaeophyceae) | |||||||||||||
| Gracilaria cornea (Rhodophyta), Ulva rigida (Chlorophyta) | S. aurata | 5, 15, and 25% | 15% | Biodiversity of microbial community was significantly reduced with highest inclusion of U. rigida. Various Lactobacillus sp. were significantly stimulated, while Vibrio sp. was reduced | [138][ | O. mykiss | 0.005, 0.01, 0.02, and 0.04% | - | 56 | Immune status and lower mortality against Yersinia rukeri by dietary SW | [68][141] | ||
| 207 | Gracilaria sp. (Rhodophyta) | D. labrax | 0.5 and 4.5% | - | 42 | ACH50 activity was enhanced, while no effect was observed on LYZ and PO activity by SW inclusion | [69][142] | ||||||
| Sargassum dentifolium (Phaeophyceae) | O. mossambicus × O. niloticus | 1, 2, and 3% | 3% | 84 | Significantly increased GOT and triglycerides level, while no impact was noticed for total plasma protein, albumin, and globulin | [70] | |||||||
| supplementation promoted the growth of | Sulfitobacter | and | ] | ] | [ | 143 | Saccharina latissimi (Phaeophyceae) | O. mykiss | 1, 2, and 4% | 1 and 2% | 84 | Significantly downregulated the expression of stress marker (gpx1b2) | [71][144] |
| Ulva prolifera, Ulva australis (formerly U. pertusa) (Chlorophyta), or G. lemaneiformis (Rhodophyta) |
Siganus canaliculatus | 12% | - | 70 | LYZ, dismutase, and acid phosphatase were significantly enhanced. Enhanced resistance against Vibrio parahaemolyticus | [72][145] | |||||||
| Ulva sp. (Chlorophyta) | O. niloticus | 5 and 10% | 10% | 68 | Significantly enhanced ACH50 activity, while no effects were observed in the cases of LYZ and PO activity | [73][146] | |||||||
| Padina gymnospora (Phaeophyceae) | Cyprinus carpio | 0.01, 0.1, or 1% | - | 21 | Remarkably improved serum LYZ, MPO, and antibody responses | [74][147] | |||||||
| Enteromorpha intestinalis (Chlorophyta) | O. niloticus | 10, 20, 30, and 40% | 20% | 42 | Significantly improved growth performance at 20% inclusion level | [75][148] | |||||||
| Sargassum fusiformis (formerly Hizikia fusiformis) (Phaeophyceae) | Paralichthys olivaceus | 0, 0.5, and 1% | - | 84 | Significantly upgraded the immune status of fish by raising the level of hepatic IL-2 and IL-6 | [76][149] | |||||||
| S. fusiforme and Ecklonia cava (Phaeophyceae) |
Paralichthys olivaceus | 6% | - | 42 | Hb level and RBC count were significantly elevated. Exhibited higher resistance against Edwardsiella tarda challenge | [77][150] | |||||||
| Ulva lactuca (Chlorophyta) and Pterocladia capillacea (Rhodophyta) | D. labrax | 5, 10, and 15% | - | 56 | P. capillacea exhibited high-stress resistance capacity compared to U. lactuca | [78][14] | |||||||
| Eucheuma denticulatum (Rhodophyta) and Sargassum fulvellum (Phaeophyceae) | P. olivaceus | 3 and 6% | 6% | 56 | Significantly lowered the level of blood cholesterol and triglycerides. Serum LYZ activity was significantly enhanced | [79][151] | |||||||
| Sargassum whitti (Phaeophyceae) |
M. cephalus | 0.5, 1.0, and 1.5.0% | - | WBC, LYZ, and RBC significantly elevated in seaweed-supplemented groups. Mortality rate decreased after exposure to Pseudomonas fluorescence | [80][152] | ||||||||
| Gracilariopsis lemaneiformis (Rhodophyta) | Siganus canaliculatus | 33% | - | 56 | LYZ and ACH50 activity was remarkably enhanced in the group provided seaweed | [81][153] | |||||||
| Ecklonia cava (Phaeophyceae) | P. olivaceus | 2, 4, and 6% | - | 42 | Serum LYZ, MPO, and NBT activities were significantly increased | [82][154] | |||||||
| Macrocystis pyrifera (Phaeophyceae) and Chondrus crispus (Rhodophyta) | Epinephelus coicoides | 0.001, 0.002, and 0.003% | - | 5 | Significantly enhanced RBC, SOD, and phagocytic activity. Exhibited resistance against V. alginolyticus | [83][155] | |||||||
| Sargassum fusiforme (formerly Hizikia fusiformis) (Phaeophyceae) | P. olivaceus | 2, 4, and 6% | - | 56 | Phagocyte activity was elevated with the increase of S. fusiforme in diet. Improved resistance to Streptococcus iniae | [84][156] | |||||||
| Ulva lactuca (Chlorophyta) Pterocladia capillacea (Rhodophyta) |
S. aurata | 5, 10, and 15% | 5 and 10% | 56 | Enhanced stress response ability | [85][157] |
Note: SW—seaweed; FM—fish meal; WG—weight gain; FCR—feed conversion ratio; PER—protein efficiency ratio; FE—feed efficiency; ADC—apparent daily co-efficient; FI—feed intake; PM—poultry meal; GOT—glutamic-acid-oxyl acetic-acid-transaminase; SOD—superoxide dismutase; GP—glutathione peroxidase; PO—phenoloxidase; LYZ—lysozyme activity; CAT—catalase activity; ACH50—alternative complement activity; MPO—myeloperoxidase; NBT—nitroblue tetrazolium; RBC—red blood cell; WBC— white blood cells; Hb—haemoglobin; AST—aspartate transaminase; ALT—alanine transaminase; ROS—reactive oxygen species; NO—nitric oxide; - — not identified.
3. Immune Status, Antioxidant Response, and Gut Health in Fish
3.1. Immunity and Disease Resistance
Disease remains a great threat to the intensive aquaculture sector, hindering industry growth and potentially leading to huge economic losses [86][158]. The main purpose of intensive aquaculture systems is to ensure maximum production within a limited culture period. In some circumstances, on-farm efficiency may be improved by operating at high stocking densities, potentially causing a deterioration of water quality to the detriment of fish health through the suppression of the immune system and the disruption of antioxidant defense mechanisms. To address these challenges, different types of drugs are commonly used for the treatment of disease [87][159]. However, their indiscriminate use has led to growing concerns for the surrounding environment as well as public health via the direct consumption of treated farmed fish or through the consumption of wild fishes located in the areas surrounding the treated aquaculture farm [88][89][160,161]. Furthermore, the rapid application of antibiotics may give rise to antibiotic-resistant bacteria [90][162] that significantly reduce the efficacy of antibiotics in controlling diseases. Therefore, it is of the utmost importance to seek possible environmentally friendly prophylactic measures. The application of seaweed-based feed ingredients as immunostimulants to strengthen the immune status of fish is viewed as a suitable alternative. An overview of the role of seaweeds as immunostimulators in fish is presented in Table 1. Mendonca et al. [91][163] revealed that 5% dietary Gracilaria domingensis (Rhodophyta) improved the immune response of juvenile mullet (Mugil liza) by modulating the activity of glycoproteins CD3 and CD4. Similarly, 6% dietary S. hornei promoted the antioxidant profile and immune capacity of black sea bream (Acanthopagrus schelegelii) [21][95]. Likewise, the green seaweed (Ulva lactuca (formerly Ulva fasciata) (Chlorophyta)) greatly improved the innate immune response of Nile tilapia through the modulation of lysozyme and phagocytic activity, as well as total WBC count and overall antioxidant status [92][164]. Similarly, the addition of red microalga (Porphyridium sp.) in the diet of pompano (Trachinotus ovatus) significantly upregulated the levels of mRNA c-type lysozyme and complement C4 while downregulating the mRNA heat shock protein (HSP70), thus playing an important role in the improvement of non-specific immune responses [93][165]. On the contrary, besides the positive role of seaweeds and their derivatives, the review of Thepot et al. [94][6] revealed no significant impacts in stimulating growth and immune responses in fish. This may be attributed to the very short (14-day) feeding trial that was likely insufficient to exhibit a significant immune response, given most immunostimulating compounds are reported after longer (49-day) feeding trials [95][166].
Alternatively, Wang et al. [96][167] observed that dietary Sargassum horneri (Phaeophyceae) did not significantly alter the lysozyme activity of juvenile turbot (Scophthalmus maximus) in comparison to the control. This variation may be caused by genetically driven species-specific responses of fish to the associated seaweed species. In addition, diseases in the aquaculture sector cost the industry in excess of USD 6 billion each year [97][168]. Fish are often subjected to various pathogenic organisms, which can lead to several diseases that negatively impact their health status and growth performance. In this situation, the utilization of seaweed and its bioactive substances as suitable alternative strategies can enhance both the cellular and humoral immune response towards disease resistance. Zeraatpisheh et al. [68][141] reported that the supplementation of Sargassum angustifolium (Phaeophyceae) in the diet of rainbow trout positively modulated fish immunity via increased hemoglobin (Hb), hematocrit (Hct), red blood cells (RBC), white blood cells (WBC), and phagocytes. Likewise, elevated lysozyme activity (LYZ), the expression of immune-related genes (e.g., il-1β, tnf-α), and the modulation of resistance to pathogenic bacterial infection from Yersinia rukeri (Pseudomonas fluorescens) were also reported. Several recent studies have also demonstrated that dietary provision of Sargassum ilicifolium (Phaeophyceae), Gracilaria sp. (Rhodophyta), Ulva ohnoi, and Sarcodia suiae (Rhodophyta) improved the nonspecific immunity and disease resistance of great sturgeon (Huso huso) [98][169], Dicentrarchus labrax [69][142], Solea senegalensis [99][170], and O. niloticus [100][171], respectively. In addition, Wang et al. [96][167] revealed that dietary Sargassum horneri (Phaeophyceae) significantly enhanced the non-specific immunity of juvenile turbot (S. maximus) and its disease resistance against Edwardsiella tarda.
3.2. Antioxidant Response
Seaweed and its extracts exhibit excellent antioxidant and immunomodulatory properties [101][102][172,173]. Inoculation of seaweed extracts (sodium alginate and carrageenan from Macrocystis pyrifera and Chondrus crispus) in grouper (Epinephelus coicoides) resulted in a significant enhancement of respiratory burst; superoxide dismutase and phagocytic activities that are the key indicators of antioxidant status [83][155]. Peixoto et al. [103][18] reported that a 2.5% inclusion of dietary Gracilaria spp. significantly promoted glutathione peroxidase (GPx) activity in European seabass, which may be attributed to the elevated levels of selenium found in Gracilaria spp. that contribute to increased GPx production [104][105][174,175]. In addition to enhanced lipid peroxidation, increases in glutathione reductase and glutathione s-transferase have been reported as a result of dietary Gracilaria inclusion [4][81], clearly indicating the influence of Gracilaria sp. inclusion with respect to the modulation of fish antioxidant profile and the stress status of fish. Moreover, in Atlantic salmon, the supplementation of Laminaria sp. not only significantly increased the total plasma antioxidant status but also activated several mitochondrial antioxidant enzymes, including catalase, superoxide dismutase (SOD), and total glutathione level [5][82]. Similar results have also been reported in rainbow trout fed diets supplemented with
Note: SW—seaweed; GI—gastrointestinal tract; - — not identified.
5. Gut Microbiota Composition
The study of fish gut microbiota has attracted significant research attention in recent years [139][208]. Gut microbiota plays an important role in fish growth, nutrition, immunity, and resistance against pathogenic microorganisms [140][141][4,209]. The fish gut acts as an assemblage of sever al microbial communities, and their activities greatly influence different aspects of fish physiology [142][210]. An overview of the role of seaweed on gut microbiota composition in fish is presented in Table 2. The microbial communities in fish guts vary greatly depending on several factors such as the physiological state of the gut, trophic level and environment, and dietary ingredients [143][144][211,212]. Seaweed has been shown to be a promising feed additive that can modulate the gut microbial composition of fish [127][196]. The dietary inclusion of Gracilaria gracilis enhanced the abundance of the microbes Sulfitobacter and Methylobacterium in the gut of European seabass [128][197]. These microbes are capable of producing short- and medium-chain fatty acids and can lower the pH of the intestine, thus playing a crucial role in suppressing pathogenic bacteria and potentially representing a promising method of enhancing disease resistance in fish [145][146][213,214]. Similarly, dietary supplementation of fucoidan, a polysaccharide derived from brown seaweed (Undaria pinnatifida), is reported to elevate the intestinal digestive enzyme activities and, thereby, modulate the intestinal microbial communities in gibel carp (Carassius auratus gibelio) when added at a level of 30 g/kg WW [120][190]. Furthermore, the provision of S. dentifolium (3 g/kg of diet DW) extract significantly lowered the abundance of pathogenic microorganisms in the gut of Pacific white shrimp (Litopenaeus vannamei) [26][100]. On the contrary, a high inclusion (8%) of G. gracilis resulted in a significant reduction of gut microbial diversity. However, these negative impacts were mitigated at a lower inclusion level (4%) [128][197]. Tapia-paniagua et al. [129][198] reported that a relatively low (<3%) dietary administration of Ulva ohnoi significantly enhanced the diversity of whole gut microbes in Senegalese sole (Solea senegalensis), while 5% U. ohnoi did not exhibit a significant influence on gut microbial diversity [127][196]. These variable results could be attributed to the specific adaptative response of different microbial communities across a range of feeding schedules, such as time and duration of feeding and feeding frequencies. Nevertheless, dietary U. ohnoi reduced the abundance of the genus Escherichia [129][198] in S. senegalensis, which could be attributed to the antibacterial properties of Ulva spp. against Escherichia coli [147][148][215,216]. Further, Xinxu et al. [137][206] reported that dietary Ulva australis (formerly Ulva pertusa) (Chlorophyta) enhanced the abundance of several bacterial species of the Firmicutes group, including Ruminococcus, Clostridium, and Lachnospiraceae in white-spotted rabbitfish (S. canaliculatus) that actively participate in the degradation of non-starch polysaccharides in the host gut [149][150][217,218]. These results indicate that seaweed inclusion in aquafeed can be beneficial up to a certain extent, while the excessive inclusion of seaweed in aquafeed can lead to negative effects, including reductions in the growth of beneficial gut bacteria, leading to poor digestion and nutrient absorption, which may weaken fish immune systems and subsequently increase susceptibility to disease. The potential impacts of dietary seaweed inclusion or their extracts on the intestinal health of fish are depicted in Figure 2.
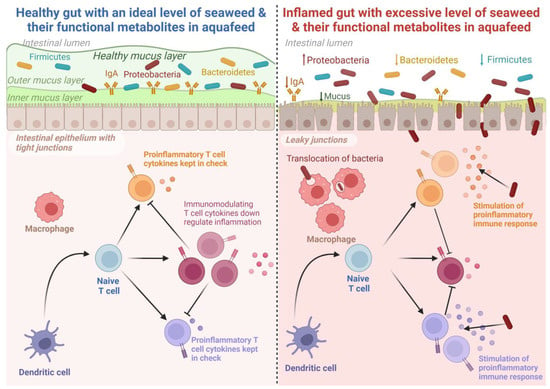
Figure 2. The potential effects of seaweed and seaweed-based functional metabolites in improving the gut health of fish. An optimal dietary inclusion of seaweed in aquafeed stimulates gut microbiota and improves immune response, whereas excessive inclusion is reported to suppress growth, mask immune response, and distort gut microbiota. Created with BioRender.com accessed on 25 September 2023.
