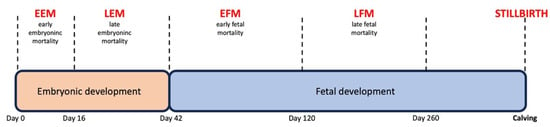
Because of the abovementioned risk of losing a successful pregnancy after a positive pregnancy diagnosis, there is a need to distinguish between dams showing a higher risk of late embryonic (days 30–42) and of early fetal (days 42–60) loss. In addition, it needs to be considered that more pregnancy losses occur in the late embryonic period. The factors responsible for pregnancy loss differ according to the developmental stage of gestation, late embryonic or early fetal. In clinical practice, there is no standard procedure available for the termination of these high-risk pregnancies as part of the management strategy.
2. Noninfectious Causes of Late Embryonic/Early Fetal Losses
2.1. Dam-Related Risk Factors
2.1.1. Declining Pregnancy Protein Concentrations
Reduced concentrations of placental lactogens in maternal blood have been identified as a marker of subsequent pregnancy loss
[5][6][8,9]. However, in a clinical setting, the diagnostic potential of this marker is limited. Maternal blood concentrations of pregnancy proteins, arising from the fetoplacental unit, are elevated and measurable from days 22 to 25 of pregnancy
[7][10]. Pregnancy-associated glycoproteins (PAGs) can be differentiated into two phylogenetic subgroups: PAG-2, mainly localized at the fetal–maternal border, and PAG-1, originating from trophoblast cells
[8][9][10][11][11,12,13,14].
The accuracy of the measurement method should also be considered when performing a clinical diagnosis and analyzing data, as results will affect decision making at the time of the initial pregnancy diagnosis. Both early RIA
[12][17] and ELISA tests
[13][14][15][16][18,19,20,21] serve to accurately determine maternal blood concentrations of PAGs and pregnancy-specific protein B (PSPB). These tests are available in laboratories and as on-farm tests and can be used on biological fluids (whole blood, serum, plasma, and milk). Their overall sensitivity is 90% to 99%, whereas cow-side tests offer good results but of lower accuracy
[17][18][22,23].
Fetal viability can be monitored through pregnancy proteins
[19][24] as a drop in the level of these proteins occurs immediately after the death of the conceptus. PSPB and bovine PAGs have different half-lives
[20][21][25,26]. Thus, a biological delay exists from the loss of the embryo/fetus and the decrease produced. In addition, differences have been detected between animals undergoing pregnancy loss and those not, and some studies have defined cut off values
[22][15]. PSPB was recently monitored in more than 7000 early pregnancies
[23][27]. The primary goal of this last study was to predict and identify factors detectable in cows carrying twins to determine the likelihood of twinning based on measured concentrations. Although plasma PSPB levels could not distinguish between cows carrying twins and singletons (2.1 vs. 2.9 ng/mL), mortality was higher in the case of singletons, yet the authors provided no explanation for the differences. In another recent study conducted in the US, plasma P4 concentrations differed in the case of unilateral twins, yet plasma PAG concentrations did not
[24][28].
2.1.2. Twin Pregnancy Diagnosis
An initial diagnosis of a twin pregnancy indicates an increased risk of pregnancy loss
[4]. An incidence of twinning among all pregnancies in dairy animals as high as 20% has been reported
[25][5]. However, twinning rates at the time of early pregnancy diagnosis range from 5% to 12%
[1], and pregnancy losses thereafter vary widely. According to recent reports, twin laterality can affect the pregnancy loss rate. Hence, a higher mortality rate has been detected for unilateral than bilateral twins (54.4% vs. 45.6%,
p < 0.0001)
[26][31], and this factor has been therefore defined as a clinically important noninfectious cause of pregnancy loss
[26][27][28][31,32,33]. In bilateral twin pregnancies, when one embryo is located at the end of the uterine horn and the other occurs in the other horn but close to the bifurcation, the contents of the allantoic sac often extend into the contralateral uterine horn
[28][33]. These cases should be assessed for loss risk as sometimes embryo reduction can be the solution to maintain the pregnancy
[25][5].
2.1.3. Number of Corpora Lutea
The number of corpora lutea present and their ultrasonographic structure are also important factors affecting pregnancy maintenance. Nonlactating non-pregnant dairy cows have smaller corpora lutea and higher peripheral blood progesterone concentrations than lactating animals
[29][30][34,35]. Corpora lutea larger than 17 mm in diameter, as determined by TRUS, are considered mature
[31][36]. In around 10% of all pregnancies, cows carrying singletons show an additional corpus luteum
[23][27]. At the time of pregnancy diagnosis, 99% of singleton pregnant animals have one corpus luteum ipsilateral to the gravid uterine horn
[4]. However, the transuterine migration of embryos has been confirmed and low numbers of contralateral corpora lutea detected in singleton pregnancies can be explained by migration of the oocyte
[32][37].
2.1.4. Ultrasonographic Findings Indicating Imminent Pregnancy Loss
Through TRUS, it has been shown that the integrity and functions of embryonic/fetal particles and vesicles are crucial for conceptus viability. However, placentomes are also detectable during fetal development. From the onset of fetal development (day 43), both the amniotic and allantoic fluids are visible and distinguishable. Alterations such as reduced placental fluid volumes, increased amniotic fluid turbidity, and improper structure of the allantoic membranes are features observed during the TRUS examination of the ongoing process of pregnancy loss. Today, TRUS can be used as a real-time diagnostic method during the initial pregnancy examination
[33][48]. When an embryonic/fetal heartbeat is lacking, a pregnancy loss diagnosis protocol should be implemented
[19][26][24,31]. An embryonic/fetal heartbeat can also be detected by means of Doppler ultrasonography
[34][35][42,49].
2.1.5. Metabolic State at the Time of Pregnancy Diagnosis
Further clinically relevant factors associated with pregnancy loss are dam-related and include milk production, body condition score (BCS), and parity. A proper balance of carbohydrate intake is essential to maintain gestation and avoid pregnancy loss. While blood glucose levels are not adequate predictors of the fate of a pregnancy, some metabolic parameters such as excessive ketone body formation and increased blood urea nitrogen may suggest a threat to pregnancy. Further, several studies have shown that the pregnancy loss rate increases with number of parities or age
[36][37][38][52,53,54]. Remarkably, higher pregnancy loss rates were observed in the third and fourth parities of Simmental cows compared to those of heifers, primiparous cows, and cows in their second lactation
[39][55]. In contrast, Fernandez-Novo et al.
[40][56] detected no effect of parity on conceptus mortality rates, reporting a 15% pregnancy loss rate in multiparous cows. However, losses as low as 3%–5% have been reported in primigravid animals
[39][41][42][55,57,58].
2.2. Environment-Related Factors
Early pregnancy can be compromised when cattle are subjected to environmental stress
[43][69]. Individual animals experience different effects, in response to the same amount of stress. Stress affects uterine health, oocyte quality, ovarian function, and the developmental capacity of the conceptus
[44][70]. Pregnancy loss in the early embryonic stage is more common than in the late embryonic or early fetal stages, and the loss of embryos younger than 16 days can be indicated by a return to estrus. This could be the reason for the relatively limited literature data regarding the role played by stress in early embryonic mortality in dairy cattle. Although stress can be accurately measured via physiological and behavioral indicators in dairy cattle
[45][71], addressing this issue is challenging in the context of a multifactorial phenomenon such as pregnancy loss.
The numerous factors that lead to stress-induced pregnancy loss in individual animals can be classified in several ways. Nutritional stressors (quantitative and qualitative, animal welfare and climate effects both cold and warm) are classified as external stress factors while internal stressors include clinical disease, endocrine imbalance, and physical trauma (mechanical shock). The clinical impacts of stress on the reproductive system are mediated by some of the abovementioned factors. Among these factors, body temperature elevation (heat stress), metabolic hormones (stress related to mammary gland milk production and nutritional factors), and/or the activation of the hypothalamus–pituitary–adrenal (HPA) axis are important
[46][72]. Activity of the HPA axis can be increased by several stressors. Examples of these stressors are new milking machines or housing conditions, negative interactions with herd mates, aversive human handling, or restraining. However, the effects of restraining, limited access to resources, and social stressors on late embryonic and early fetal development in dairy cattle are not as well understood.
Environmental temperature has a clinically obvious effect on reproductive outcomes both in herds and individual animals. In a recent study by García-Ispierto et al.
[47][78], a strong link was observed between pregnancy loss and heat stress. The likelihood of late embryonic mortality was increased by a factor of 1.05 for each additional unit of mean maximum temperature–humidity index increase recorded between days 21 and 30 of gestation. This increased index is probably also responsible for the loss of a unilateral twin pregnancy
[27][32], which often occurs from days 60 to 90
[3]. The mean maximum temperature–humidity index has also been associated with higher ambient temperatures by other authors
[26][31].
Transporting animals can also cause a high level of stress that could result in late embryonic/early fetal death. Based on the limited data available, it seems that the risk of mortality is higher in cows carrying later-stage embryos or early-stage fetuses compared to early embryos (less than 16 days of pregnancy).
3. Conclusions
Several internal factors such as body condition or milk production serve to explain stress-mediated pregnancy loss in the individual animal. In the context of animal production, other effects may influence the success of pregnancy. Hence, the final clinical outcome can be predicted as the balanced consequence of these factors. This highlights the need for accurate diagnoses of pregnancy and of pregnancy loss. Pregnancy loss assessment commences in the initial pregnancy diagnosis exam and should consider all possible factors that could negatively affect the developing pregnancy and potentially result in its loss. The early identification of animals with a higher risk of losing their pregnancy offers economic benefits. This can be performed as early as in the initial pregnancy diagnosis. Avoiding negative consequences will also lead to improved herd profitability and reduced antibiotic usage. The different pregnancy determination methods currently available have different advantages in predicting pregnancy losses. Transrectal ultrasonography has the benefit of real-time observation of the viable embryo.