Autoimmune dermatological diseases (AIDD) encompass a diverse group of disorders characterized by aberrant immune responses targeting the skin and its associated structures. In recent years, emerging evidence suggests a potential involvement of the renin–angiotensin system (RAS) in the pathogenesis and progression of these conditions. RAS is a multicomponent cascade, primarily known for its role in regulating blood pressure and fluid balance. All of the RAS components play an important role in controlling inflammation and other immune responses. Angiotensin II, the main effector, acts on two essential receptors: Angiotensin Receptor 1 and 2 (AT1R and AT2R). A disturbance in the axis can lead to many pathological processes, including autoimmune (AI) diseases. AT1R activation triggers diverse signaling cascades involved in inflammation, fibrosis and tissue remodeling.
- RAS
- autoimmune diseases
- dermatology
- psoriasis
- systemic sclerosis
- vitiligo
- lupus erythematosus
1. Introduction
23. The Renin–Angiotensin–Aldosterone System
The central role of RAS is to maintain blood pressure and body fluid homeostasis [1]. The kidney is the main source of prorenin, the precursor of renin. Low arterial pressure, low sodium chloride and activation of beta-1 adrenoceptor lead to the release of renin from the juxtaglomerular cells [2]. Renin is a proteinase that hydrolyzes angiotensinogen (also called renin substrate), a plasma alfa-2-globulin synthesized by the liver and released into the blood flow. The Ang I resulting is a mild vasoconstrictor agent with no significant changes in blood pressure homeostasis. Thus, Ang I is further transformed into Ang II by ACE, present in lung capillaries, kidney and endothelial cells (ECs) (Figure 1) [3][4][5][3,4,5]. Chymase is a serine protease with a significant role in the conversion of Ang I into Ang II through a non-ACE pathway. This enzyme is found in mast cells, vascular ECs and cardiac fibroblasts, thereby serving as a primary contributor to the production of Ang II within the tissues [6][7][6,7]. Ang II, a very potent vasoconstrictor, exerts its effects through the activation of two G-protein-coupled receptors (GPCRs): AT1R and AT2R. AT2Rs appear especially in the fetal period, and their number decreases shortly after birth. Contrarily, AT1Rs appear mainly in the adult organism [4][8][4,8]. Natural antibodies (Abs) against GPCRs, involved in physiological homeostasis, including immune responses, have been identified in healthy individuals. However, when the levels or functions of these Abs become dysregulated, pathological mechanisms that contribute to the development of AI diseases, including systemic sclerosis, can result [9]. The Ang II-AT1R pathway is essential for survival [10]. In vascular smooth muscle cells (VSMCs), Ang II binds to AT1R, activating phospholipase C and raising intracellular [Ca+2], leading to vasoconstriction [3][4][3,4]. Activating AT2R, Ang II lowers blood pressure by vasodilatation and nitric oxide (NO) release [11]. Also, AT2R is involved in wound healing and tissue remodeling [12]. Aldosterone, the final element in the RAS, stimulates Na+ reabsorption [3][4][3,4]. Significantly, Aldosterone and Ang II are implicated in the production of extracellular matrix (ECM) proteins, including fibronectin, collagen I and plasminogen activator inhibitor proteins. These actions suggest an important role of RAS in tissue fibrosis [13].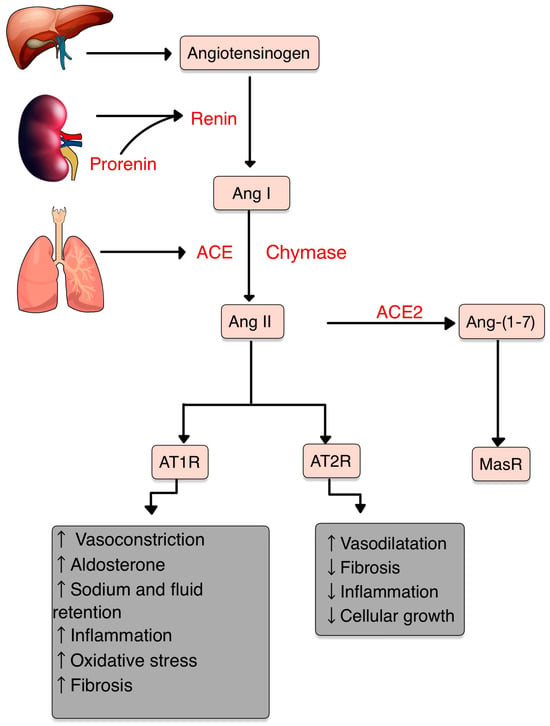
34. RAS and Inflammation
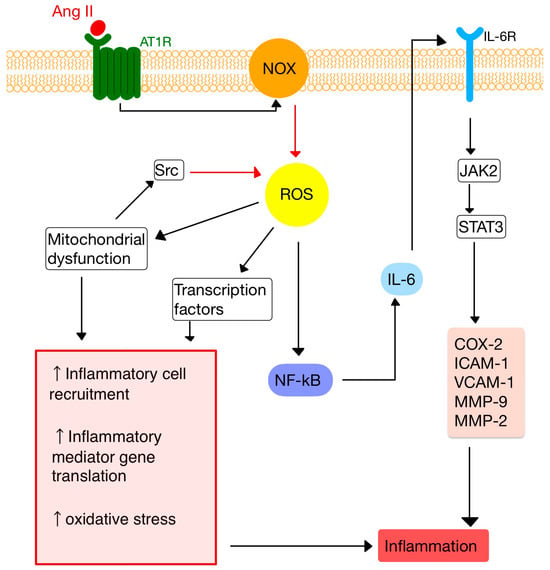
45. RAS and the Immune System
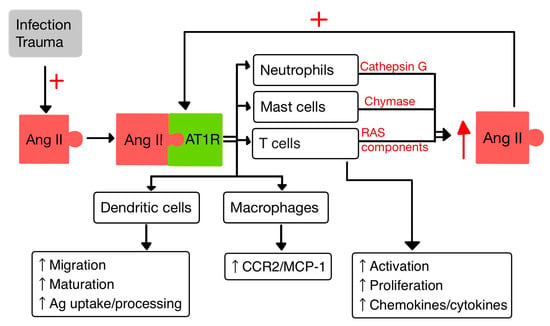
4.1. T Cells
5.1. T Cells
4.2. Dendritic Cells
5.2. Dendritic Cells
Dendritic cells (DCs) are specialized APCs that have a critical role in regulating the innate and also the adaptive immune responses [36][37][40,41]. In a study by Meng et al. [36][40], it was observed that Ang II exerts contrasting effects on DCs. On one hand, Ang II inhibits the phagocytic activity and proliferation of DCs. However, on the other hand, it promotes the maturation and the migration of DCs and also the expression of pro-inflammatory cytokines. Additionally, Ang II stimulates the T cell proliferation mediated by DCs [36][40].4.3. Macrophages
5.3. Macrophages
Macrophages and their precursors, known as monocytes, are white blood cells specialized in clearing away cellular debris and pathogens through phagocytosis. Additionally, they possess the ability to trigger and activate other immune cells to respond to invading pathogens [14]. Aldosterone aims at monocytes/macrophages and promotes the activation/migration of these cells in the ECs by increasing the expression of VCAM-1 and ICAM-1 [37][41]. It was proven that the activity of AT1R in M1 macrophages promotes polarization, which accelerates the inflammation with progression of tissue damage [38][42]. Likewise, Ang II upregulates the expression of monocyte chemoattractant protein-1 (MCP-1) and one of its receptors, CCR2 [14][39][14,43].4.4. Neutrophils
5.4. Neutrophils
PMNs, as the first responding cells to invading pathogens, play a crucial role in providing early immune protection. PMN bactericidal activity is increased by ACE present within them, regardless of the involvement of the Ang II/AT1R pathway. By interacting with AT1R, Ang II releases IL-8, which stimulates PMN recruitment and infiltration. Also, when stimulated by Ang II, PMNs produce oxidative bursts [10][40][10,44]. Cathepsin G (CatG), found in macrophages and PMNs, is a lysosomal protease that is upregulated in response to signals linked to infection and inflammation. CatG can elevate the local generation of Ang II by converting both angiotensinogen and Ang I to Ang II [10][41][42][10,45,46].56. RAS in the Skin
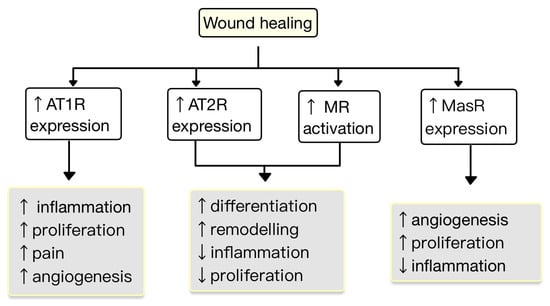
New research findings have unveiled the presence of a local RAS within the skin, where ACE has a role in the regulation of inflammation and autoimmunity [43][47]. The components of the RAS are situated in cutaneous and subcutaneous layers. They are crucial in various skin-related conditions, such as inflammation, fibrosis, scar formation and certain types of skin cancers [44][48]. Components of RAS are expressed in the human skin. Initial studies highlighting the presence of a local RAS in the skin revealed that skin cells, particularly keratinocytes, possess the ability to produce Ang II (as well as potentially other angiotensins) independently of the systemic circulation’s supply of RAS components. Keratinocytes are abundant in AT1R throughout all epidermal layers. AT1 receptors are also expressed in the hair follicles and sweat glands. In dermis, fibroblasts express AT1R, AT2R, MAS, angiotensinogen, renin, ACE, Ang II, and mast cells express chymase. In hypodermis, the subcutaneous fat expresses the same components of RAS as in dermis plus ACE2 [45][46][47][49,50,51]. RAS activity is involved in cell proliferation, differentiation, tissue remodeling and skin photoaging [48][49][52,53]. Stimulation of AT1R triggers cellular processes, including cell proliferation, migration, collagen synthesis and angiogenesis. Contrarily, the AT2R inhibit these actions by blocking the synthesis of certain pro-inflammatory molecules, like TGF-β, TNF-α and IL-6. Therefore, the interplay between AT1R and AT2R within RAS provides a fragile balance in regulating these cellular functions and inflammatory responses in the skin [50][54]. The expression of RAS components is upregulated in human wounded skin. Ang receptors have been implicated in wound healing and scar formation of the skin (Figure 4) [45][49]. Impaired wound healing has been associated with disruptions in the function of AT1R. In both aging and diabetes, RAS dysregulation occurs, characterized by high AT1R expression and low AT2R expression. This modification in the AT1R/AT2R ratio is linked with a reduction in epidermal thickness, collagen degeneration, dermal layer fractures and subcutaneous fat atrophy [51][55]. Research studies revealed that valsartan (an ARB) exhibits the highest level of skin penetration among other ARBs. Topical application of 1% valsartan gel has shown significant enhancement in wound healing. Researchers found that the rate of wound healing is superior while using topical valsartan compared to losartan. The beneficial effects of valsartan gel were mediated through the activation of AT2R, as the healing effect was absent in mice lacking AT2R. Conversely, the application of a 5% captopril gel resulted in a notable delay in the process of wound healing [44][48].