Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Wei Wang and Version 2 by Lindsay Dong.
The amplified employment of rigid polyurethane foam (RPUF) has accentuated the importance of its flame-retardant properties in stimulating demand. Thus, a compelling research report is essential to scrutinize the recent progression in the field of the flame retardancy and smoke toxicity reduction of RPUF.
- rigid polyurethane foam
- flame retardancy
- smoke toxicity suppression
- flame retardants
1. Introduction
Rigid polyurethane foam (RPUF) is a highly adaptable type of polymer foam that finds utility across a diverse array of industries and everyday household applications, such as building insulation and the structural portions of roofs, walls, floors, and furniture, primarily due to its excellent insulation properties, durability, lightweight nature, and flexibility in customization [1][2][3][4][5][6][7][1,2,3,4,5,6,7].
Rigid polyurethane foam is a type of foam insulation made by combining two liquid components, a polyol (a type of alcohol) and an isocyanate (a type of chemical compound), which react and expand to form a solid foam material [8][9][10][11][10,11,12,13]. In regard to flammability, the cellular structure and organic composition of RPUF make it combustible and susceptible to burning upon exposure to high temperatures or flames [12][13][14][15][14,15,16,17]. Moreover, the combustion of RPUF may result in the emission of toxic smoke and gases, such as carbon monoxide (CO), nitrous oxides (NOx), and hydrogen cyanide (HCN), which can be hazardous to human health [16][18]. For this reason, it is crucial to explore flame-retardant RPUF and to expand its practical applications [5][17][18][19][20][21][5,19,20,21,22,23].
There are three primary approaches being utilized to address the limitations of RPUF, as depicted in Table 1. The first method is copolymerization, which entails the chemical modification of the surface and core of the polymer [22][23][24,25]. The final two methods involve adding flame-retardant (FR) additives to the polymer matrix through mixing or coating [5][19][24][25][26][27][5,21,26,27,28,29]. Meanwhile, the first option has been demonstrated to be the durable and feasible approach for pristine RPUF. These flame retardants are primarily composed of organic compounds which possess a flame-retardant segment that can create covalent bonds with RPUF [28][30]. As a result, the integration of flame retardants into RPUF composites can lead to a considerable improvement in the compatibility between the polymer and the flame retardants as well as provide additional benefits such as enhanced mechanical properties, improved thermal stability, and better compatibility with other additives [29][30][31,32].
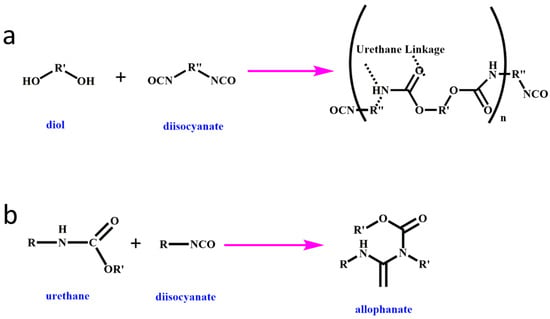
2. Reactive-Type Flame Retardants
The process of forming RPUF involves various reactions, including urethane formation, crosslinking reactions, and foaming reactions facilitated using a chemical blowing agent. The formation of the urethane linkage is illustrated in Figure 1a, while Figure 1b shows how the urethane group reacts with an isocyanate group to create allophanate, which results in chemical crosslinking. Reactive flame retardants demonstrate favorable interfacial compatibility with the matrix due to their chemical bonding interactions, resulting in minimal impact on the mechanical properties of RPUF. Meanwhile, reactive flame retardants containing multiple hydroxyl, amino, or epoxy groups can serve as polyols in the curing process of RPUF, providing flame retardant properties through the presence of phosphorus, nitrogen, or sulfur elements in their structure [31][32][33][34][35][8,41,46,47,48]. Additionally, reactive flame retardants with chemical bonding interactions are highly durable in industrial applications, as they prevent migration from the RPUF matrix.
Figure 1.
Basic reaction scheme for (
a
) urethane and (
b) allophanate formation.
2.1. Incorporation of Phosphorus-Containing Groups
Phosphorous-containing flame-retardant polyols are a type of reactive flame retardants, which can be used as a substitute for conventional polyols in the preparation of RPUF to enhance its flame-retardant properties [32][36]. The effectiveness of phosphorus-based flame retardants in minimizing the flammability of polyurethane foam has been well established, leading to its widespread adoption in industries ranging from construction and transportation to electronics [30][37]. Polyols containing phosphorus-based flame retardants possess multiple hydroxyl groups that can actively engage in the curing reaction of polyurethane foam. The incorporation of phosphorus-containing flame retardants in polyols enables the formation of chars and reduces the release of flammable gases during combustion [38].
One of the studies in this area focused on the application of biobased flame-retardant polyols. In their study, Bhoyate et al. [39] explored the fire safety of a polyol sourced from limonene, which was chemically modified with phenyl phosphonic acid. According to their findings, adding 1.5 wt.% of phosphorus through chemical modification could decrease the self-extinguishing time from 81 s to 11.2 s.
Currently, green materials such as plant oil, tung oil, and lignin have been chosen as the primary synthetic resources. However, the flame-retardant performance of biobased polyols is hindered by their long-chain structure, resulting in the low content of flame-retardant elements (phosphorus, nitrogen, silicon, etc.). Zhou et al. [40] synthesized tung-oil-based polyols through the ring-opening reaction of epoxidized tung oil and silane-coupling agents. Although the limiting oxygen index of the biobased RPUF prepared from these polyols increased from 19.0 vol.% to 22.6 vol.%, the improvement in the flame retardancy was not significant. This can be attributed to the low phosphorus content in long-chain biobased polyols.
In another approach, flame-retardant polyols with short chains are utilized for the synthesis and application of RPUF due to their elevated phosphorus content. Zou et al. [41] developed a hard-segment flame retardant (HSFR) to enhance the fire resistance of RPUF. By incorporating 13.8 wt.% of THPO, the resulting system was able to achieve a UL-94 V-0 rating, with an LOI ranging from 17.0 to 25.5%. During combustion, the HSFR can produce PO• and PO
) allophanate formation.
2.1. Incorporation of Phosphorus-Containing Groups
Phosphorous-containing flame-retardant polyols are a type of reactive flame retardants, which can be used as a substitute for conventional polyols in the preparation of RPUF to enhance its flame-retardant properties [41,49]. The effectiveness of phosphorus-based flame retardants in minimizing the flammability of polyurethane foam has been well established, leading to its widespread adoption in industries ranging from construction and transportation to electronics [32,50]. Polyols containing phosphorus-based flame retardants possess multiple hydroxyl groups that can actively engage in the curing reaction of polyurethane foam. The incorporation of phosphorus-containing flame retardants in polyols enables the formation of chars and reduces the release of flammable gases during combustion [51].
One of the studies in this area focused on the application of biobased flame-retardant polyols. In their study, Bhoyate et al. [52] explored the fire safety of a polyol sourced from limonene, which was chemically modified with phenyl phosphonic acid. According to their findings, adding 1.5 wt.% of phosphorus through chemical modification could decrease the self-extinguishing time from 81 s to 11.2 s.
Currently, green materials such as plant oil, tung oil, and lignin have been chosen as the primary synthetic resources. However, the flame-retardant performance of biobased polyols is hindered by their long-chain structure, resulting in the low content of flame-retardant elements (phosphorus, nitrogen, silicon, etc.). Zhou et al. [55] synthesized tung-oil-based polyols through the ring-opening reaction of epoxidized tung oil and silane-coupling agents. Although the limiting oxygen index of the biobased RPUF prepared from these polyols increased from 19.0 vol.% to 22.6 vol.%, the improvement in the flame retardancy was not significant. This can be attributed to the low phosphorus content in long-chain biobased polyols.
In another approach, flame-retardant polyols with short chains are utilized for the synthesis and application of RPUF due to their elevated phosphorus content. Zou et al. [56] developed a hard-segment flame retardant (HSFR) to enhance the fire resistance of RPUF. By incorporating 13.8 wt.% of THPO, the resulting system was able to achieve a UL-94 V-0 rating, with an LOI ranging from 17.0 to 25.5%. During combustion, the HSFR can produce PO• and PO
2• radicals in the vapor phase, which then react with flammable free radicals and impede segment decomposition.
• radicals in the vapor phase, which then react with flammable free radicals and impede segment decomposition.
2.2. Incorporation of Nitrogen-Containing Groups
The incorporation of nitrogen-containing groups is another effective approach for flame retardancy in polyurethane foam [59,60,61]. These compounds are usually incorporated into the polyol component during the production process, which then react with isocyanates to form polyurethane foam with improved flame retardancy [62]. During combustion, these compounds release nonflammable gases, such as nitrogen or ammonia, when exposed to high temperatures, diluting the flammable gases and reducing the combustibility of the foam [63,64,65]. Nevertheless, the flame-retardant effectiveness of nitrogen-based compounds is typically inferior to that of phosphorus-containing ones due to their single flame-retardant function. Melamine-based polyols are a type of reactive flame retardant used in the production of RPUF due to their high nitrogen content [66,67]. These polyols are synthesized by reacting melamine with an excess of formaldehyde and an alcohol or polyol, resulting in a highly crosslinked and thermally stable polymer. Hu et al. [62] developed a melamine-based polyol (MADP) and incorporated it into the RPUF matrix, and the interactions between the PU-NCO groups. The findings from their study demonstrated a notable improvement in the LOI values (increasing from 19.0% to 28.5%) with the implementation of the flame-retardant system. This enhancement promotes the development of protective char layers and reduces the concentration of flammable gases in the gaseous phase.2.2. Incorporation of Nitrogen-Containing Groups
The incorporation of nitrogen-containing groups is another effective approach for flame retardancy in polyurethane foam [42][43][44]. These compounds are usually incorporated into the polyol component during the production process, which then react with isocyanates to form polyurethane foam with improved flame retardancy [45]. During combustion, these compounds release nonflammable gases, such as nitrogen or ammonia, when exposed to high temperatures, diluting the flammable gases and reducing the combustibility of the foam [46][47][48]. Nevertheless, the flame-retardant effectiveness of nitrogen-based compounds is typically inferior to that of phosphorus-containing ones due to their single flame-retardant function.
2.3. Incorporation of Sulfur-Containing Groups
Melamine-based polyols are a type of reactive flame retardant used in the production of RPUF due to their high nitrogen content [49][50]. These polyols are synthesized by reacting melamine with an excess of formaldehyde and an alcohol or polyol, resulting in a highly crosslinked and thermally stable polymer. Hu et al. [45] developed a melamine-based polyol (MADP) and incorporated it into the RPUF matrix, and the interactions between the PU-NCO groups. The findings from their study demonstrated a notable improvement in the LOI values (increasing from 19.0% to 28.5%) with the implementation of the flame-retardant system. This enhancement promotes the development of protective char layers and reduces the concentration of flammable gases in the gaseous phase.
Sulfur-based flame retardants represent an alternative category of reactive flame retardants employed in the manufacture of polyurethane foam. These compounds have the advantages of having a low cost, being effective, and having a low impact on the mechanical properties of the foam. A sulfur-containing polyol was synthesized by Bhoyate et al. [49] to create flame-retardant polyurethane foams with improved compressive strength without affecting the foam morphology or closed cell content. The modified RPUF exhibited a self-extinguishing time that was decreased from 94.0 s to 1.7 s when the phosphorus content reached 1.5 wt.% in contrast to the pristine RPUF.
3. Additive-Type Flame Retardants
2.3. Incorporation of Sulfur-Containing Groups
3.1. Addition of Phosphorous-Containing Flame Retardants
Sulfur-based flame retardants represent an alternative category of reactive flame retardants employed in the manufacture of polyurethane foam. These compounds have the advantages of having a low cost, being effective, and having a low impact on the mechanical properties of the foam. A sulfur-containing polyol was synthesized by Bhoyate et al. [36] to create flame-retardant polyurethane foams with improved compressive strength without affecting the foam morphology or closed cell content. The modified RPUF exhibited a self-extinguishing time that was decreased from 94.0 s to 1.7 s when the phosphorus content reached 1.5 wt.% in contrast to the pristine RPUF.
The addition of phosphorous-containing flame retardants (P-FRs) in rigid polyurethane foam is a common approach to enhance its fire resistance. Phosphorous-containing compounds are considered effective flame retardants due to their ability to promote char formation in the condensed phase and to decrease the release of flammable gases in the condensed phase during combustion [75]. Examples of commonly used P-FRs in rigid polyurethane foam include ammonium polyphosphate (APP), dimethyl methylphosphonate, red phosphorous, and resorcinol bis(diphenyl phosphate) [63,76]. These flame retardants can provide varying degrees of fire resistance depending on their chemical structure, loading level, and processing conditions. It has been observed that the effectiveness of flame retardancy can be influenced by the valence state of phosphorus.
3. Additive-Type Flame Retardants
DOPO and its derivatives have recently emerged as P-FRs for the RPUF matrix, which release phosphorus species (PO•) and scavenge H• and OH• radicals in the flame to prevent the thermal degradation of polymers [77]. In a study by Zhang et al. [78], a new flame retardant called DOPO-BA was synthesized and added to a rosin-based RPUF matrix. When 20 wt.% DOPO-BA was incorporated, the LOI value increased from 20.1% to 28.1%. However, there was a significant reduction in the total smoke release.3.1. Addition of Phosphorous-Containing Flame Retardants
3.2. Addition of Phosphorus–Nitrogen-Based Flame Retardants
The addition of phosphorous-containing flame retardants (P-FRs) in rigid polyurethane foam is a common approach to enhance its fire resistance. Phosphorous-containing compounds are considered effective flame retardants due to their ability to promote char formation in the condensed phase and to decrease the release of flammable gases in the condensed phase during combustion [51]. Examples of commonly used P-FRs in rigid polyurethane foam include ammonium polyphosphate (APP), dimethyl methylphosphonate, red phosphorous, and resorcinol bis(diphenyl phosphate) [46][52]. These flame retardants can provide varying degrees of fire resistance depending on their chemical structure, loading level, and processing conditions. It has been observed that the effectiveness of flame retardancy can be influenced by the valence state of phosphorus.
Nitrogen-containing flame retardants (N-FRs) are another class of commonly used flame retardants in the RPUF matrix that work by releasing nitrogen species in the gas phase during combustion, which can act as diluents fuels, oxygen, and free radicals in the flame [59]. The most used N-FRs in RPUF include melamine, melamine cyanurate, melamine phosphate, and guanidine derivatives. These N-FRs can provide excellent an flame retardancy performance in RPUF, especially when used in combination with phosphorus-containing flame retardants. The addition of N-FRs can also improve other properties of RPUF, such as its mechanical properties and thermal stability, while minimizing the smoke and toxic gas released during combustion.
DOPO and its derivatives have recently emerged as P-FRs for the RPUF matrix, which release phosphorus species (PO•) and scavenge H• and OH• radicals in the flame to prevent the thermal degradation of polymers [53]. In a study by Zhang et al. [54], a new flame retardant called DOPO-BA was synthesized and added to a rosin-based RPUF matrix. When 20 wt.% DOPO-BA was incorporated, the LOI value increased from 20.1% to 28.1%. However, there was a significant reduction in the total smoke release.
3.2. Addition of Phosphorus–Nitrogen-Based Flame Retardants
Nitrogen-containing flame retardants (N-FRs) are another class of commonly used flame retardants in the RPUF matrix that work by releasing nitrogen species in the gas phase during combustion, which can act as diluents fuels, oxygen, and free radicals in the flame [42]. The most used N-FRs in RPUF include melamine, melamine cyanurate, melamine phosphate, and guanidine derivatives. These N-FRs can provide excellent an flame retardancy performance in RPUF, especially when used in combination with phosphorus-containing flame retardants. The addition of N-FRs can also improve other properties of RPUF, such as its mechanical properties and thermal stability, while minimizing the smoke and toxic gas released during combustion.
A new phosphorus–nitrogen intumescent flame retardant (DPPM) was synthesized by Guo et al. [30][32]. The addition of only 9% DPPM was sufficient to enable RPUF to achieve a UL-94 V-0 rating and a limit oxygen index of 29%. Hexa(phosphitehydroxylmethylphenoxyl) cyclotriphosphazene (HPHPCP, depicted in Figure 25) is another flame retardant that has been successfully synthesized and incorporated into rigid polyurethane foam [30][32]. HPHPCP contains multifunctional groups that introduce crosslinking into the foam structure, thereby improving its thermal stability and compressive strength. The addition of HPHPCP to DPPM-RPUF resulted in an LOI of 29.5% and a UL-94 V-0 rating, which can be attributed to the surface pyrolysis of RPUF.
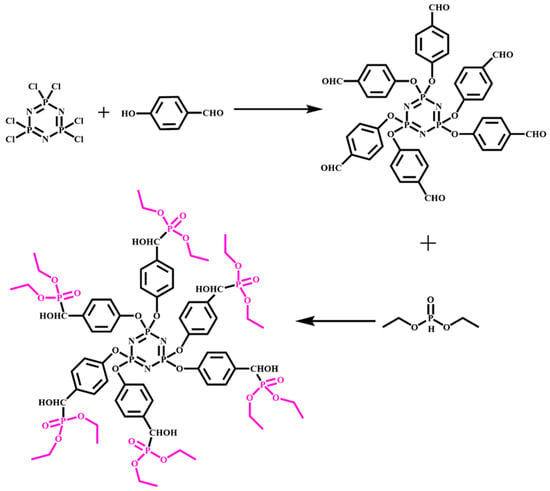

Figure 5. The synthesis of reactive flame-retardant HPHPCP.
3.3. Addition of Expandable Graphite and Derivatives
Expandable graphite (EG) and derivatives have emerged as a promising flame retardant for rigid polyurethane foam (RPUF) due to their exceptional fire-resistant properties [55][56][57]. Expandable graphite (EG) is produced by introducing sulfuric acid, acetic acid, or nitric acid into the crystalline structure of graphite. This process results in a unique material with exceptional thermal expansion properties when exposed to heat [58]. As a result, EG effectively decreases the flammability and heat release of RPUF.
When considering the utilization of RPUF in refrigerators, it is essential that the material exhibits a thermal conductivity falling within the range of 19 to 22 mW/(m K) and a compressive strength exceeding 110 kPa. Akdogan et al. [59] developed a flame-retardant RPUF using 15 wt.% EG and 5 wt.% ammonium pentaborate (APB). The outcomes revealed that this RPUF exhibited a remarkable 42.8% reduction in the THR and a 77.0% decrease in the TSR compared to the pristine foam.
3.4. Addition of Nanoclay and Other Nanoparticles
Adding nanoclay and other nanoparticles to both flexible and rigid polyurethane foam is a promising strategy for decreasing the production of smoke particles and toxic gases during combustion [60][61][62]. Clay nanosheets are frequently used as nanoparticle fillers in polymer nanocomposites because of their low cost, widespread availability, and flexibility. Incorporating nanoclay (a two-dimensional nanoparticle) into rigid polyurethane foam can form a physical barrier that obstructs gas diffusion and heat transfer, resulting in reduced smoke production and increased thermal stability [63][64][65]. Similarly, nanoparticles, such as cuprous oxide (Cu The synthesis of reactive flame-retardant HPHPCP.
3.3. Addition of Expandable Graphite and Derivatives
Expandable graphite (EG) and derivatives have emerged as a promising flame retardant for rigid polyurethane foam (RPUF) due to their exceptional fire-resistant properties [82,83,84]. Expandable graphite (EG) is produced by introducing sulfuric acid, acetic acid, or nitric acid into the crystalline structure of graphite. This process results in a unique material with exceptional thermal expansion properties when exposed to heat [85]. As a result, EG effectively decreases the flammability and heat release of RPUF.
When considering the utilization of RPUF in refrigerators, it is essential that the material exhibits a thermal conductivity falling within the range of 19 to 22 mW/(m K) and a compressive strength exceeding 110 kPa. Akdogan et al. [87] developed a flame-retardant RPUF using 15 wt.% EG and 5 wt.% ammonium pentaborate (APB). The outcomes revealed that this RPUF exhibited a remarkable 42.8% reduction in the THR and a 77.0% decrease in the TSR compared to the pristine foam.
3.4. Addition of Nanoclay and Other Nanoparticles
Adding nanoclay and other nanoparticles to both flexible and rigid polyurethane foam is a promising strategy for decreasing the production of smoke particles and toxic gases during combustion [88,89,90]. Clay nanosheets are frequently used as nanoparticle fillers in polymer nanocomposites because of their low cost, widespread availability, and flexibility. Incorporating nanoclay (a two-dimensional nanoparticle) into rigid polyurethane foam can form a physical barrier that obstructs gas diffusion and heat transfer, resulting in reduced smoke production and increased thermal stability [34,35,91]. Similarly, nanoparticles, such as cuprous oxide (Cu2
O), titanium dioxide (TiO
2
), nickel oxide (NiO), and silica (SiO
2), have also been investigated for their potential in reducing smoke production and toxic gas emissions during rigid polyurethane foam’s combustion [66][67][68]. These nanoparticles act as flame retardants by catalyzing the formation of integral and compact chars, reducing the production of volatile compounds, and increasing the thermal stability of RPUF.
), have also been investigated for their potential in reducing smoke production and toxic gas emissions during rigid polyurethane foam’s combustion [94,95,96]. These nanoparticles act as flame retardants by catalyzing the formation of integral and compact chars, reducing the production of volatile compounds, and increasing the thermal stability of RPUF.
