Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Fanny Huang and Version 1 by Giuseppe Marongiu.
Worldwide, the number of revisions to total knee arthroplasty procedures is increasing. Revision surgery is a challenging procedure, required for the management of bone loss after removal of the first implant. Although further long-term follow-up studies are needed, the use of cones in revisions of total knee arthroplasty yields reliability in fixation and stability to restore joint lines, especially in challenging surgeries with poor bone stock. The introduction of 3D-printed cones in revision surgery seems to be advantageous for AORI type III bone defects, especially in reducing intraoperative complications and procedure times.
- revision surgery
- total knee arthroplasty
- bone loss
1. Introduction
Total knee arthroplasty (TKA) is the gold standard for treating late-stage knee osteoarthritis. The advancing age and the increasing level of obesity of the global population are the main risk factors for hip and knee osteoarthritis, which will become one of the leading causes of global disability [1].
Between 2001 and 2016 in Italy, total primary knee arthroplasty exhibited an increment of 262%, with an average annual growth rate of 6.6%. Romanini et al. calculated that in Italy by 2050, the incidence rate of TKA will rise by 45% with respect to 2017 [2]. Worldwide, an increase of 143% by 2050 is expected.
In the last forty years, innovation in the prosthetic field in terms of designs, materials, instrumentation, and surgical techniques has improved survivorship of TKA. Recent studies have demonstrated a positive survivorship of 25 years in 82.3% of first implants [3]. However, one in five patients are reportedly still not satisfied with the results of surgery [4]. This inevitably indicates increases in the revision total knee arthroplasty rate.
In the United States between 2005 and 2006, 60,355 revisions of TKA were performed; the major indications were periprosthetic infection, aseptic loosening, and implant failure. Subsequently, studies have demonstrated similar trends with substantial reductions in the number of failures secondary to polyethylene wear [5].
Revision surgery and the re-revision of TKA are very challenging procedures, fraught with problems and complications. Bone defects secondary to aseptic and septic failure, or iatrogenic bone loss after first implant removal, significantly jeopardize the stability of the implant and the final success of the revision surgery [6]. The introduction of metal augments for the treatment of bone defects seems to improve load distribution, granting better stability and fixation of the implant, and enhancing longevity. Nevertheless, periprosthetic infections remain the main cause for the failure and re-revision of TKA.
2. Bone Defects: Classification System
Key challenges in revision surgery are bone defects and their management.
In addition to preoperative evaluation of the patient with historical and clinical examination, the assessment and classification of bone loss before surgery are vital, especially to predict outcomes, to choose the right type of implant, and to avoid unforeseen intraoperative problems. Radiological studies are required, such as a weight bearing imaging with anteroposterior, lateral, and Merchant patellar views. CT scans may be useful to estimate bone loss and bone prosthesis interfaces more accurately [7]. A proper classification system could help surgeons evaluate bone defects before surgery to decide on the best option for management and treatment.
In the contemporary literature, the most commonly used classification, clinical trials, and treatment guideline protocols on knee revision surgery is the Anderson Orthopaedic Research Institute (AORI), described by Engh in 1997. It describes bone loss according to the size, localization, and soft tissue involvement from preoperative radiographs and is either confirmed or changed intraoperatively (Table 1). Type I are lesions are limited to cancellous bone with intact metaphysis and do not compromise the stability of the component. Type II involves metaphysis, affecting one condyle or tibial plateau (IIA) or two condyles or tibial plateaus (IIB). Type III lesions, beyond the metaphyseal bone which is deficient, involve the attachments of collateral ligaments and patellar tendon [8,9][8][9].
Table 1. Description, characteristics, and treatment options of bone deficiencies in rTKA by Anderson Orthopaedic Research Institute (AORI) classification.
| AORI Classification | Type of Lesion | Treatment Options |
|---|---|---|
| Type I | Limited to cancellous bone Metaphysis intact |
Cement, cement with screws, autograft or allografts |
| Type II | Metaphysis damaged | Allograft, modular metal augments, porous metallic cones, sleeves |
| Type IIa | Involves one femoral or tibial condyle | |
| Type IIb | Involves both femoral or tibial condyles | |
| Type III | Metaphysis severely deficient; bone loss that comprises a major portion of the condyle or plateau |
Allograft, metallic cones and sleeves, mega-prosthesis, modular stems |
Although the AORI classification is the gold standard for the treatment choice and is simple to use, it has inherent limitations: AORI classifications typically underestimate bone loss [10]. This method does not quantify diaphyseal bone loss and only partially quantifies the metaphyseal area [11].
For this reason, several authors have proposed new classification systems or modified AORI classifications, although most of them are complex and have not been widely adopted. Rosso et al. modified the AORI classification with the intraoperative evaluation of epiphysis and metaphysis bone, distinguishing good bone quality (G), i.e., whether there was good cancellous bone and bleeding after bone preparation; sclerotic bone (S), i.e., whether there was a marble aspect of the bone with poor vascularization; and osteoporotic bone (O), i.e., poor bone quality [6].
Stambough et al. proposed an AORI modification in which Type IIA and Type III tibial defects are subdivided into contained and uncontained defects [12].
According to the concept of fixation, proposed by Morgan-Jones, there are three anatomical zones (epiphysis, metaphysis, and diaphysis) that are important for stability in revision surgery. In most revisions, the epiphysis and metaphysis are compromised, and the choice of an appropriate revision implant require a multizone approach [13].
Based on this concept, Jang S.J. et al. proposed an algorithm that identifies bone revision zones and cone placements with excellent accuracy and reliability through the use of deep learning, a subset of artificial intelligence [14].
Despite the efforts and the proposed modification, AORI classification remains a reliable system for femoral bone loss and substantial tibial bone loss [10,15][10][15].
3. Treatment Options
The purpose of classification systems is to suggest an appropriate treatment based on the bone defect. Cement should be used in cases of limited bone defects (AORI type I), peripheral deficiency up to 10%, central and cystic defects, and especially in cancellous non-sclerotic bone. The use of screws in addition to cement unloads the joint line and the cement–bone interface. Unfortunately, the outcomes of this technique are not satisfying; the use of a relatively large amount of cement could lead to thermal necrosis of the bone interface. Furthermore, the cement mass may exhibit failure due to its low resistance to stress and compression [16]. Autograft or allograft bones should be used in small and contained defects, especially in young patients, due to their osteoconductive properties. Both exhibit better load transfer compared with cement. Autografts are only used for small defects due to their limited availability. The use of allografts showed great results in terms of stability and survivorship for the management of severe tibial bone defect in revision surgery [17]; however, since large allografts have numerous disadvantages such as a lack of revascularization and inadequate remodelling [18], the introduction of other treatment options in revision surgery has become necessary.
The surgical choice for small uncontained defects (AORI IIa–IIb) is based on the size and location of the bone defect. Modular metal augments in wedges or blocks could be used for defects of up to 25 mm, and are particularly useful in tibial plateau deflections or posterior femoral condyles bone defects. These augments provide prompt support but often require additional resection to fit to the host bone [19].
Nowadays, there are outstanding options for the treatment of large, uncontained bone defects (AORI Type III); the use of tantalum or titanium cones and sleeves is widespread in knee surgery revisions. Sleeves and cones are metal devices that induce stability in the prosthesis with a cementless metaphyseal anchorage (Figure 1). Cones are first implanted press-fit into the defect after its preparation; then, the prosthesis is cemented into the cone [8]. Sleeves are bonded to the stem with a morse taper and inserted as a press-fit implant after preparing the host bone with a broach. The choice between one or the other is dictated by surgeon preference, shape, and size of the defect [20]. On the other hand, surgical techniques implanting the sleeve require the use of broaches that achieve alignment of the implant by the intramedullary channel are difficult to manage in patients with canal–epiphyseal mismatch. Moreover, metaphyseal sleeves need strong contact with the host bone, which is difficult and highly surgery demanding in cases of uncontained defects [21].

Figure 1. Metaphyseal modular implants for bone defection repair: (a) porous metal cones (Stryker, Mahwah, NJ, USA); (b) porous metal sleeves (DePuy Orthopedics, Warsaw, IN, USA).
4. Metallic Cones and Materials
First-generation metaphyseal cones are metal cone-shaped constructs composed of trabecular tantalum, which is a highly porous metal with excellent properties such as porosity, strength, flexibility, and biocompatibility. Tantalum was introduced in 1997. Since then, it has been widely used in hip orthopaedic reconstructive surgery and, in recent decades, in reconstructive surgery of the knee [22,23][22][23]. Porous tantalum is an excellent biomaterial with mechanical and biological properties for augmentation because of its similar elasticity to subchondral bone, low stiffness, high coefficient of friction that enables early weight bearing, and its excellent structure with a stable oxide that interacts with the surrounding tissue, facilitating osteoconduction and the development of bone-like apatite layers [24,25][24][25]. Moreover, porous tantalum foam seems to provide initial implant stability on loads while bone ingrowth occurs [26,27][26][27]. Bobyn et al. studied canine models of the characteristics of bone ingrowth using porous tantalum biomaterial and concluded that porous tantalum functions as a scaffold. In fact, the regions of bone-to-implant contact increase over time, producing osteons within the pores at a later time [23]. The elastic modulus of tantalum varies based on the porosity, and in general ranges between 2 and 20 GPa, imitating a cortical bone modulus of 3–30 GPa [28]. Metallic properties such as the surface area, porosity, hydrophobicity, chemistry, and microenvironmental factors are fundamental to surgery outcomes because they interact with the host bone and blood cells. Pure tantalum increases host white blood cell activation and seems to have a “bacteriophobic effect”, reducing S. aureus adhesion compared with titanium alloy, polished stainless steel, and tantalum-coated stainless steel implants, according to studies conducted by Schildhauer [29,30][29][30].
In the last decade, further improvements have been reached for the treatment of severe bone defects, with the production of 3D-printed cones. Second-generation cones are highly porous taper titanium cones produced from titanium powder using 3D-printed technology based on a computed tomography database. These cones have porous surface layers that provide bone ingrowth and reduce implant–bone mismatch. The implantation is made easier by specific instrumentation that enables the preparation of medial and lateral lobed portions to achieve a better fit [31,32][31][32]. These second-generation cones are implanted with a facilitated system of cannulated ream to prepare host bone to better fit the cone compared with the first-generation cones. Titanium and tantalum could also be coated with calcium phosphate to improve osseointegration and the bioactivity of augments [33]. According to Rambani R. et al., tantalum seems to be superior to titanium in terms of fewer radiolucencies, survivorship, osteointegration, decreasing osteolysism, and mechanical loosening [34]. On the other hand, titanium and its alloys reduce stress shielding compared with Co–Cr alloys because of their lower elastic moduli; however, this can cause stiffness mismatch to bone. The use of porous material overcomes this drawback and achieves stable long-term fixation [35].
Modern technology is constantly evolving; thus, surgeons have the possibility to use patient-specific 3D-printed titanium cones. After a knee CT or MRI, a computer reconstruction is created, and planning is performed with specialized computer-aided design software. A virtual model is used to mark the areas that could be removed or smoothed during surgery to facilitate the implantation. The model is then converted into a series of sliced 2D layers. Subsequently, the cones are printed layer by layer from titanium powder using a computer-controlled 3D printer. Grinding, coating, and/or surface oxidation are conducted in the postprocessing step. The production time of patient-specific cones could necessitate a variable time spanning from 2 to 8 weeks, including design, production, and logistics [36].
To date, several methods are used for titanium alloy 3D printing. The electron beam melting (EBM) technique is the most common 3D printing technique used to produce orthopaedic devices with high strength and low impurities. Other common techniques are selective laser sintering (SLS), which is the fastest production method in the industry but requires a longer post-printing process; and the selective laser melting (SLM) method, which yields higher accuracy but lower speed compared with SLS [37].
5. Surgical Technique
Even though the use of cones showed excellent results, the limited sizes and geometries of these devices could make the surgery technically challenging in matching the cone with the defect.
Surgical techniques that use metaphyseal standard cones consist of preparing the host bone with high-speed round or pencil-tip burrs and selecting the best size and shape of the cone using plastic trials. The resections are hand-made; therefore, care should be taken not to over-resect the bone. The definitive cone is then press-fitted into the metaphyseal bone and the stability of the porous tantalum is assessed during impaction. Figure 1 depicts voids between the host bone and the external surface of the cone then being filled with morselized cancellous bone grafts or demineralized bone matrixes [24,38][24][38]. Stepped cones are available when the bone defect is not centred, and could be used in combination with offset stems to restore the correct alignment [39]. Even though commercially available cones come in different shapes and measures, one of the major critiques in the use of metal standard cones is fitting to the remaining bone after TKA removal; in fact, standard cones usually do not fit to the individual patient’s gap and anatomy. The preparation of the host bone with burr in addition to removing remaining bone stock to adapt the cone makes the surgical technique more demanding and increases the risk of intraoperative fractures.
Surgical techniques for the implantation of 3D cones consist of preparing the intramedullary canal with sequential reaming until a correct fit is achieved. Then, the reamer is left in the canal and used as a guide for a conical reamer of the corresponding cone size. Single conical reamers could be used for symmetric defects or an instrumented system to fill medial or lateral defects. After choosing the right cone that matches the bone defect, the definitive cone is impacted (Figure 2). Rarely is a bone graft needed [40].
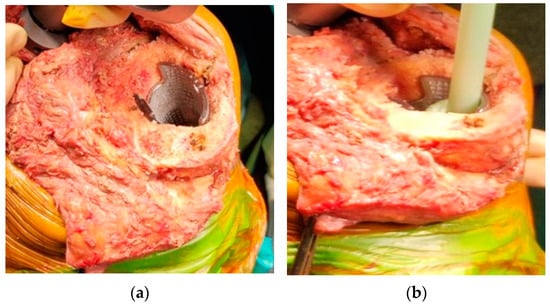
Figure 2. Surgical technique: (a) the definitive cone is press-fitted into the metaphyseal bone; (b) the prosthesis is then cemented into the cone.
References
- Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C.L.; et al. The global burden of hip and knee osteoarthritis: Estimates from the Global Burden of Disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1323–1330.
- Romanini, E.; Decarolis, F.; Luzi, I.; Zanoli, G.; Venosa, M.; Laricchiuta, P.; Carrani, E.; Torre, M. Total knee arthroplasty in Italy: Reflections from the last fifteen years and projections for the next thirty. Int. Orthop. (SICOT) 2019, 43, 133–138.
- Evans, J.T.; Walker, R.W.; Evans, J.P.; Blom, A.W.; Sayers, A.; Whitehouse, M.R. How long does a knee replacement last? A systematic review and meta-analysis of case series and national registry reports with more than 15 years of follow-up. Lancet 2019, 393, 655–663.
- Bourne, R.B.; Chesworth, B.M.; Davis, A.M.; Mahomed, N.N.; Charron, K.D. Patient satisfaction after total knee arthroplasty: Who is satisfied and who is not? Clin. Orthop. Relat. Res. 2010, 468, 57–63.
- Bozic, K.J.; Kurtz, S.M.; Lau, E.; Ong, K.; Chiu, V.; Vail, T.P.; Rubash, H.E.; Berry, D.J. The epidemiology of revision total knee arthroplasty in the United States. Clin. Orthop. Relat. Res. 2010, 468, 45–51.
- Rosso, F.; Cottino, U.; Dettoni, F.; Bruzzone, M.; Bonasia, D.E.; Rossi, R. Revision total knee arthroplasty (TKA): Mid-term outcomes and bone loss/quality evaluation and treatment. J. Orthop. Surg. Res. 2019, 14, 280.
- Gonzalez, M.H.; Mekhail, A.O. The Failed Total Knee Arthroplasty: Evaluation and Etiology. J. Am. Acad. Orthop. Surg. 2004, 12, 436–446.
- Mancuso, F.; Beltrame, A.; Colombo, E.; Miani, E.; Bassini, F. Management of metaphyseal bone loss in revision knee arthroplasty. Acta Biomed. 2017, 88, 98–111.
- Engh, G.A. Classification of Bone Defects Femur and Tibia. In Knee Arthroplasty Handbook; Scuderi, G.R., Tria, A.J., Eds.; Springer: New York, NY, USA, 2006; pp. 116–132.
- Mulhall, K.J.; Ghomrawi, H.M.; Engh, G.A.; Clark, C.R.; Lotke, P.; Saleh, K.J. Radiographic Prediction of Intraoperative Bone Loss in Knee Arthroplasty Revision. Clin. Orthop. Relat. Res. 2006, 446, 51–58.
- Belt, M.; Smulders, K.; van Houten, A.; Wymenga, A.; Heesterbeek, P.; van Hellemondt, G. What Is the Reliability of a New Classification for Bone Defects in Revision TKA Based on Preoperative Radiographs? Clin. Orthop. Relat. Res. 2020, 478, 2057–2064.
- Stambough, J.B.; Haynes, J.A.; Barrack, R.L.; Nunley, R.M. Acetabular wedge augments for uncontained tibial plateau defects in revision total knee arthroplasty. Arthroplast. Today 2018, 4, 313–318.
- Morgan-Jones, R.; Oussedik, S.I.; Graichen, H.; Haddad, F.S. Zonal fixation in revision total knee arthroplasty. Bone Jt. J. 2015, 97-B, 147–149.
- Jang, S.J.; Flevas, D.A.; Kunze, K.N.; Anderson, C.G.; Fontana, M.A.; Boettner, F.; Sculco, T.P.; Baldini, A.; Sculco, P.K. Standardized Fixation Zones and Cone Assessments for Revision Total Knee Arthroplasty Using Deep Learning. J. Arthroplast. 2023, 38, S259–S265.
- Khan, Y.; Arora, S.; Kashyap, A.; Patralekh, M.K.; Maini, L. Bone defect classifications in revision total knee arthroplasty, their reliability and utility: A systematic review. Arch. Orthop. Trauma. Surg. 2023, 143, 453–468.
- Brooks, P.J.; Walker, P.S.; Scott, R.D. Tibial Component Fixation in Deficient Tibial Bone Stock. Clin. Orthop. Relat. Res. 1984, 184, 302–308.
- Engh, G.A.; Ammeen, D.J. Use of Structural Allograft in Revision Total Knee Arthroplasty in Knees with Severe Tibial Bone Loss. J. Bone Jt. Surg.-Am. Vol. 2007, 89, 2640–2647.
- Stevenson, S.; Li, X.Q.; Davy, D.T.; Klein, L.; Goldberg, V.M. Critical biological determinants of incorporation of non-vascularized cortical bone grafts. Quantification of a complex process and structure. J. Bone Jt. Surg. Am. 1997, 79, 1–16.
- Sheth, N.P.; Bonadio, M.B.; Demange, M.K. Bone Loss in Revision Total Knee Arthroplasty: Evaluation and Management. J. Am. Acad. Orthop. Surg. 2017, 25, 348–357.
- Zanirato, A.; Formica, M.; Cavagnaro, L.; Divano, S.; Burastero, G.; Felli, L. Metaphyseal cones and sleeves in revision total knee arthroplasty: Two sides of the same coin? Complications, clinical and radiological results—A systematic review of the literature. Musculoskelet. Surg. 2020, 104, 25–35.
- Matar, H.E.; Bloch, B.V.; James, P.J. Role of metaphyseal sleeves in revision total knee arthroplasty: Rationale, indications and long-term outcomes. J. Orthop. 2021, 23, 107–112.
- Christie, M.J. Clinical applications of Trabecular Metal. Am. J. Orthop. 2002, 31, 219–220.
- Bobyn, J.D.; Stackpool, G.J.; Hacking, S.A.; Tanzer, M.; Krygier, J.J. Characteristics of bone ingrowth and interface mechanics of a new porous tantalum biomaterial. J. Bone Jt. Surg. Br. Vol. 1999, 81-B, 907–914.
- Lachiewicz, P.F.; Watters, T.S. Porous metal metaphyseal cones for severe bone loss: When only metal will do. Bone Jt. J. 2014, 96-B, 118–121.
- Levine, B.; Sporer, S.; Valle, C.; Jacobs, J.; Paprosky, W. Porous Tantalum in Reconstructive Surgery of the Knee—A Review. J. Knee Surg. 2010, 20, 185–194.
- Cohen, R. A porous tantalum trabecular metal: Basic science. Am. J. Orthop. 2002, 31, 216–217.
- Zardiackas, L.D.; Parsell, D.E.; Dillon, L.D.; Mitchell, D.W.; Nunnery, L.A.; Poggie, R. Structure, metallurgy, and mechanical properties of a porous tantalum foam. J. Biomed. Mater. Res. 2001, 58, 180–187.
- Balla, V.K.; Bodhak, S.; Bose, S.; Bandyopadhyay, A. Porous tantalum structures for bone implants: Fabrication, mechanical and in vitro biological properties. Acta Biomater. 2010, 6, 3349–3359.
- Schildhauer, T.A.; Peter, E.; Muhr, G.; Köller, M. Activation of human leukocytes on tantalum trabecular metal in comparison to commonly used orthopedic metal implant materials. J. Biomed. Mater. Res. 2009, 88A, 332–341.
- Schildhauer, T.A.; Robie, B.; Muhr, G.; Koller, M. Bacterial Adherence to Tantalum Versus Commonly Used Orthopedic Metallic Implant Materials. J. Orthop. Trauma 2006, 20, 476–484.
- Denehy, K.M.; Abhari, S.; Krebs, V.E.; Higuera-Rueda, C.A.; Samuel, L.T.; Sultan, A.A.; Mont, M.A.; Malkani, A.L. Metaphyseal Fixation Using Highly Porous Cones in Revision Total Knee Arthroplasty: Minimum Two Year Follow Up Study. J. Arthroplast. 2019, 34, 2439–2443.
- Black, J. Biologic performance of tantalum. Clin. Mater. 1994, 16, 167–173.
- Ohlmeier, M.; Lausmann, C.; Wolff, M.; Abdelaziz, H.; Gehrke, T.; Citak, M. Preliminary clinical results of coated porous tibia cones in septic and aseptic revision knee arthroplasty. Arch. Orthop. Trauma. Surg. 2021, 141, 555–560.
- Rambani, R.; Nayak, M.; Aziz, M.S.; Almeida, K. Tantalum Versus Titanium Acetabular Cups in Primary Total Hip Arthroplasty: Current Concept and a Review of the Current Literature. Arch. Bone Jt. Surg. 2022, 10, 385–394.
- Ryan, G.; Pandit, A.; Apatsidis, D. Fabrication methods of porous metals for use in orthopaedic applications. Biomaterials 2006, 27, 2651–2670.
- Cherny, A.A.; Kovalenko, A.N.; Kulyaba, T.A.; Kornilov, N.N. A prospective study on outcome of patient-specific cones in revision knee arthroplasty. Arch. Orthop. Trauma. Surg. 2021, 141, 2277–2286.
- Meng, M.; Wang, J.; Huang, H.; Liu, X.; Zhang, J.; Li, Z. 3D printing metal implants in orthopedic surgery: Methods, applications and future prospects. J. Orthop. Translat. 2023, 42, 94–112.
- Meneghini, R.M.; Lewallen, D.G.; Hanssen, A.D. Use of Porous Tantalum Metaphyseal Cones for Severe Tibial Bone Loss During Revision Total Knee Replacement. J. Bone Jt. Surg.-Am. Vol. 2008, 90, 78–84.
- Radnay, C.S.; Scuderi, G.R. Management of Bone Loss: Augments, Cones, Offset Stems. Clin. Orthop. Relat. Res. 2006, 446, 83–92.
- Chalmers, B.P.; Malfer, C.M.; Mayman, D.J.; Westrich, G.H.; Sculco, P.K.; Bostrom, M.P.; Jerabek, S.A. Early Survivorship of Newly Designed Highly Porous Metaphyseal Tibial Cones in Revision Total Knee Arthroplasty. Arthroplast. Today 2021, 8, 5–10.
More
