Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Jelica Grujic-Milanovic and Version 2 by Rita Xu.
Cardiovascular diseases (CVDs) are a group of diseases with a very high rate of morbidity and mortality. The clinical presentation of CVDs can vary from asymptomatic to classic symptoms such as chest pain in patients with myocardial infarction. Current therapeutics for CVDs mainly target disease symptoms. The most common CVDs are coronary artery disease, acute myocardial infarction, atrial fibrillation, chronic heart failure, arterial hypertension, and valvular heart disease.
- cardiovascular diseases
- oxidative stress
- natural products
- atioxidants
1. Introduction
Cardiovascular diseases (CVDs) are a group of diseases of the heart and blood vessels that contribute most to morbidity and mortality in the human population [1][2][1,2]. Atherosclerosis and arterial thrombosis lead to ischemic damage of different organs such as the heart, brain, kidneys, and eyes, which can induce different failures of these organs [3]. The incidence of CVDs doubled in the last three decades, from 271 million in 1990 to 523 million in 2020, with an extremely high mortality rate of over 32% [4]. Over the past 30 years, mortality from CVDs has steadily increased. Today, one person dies every half a minute from CVDs indicating the devastating fact that one-third of all deaths in the world are due to CVDs [5]. Among the main modifiable risk factors that contribute to the development and prognosis of CVDs are a combination of different psychosocial factors: socioeconomic, behavioural, unhealthy diet, physical inactivity, illicit substance use, smoking, and environmental risk factors are of most importance.
Other nonmodifiable factors may also affect the risk of CVDs, such as genetic predisposition, ethnicity, gender, and age [6].
The clinical presentation of CVDs can vary from asymptomatic in patients with atherosclerosis [7], or often with arterial hypertension [8][9][8,9], or manifest as unspecified symptoms such as weakness, light-headedness, and nausea, or classic symptoms such as chest pain in patients with coronary artery disease (CAD) [10] or acute coronary syndromes (i.e., acute myocardial infarction) [11][12][11,12]. Different etiologic and clinical symptoms of CVDs share some common features at the cellular and molecular levels: chronic inflammation [13], mitochondrial dysfunction [14][15][16][14,15,16], and oxidative damage [17] to biomolecules including proteins, lipids, and nucleic acids. These factors are believed to be a progressive process that may occur as early as childhood [18].
Numerous studies in the past decades have been performed to develop better therapeutic strategies, but current medications for CVDs mainly target disease symptoms like therapeutics for CAD disease [19][20][21][22][19,20,21,22], acute myocardial infarction [23], atrial fibrillation [24][25][26][24,25,26], chronic heart failure [27][28][27,28], and arterial hypertension [29][30][31][32][29,30,31,32], respectively. Physicians should be careful in choosing the right kind of treatment depending on the type of disease that a patient has. Especially since certain therapeutics are not effective enough in the treatment of certain CVDs or show intolerance or side effects. Therefore, it is important to improve prevention and early diagnosis and develop therapeutic options to reduce the currently very high risk of CVDs. In recent years, the search for active ingredients from natural products and plant sources for the treatment, prevention and/or supportive therapy of various types of cardiovascular disease has become a hotspot.
The World Health Organization (WHO) estimates that approximately 75% of the world medical market consists of phytomedicine [2]. Numerous therapeutics approved by the Food and Drug Administration (FDA) used today to treat the most common CVDs have been extensively studied in preclinical and clinical studies. The efficacy of herbal medicine has been carefully reviewed in the preclinical field; no comparative studies have been found to confirm the efficacy of natural products compared to FDA-approved therapeutics for the treatment of CVDs.
2. Most Frequent Cardiovascular Diseases
CVDs is an umbrella term for all diseases of the heart and circulation [1]. The pathophysiology of the occurrence of CVDs depends on a whole range of different factors (Figure 1). Numerous studies have shown that several potential mechanisms, including endothelial dysfunction, inflammation, oxidative stress, atherosclerosis, dysregulated haemostasis, cardiac stress, and epigenetics, play a role in the development of vascular and cardiac damage [33]. The most common types of heart diseases are CAD including acute coronary syndromes, atrial fibrillation, chronic heart failure, valvular heart disease, arterial hypertension, and congenital heart disease [34].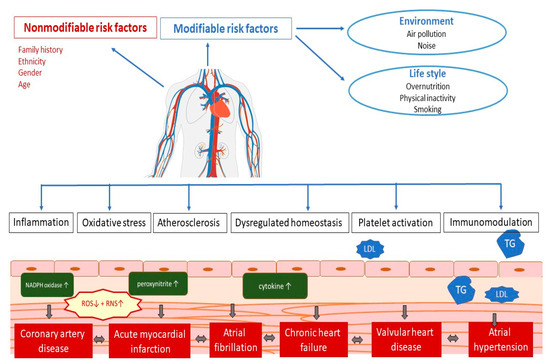
Figure 1. Pathophysiology of cardiovascular disease. LDL—low-density cholesterol; TG—triglycerides; ROS—reactive oxygen species; RNS—reactive nitrogen species.
2.1. Coronary Artery Disease
Coronary artery disease (CAD) is the most common CVD. Coronary atherosclerosis is a slow process that leads to the gradual intima thickening of the coronary arteries and subsequent development of atherosclerotic plaques that might be stable or prone to rupture due to inflammation. Atherosclerosis is the main factor that affects artery blood flow and leads to myocardial ischemia [7]. Coronary stenosis or occlusion may occur as a result of the formation of an intraluminal coronary thrombus [35]. Worldwide, an estimated 200 million people have CAD, and one in six deaths are caused by this disease [36]. In people with suspected CAD, the first option in a diagnosis is clinical diagnosis along with laboratory tests, electrocardiogram, exercise stress test, echocardiogram, and cardiac CT angiography [37].2.1.1. Treatment of Coronary Artery Disease Using Approved Drugs
Clinical guidelines for CAD treatments recommend a combination of lifestyle changes, pharmacological treatment, and, in some cases, cardiac interventions [21][38][39][21,38,39]. Lifestyle modification includes a healthy diet, smoking cessation, optimal physical activity, and stress management (Figure 2). As the development of CAD includes several risk factors such as hyperlipidaemia, obesity, diabetes mellitus, arterial hypertension, and smoking [16], pharmacological treatment includes target antiplatelet agents such as acetylsalicylic acid, clopidogrel, and blockers of adrenergic β receptors (beta blockers), hypolipemic drugs such as statins, fibrates or proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibitors, calcium channel blockers, organic nitrates, and various antihypertensive drugs (Figure 2) [21].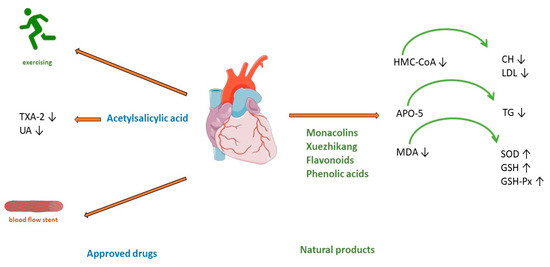
Figure 2. Treatment of coronary artery disease. TXA-2—Thromboxane A2; UA—uric acid; HMG-CoA—(3-hydroxy-3-methylglutaryl-coenzyme A) reductase; APO5—apolipoprotein A5; MDA—malondialdehyde; CH—cholesterol; LDL—low-density cholesterol; TG—triglyceride; SOD—superoxide dismutase; GSH—glutathione; GSH—glutathione peroxidase.
2.1.2. Treatment of Coronary Heart Disease Using Natural Products
Red yeast rice has been used as a herbal supplement for lowering cholesterol and lipoprotein in human blood. It is made by fermenting white rice with the yeast Monascus purpureus. Monacolin K is chemically like the cholesterol-lowering drug lovastatin. It acts by competitively inhibiting HMG-CoA (3-hydroxy-3-methylglutaryl-coenzyme A) reductase, the rate-limiting enzyme of the pathway of cholesterol synthesis (Figure 2). A meta-analysis of 6663 patients (from 20 randomized clinical trials) treated with red yeast rice extract showed a reduction in low-density cholesterol (LDL) [43][44]. The applied dose varies from 4.8 to 24 mg of monacolin K (1200–2400 mg of red yeast rice). The advantage of this treatment shows a significant reduction in the incidence of kidney injury and liver abnormalities compared with standard statin therapy [43][44]. However, research stated the limitation that reporting of adverse events was insufficient in most of studies. Thus, red yeast rice may be an effective treatment for reducing cardiovascular risk in statin-tolerant patients only when a mild profile of adverse reaction is confirmed [44][45]. Another meta-analysis of 15 high-quality randomized clinical trials with red yeast rice applied in doses of 200–4800 mg daily showed its efficacy and safety in the treatment of hyperlipidaemia. Hypertriglyceridemia represents an independent risk of coronary heart disease [45][46], but in most patients with this disease, high-intensity statin therapy is not useful because of the high incidence of statin intolerance [46][47], so treatment with Xuezhikang, may be a better alternative (Figure 2). Xuezhikang, an extract of Monascus purpureus, contains monacolins, PUFAs, flavonoids, and ergosterol. Xuezhikang is a supplementary product approved by the US Food and Drug Administration and has an excellent lowering performance on triglyceride and LDL-C levels (Figure 2). In coronary heart disease patients, 6 weeks of treatment with Xuezhikang extract (1200 mg/daily) resulted in a significant reduction in cholesterol, LDL-C, and triglycerides levels [47][48]. A review of 22 clinical randomized trials (most of them published in Chinese) showed that Xuezhikang is safe and effective in reducing cardiovascular events in coronary heart disease complicated by dyslipidaemia [48][49]. In rat models of high-fructose-diet-induced hypertriglyceridemia, Xuezhikang (XZK) was compared with simvastatin. Xuezhikang had a similar effect to simvastatin in lowering LDL-C, but a significantly higher hypotriglyceridaemic performance was attributed to the upregulation of apolipoprotein A5 (apoA5) via the peroxisome proliferator-activated receptor α (PPARα) signalling pathway [49][50]. Xuezhikang contributes to greater triglyceride reduction than simvastatin in hypertriglyceridemia rats by apoA5 elevation in hepatocytes [49][50]. Apo A5 is a target gene of PPARa and an important regulator of triglyceride metabolism [50][51]. Numerous studies have demonstrated the antioxidant effects of flavonoids. In a rat model of hyperlipidaemia, the administration of flavonoids from the seed of Amygdalus mongolica significantly lowered total cholesterol (TC), LDL-C, and the atherosclerosis index (Figure 2) [51][52]. The hypocholesterolaemic activity of the extract could be attributed to the fact it reduced malondialdehyde (MDA) and significantly increased activities of the antioxidant enzymes superoxide dismutase (SOD), glutathione (GSH), and glutathione peroxidase (GSH-Px) (Figure 2) [51][52]. In a meta-analysis of 39 prospective cohort studies (23,664 individuals with CHD), the intake of quercetin and kaempferol was linearly associated with a lower risk of CHD [52][53]. The lowest risk was observed in individuals whose intake was up to 12–14 mg/day of quercetin. Four phenolic acids are major compounds present in the methanolic extract of Quercus acutissima fruit (QF): caffeic acid, ellagic acid, gallic acid, and protocatechuic acid [53][54]. A recent investigation confirmed the important role of QF in cellular functions, such as gene regulation, cytoskeleton dynamics, receptor signalling, and cellular metabolism [54][55]. The anti-obesity, anti-hyperlipidaemic, anti-cholesterol, and anti-oxidative effects of QF are associated with the inhibition of acetylation, an important factor included in metabolic regulation (Figure 2) [55][56]. Saponin shows antiatherosclerosis activity by regulating lipid metabolism. A randomized controlled trial with Panax notoginseng saponins on 84 patients with CAD showed anti-lipidemic and anti-inflammatory effects. After 30 days of treatment with this saponin, high-density lipoprotein significantly increased, and white blood cell count decreased significantly [56][57]. An important mechanism of Panax notoginseng in vitro activity changes the methylation of miR-194, its promoter, and MAPK, FAS, RAS, and FOS, and significantly decreases the apoptosis rate of HUVECs cells [56][57]. The compound of Panax notoginseng saponin is available on drug markets as an over-the-counter drug in China and around the world [57][58]. Hydroxysaf flower yellow A is a c-glycosyl compound, a member of phenols, extracted from safflower (Carthamus tinctorius L.) which shows excellent therapeutic effects on CVDs by different mechanisms, is antioxidative, and has free radical scavenging abilities and anti-inflammatory activity. In models of atherosclerosis, it can suppress foam cell formation, vascular endothelial cell dysfunction, vascular smooth muscle cell proliferation and migration, and platelet activation by regulation of the reverse cholesterol process, fatty acids synthesis, and regulation of oxidative stress parameters [58][59]. Hydroxysaf flower yellow A reduces vascular inflammation by regulating the expression of NF-kappaB, Bax/Bcl-2, and TLR4/Rac1/Akt, PI3K/Akt/mTOR signalling pathways [58][59].2.2. Acute Myocardial Infarction
Acute myocardial infarction occurs when the blood supply to the heart is interrupted. In this situation, the heart is no longer supplied with sufficient oxygen and nutrients, so the muscle begins to die. In many cases, myocardial infarction is not fatal, especially if patients receive early treatment [11]. Myocardial infarction is the leading cause of death worldwide, with a prevalence approaching 3 million people [12].2.2.1. Treatment of Acute Myocardial Infarction Using Approved Drugs
The type of acute myocardial infarction (AMI) depends on the degree of coronary artery occlusion (Figure 3). The traditional recommendation for patients is to take one nitro-glycerine dose sublingually, 5 min apart, for up to three doses before admission to the emergency department [59][64]. After AMI, it is crucial to improve cardiac function and prevent postinfarction pathophysiologic remodelling [11]. Timely revascularization of the heart after AMI depends on the infarct size; therefore, an adequate reaction of physicians is very important. Standard treatment includes the use of antiplatelets and/or anticoagulants, beta-blockers, antiarrhythmics, opiate analgesics, antihypertensives such as angiotensin-converting enzyme (ACE) inhibitors, diuretics or calcium channel blockers, and oxygen therapy. Even prognosis most often depends on the type of AMI and administration of thrombolytic treatment or PCI [23]. Consequently, many patients in which this approach is used still progress to cardiac hypertrophy and heart failure.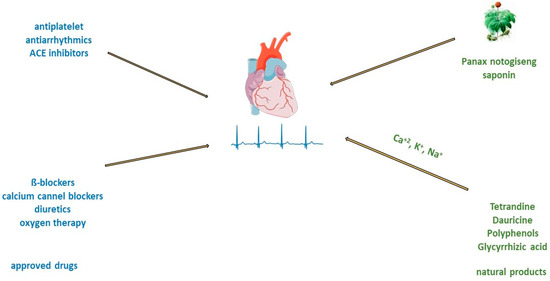
Figure 3. Treatment of acute myocardial infarction and atrial fibrillation with approved drugs or natural products.
2.2.2. Treatment of Acute Myocardial Infarction Using Natural Products
Saponin from Panax notoginseng exerts a cardioprotective effect in acute myocardial infarction [60][65]. In traditional medicine, the freeze-dried extract of Panax notoginseng for intravenous administration is used in the clinic for the prevention and treatment of cerebral ischemic injuries [61][66]. In addition, preclinical studies have shown the antioxidant and anti-inflammatory properties of this saponin [62][63][67,68]. Administration of Panax notoginseng injection to patients with myocardial infarction improved survival and cardiac function and decreased infarct size by direct inhibition of platelet aggregation and improved endothelial cell migration and angiogenesis (Figure 3). Panax notoginseng treatment significantly lowers lactate dehydrogenase and cardiac troponin I concentrations in the plasma of mice with MI. The mechanism of Panax notoginseng is manifested through the phosphorylation of AMPK and CaMKII in cardiomyocytes which induces autophagy [60][65]. Salvianolic acid B extracted from Salvia miltiorrhiza Bunge, promote angiogenesis in the marginal zone of MI by increasing the expression of VEGF [64][69]. In large myocardial infarction of rats, pretreatment with salvianolic acid B promotes the differentiation of mesenchymal stem cells into endothelial cells and has greater effects than the angiotensin-converting enzyme inhibitor benazepril [65][70]. In hyperlipidaemic animals with myocardial ischemia/reperfusion, hydroxysafflower yellow A inhibited the NF-κB signalling pathway, TLR4 signalling pathway, and phosphorylation of p38 [66][71]. Experimental acute myocardial ischemic models reduced serum levels of inflammatory factors such as TNF-alpha, IL-1β, and IL-18, reduced NLRP3 inflammasome expression, and induced autophagy [67][72].2.3. Atrial Fibrillation
Atrial fibrillation is a disorder of myocardial electrical conductivity that causes arrhythmia with various heart rhythms and rates [24]. As a result, too little blood is transported into the heart chambers (ventricles). This increases the risk of lung congestion and atrial thrombosis, as well as systemic thrombosis that causes a stroke. Uncontrolled atrial fibrillation can lead to chronic and acute heart failure [68][77]. The prevalence of atrial fibrillation ranged from 0.5% to 9% for people aged 50 to 90 years, respectively [42][69][42,78]. Causes of atrial fibrillation include sinus node dysfunction, coronary artery disease, rheumatic heart disease, arterial hypertension, hyperthyroidism, and alcohol [42]. Pathophysiological changes in atrial fibrillation include electrical remodelling, impaired atrial structure, autonomic nerve dysfunction, metabolic abnormalities, oxidative stress, etc. [69][78].2.3.1. Treatment of Atrial Fibrillation Using Approved Drugs
Treatment of atrial fibrillation usually includes rate and rhythm control, anticoagulation, and left atrial appendage closure. There is consensus that in patients with acute atrial fibrillation, parenteral anticoagulants such as heparin must be administered before cardioversion to reduce the risk of embolism [70][79]. Guidelines from various professional societies (The European Association of Cardio-Thoracic Surgery, American Heart Association (AHA)/American College of Cardiology (ACC)/Heart Rhythm Society (HRS)) recommend catheter ablation to restore sinus rhythm in patients with atrial fibrillation [71][80]. Patients with atrial fibrillation have an impaired quality of life and an increased risk of stroke, heart failure, cardiomyopathy, and acute coronary syndrome [70][79]. Administration of beta-blockers and calcium channel blockers is recommended as a first-line treatment for rate control of atrial fibrillation (Figure 3) [24]. Oral or intravenous application of different antiarrhythmic drugs, amiodarone, digoxin, flecainide, and ibutilide, increase the likelihood of reversion to sinus rhythm and can cause ventricular arrhythmias [25][26][72][73][25,26,81,82]. In addition, there are limitations such as that flecainide and propafenone should not be used in people with ischemic heart disease [25][26][25,26]. Treatment with verapamil, diltiazem, and digoxin may control heart rate, but they are unlikely to restore sinus rhythm [73][82]. On the other side, the long-time application of amiodarone can cause hepatotoxicity, interstitial lung disease, and thyroid dysfunction [74][83]. Therefore, the search for antiarrhythmic drugs from natural sources has been one of the priorities of scientists in recent years.2.3.2. Treatment of Atrial Fibrillation and Natural Compounds
There are many electrolytes in the human body; however, some of them, such as potassium, calcium, and sodium, play an important role in regulating signal transduction and ion transport across cell membranes. In patients with atrial fibrillation, due to electrolyte imbalance, the expression of ion channel proteins as well as gene transcription is altered, and fibrosis develops [75][84]. In an animal model of middle cerebral artery occlusion, saponin extracted from the roots of Panax notoginseng has significant antiarrhythmic and antiplatelet effects, regulates glycoprotein Ib-α, and reduces von Willebrand factor (VWF)-mediated platelet adhesion [76][85]. Myocardial tissue from the right and left atria of patients with atrial fibrillation after treatment with saponin increases in mitochondrial respiration rate [77][86]. The other group of saponins, ginsenosides, exert antiarrhythmic effects by modulating intracellular Ca2+ signalling through the inhibition of Ca2+ channels [78][87], or by regulating sodium, potassium, and calcium channels [79][88], or inhibiting collagen deposition in cardiomyocyte (Figure 3) [80][89]. Alkaloids are widely distributed in advanced plants and contain at least one nitrogen group. One of them, berberine, inhibits the occurrence of atrioventricular re-entrant tachycardia by regulating potassium and calcium ion channels and cyclic nucleotide-gated cation channels activated by hyperpolarization [81][90], or prolongs action potential duration and the effective refractory period in cardiac myocytes of rabbits [82][91]. Another alkaloid, tetrandrine, is antiarrhythmic by the inhibition of calcium, potassium, and sodium channels. An in vitro study of tetrandrine at a dosage of 100 µmol/L in rat cardiomyocytes, reduced Ca2+ influx into the sarcolemma and inhibited Ca2+ uptake into the sarcoplasmic reticulum by inhibiting ATP [83][92]. The significantly low dosage of tetrandrine, 15 µmol/L, increased the opening frequency and prolonged the opening time of calcium-activated potassium channels [84][93]. In a concentration-dependent manner (25, 125, 250, 400, 1000, and 2500 μmol/L) guanfacine blocked the L-type calcium channel and inhibited potassium currents in rat ventricular myocytes [85][94]. Dauricine reduced intracellular Ca2+ concentration by Na+-K+-ATPase and Ca2+-Mg2+-ATPase activation [86][95]. Matrine at a high concentration of 100 μM inhibited the expression of the human ether-a-go-go-related gene (hERG), encoded the rapidly activating, delayed rectifier potassium channel (IKr) important for cardiac repolarization, and at a low concentration of 1 μM, martine promoted hERG expression in rat cardiomyocytes. Indeed, matrine prolonged the action potential duration and the effective refractory period of cardiomyocytes [87][96]. Polyphenols are secondary metabolites widely distributed in the skin, roots, and leaves of fruits and medicinal plants. In vitro, cardiac arrhythmias caused by oxidative stress and calcium overload were significantly reduced in guinea pigs’ ventricular myocytes after treatment with resveratrol. Resveratrol reduced oxygen-free radical production, prevented the activation of calmodulin-activated protein kinase II, and inhibited L-type calcium channels [88][97]. Hydrogen-peroxide-induced ischemic arrhythmias in ventricular myocytes were reduced after resveratrol treatment by decreasing sodium concentration and reversing the sodium–calcium exchange current [89][98]. Puerarin protected rats’ ventricular myocytes against ischemia and reperfusion injury by regulating the calcium-activated potassium channel and activating protein kinase C [90][99].2.4. Chronic Heart Failure
Heart failure is a chronic, long-term condition in which the heart can no longer provide sufficient minute volume. This leads to circulus viciousness in terms of fluid retention starting from the legs, abdomen, and lungs to general edema (anasarca) in association with other symptoms of chronic heart failure. Chronic heart failure has increased to an estimated 37.7 million people, and almost 50% of these patients die within 5 years after diagnosis [91][101]. The risk increases with age, obesity, diabetes, smoking, alcohol abuse, or cocaine use. The guidelines of the American College of Cardiology Foundation (ACCF)/American Heart Association (AHA) defined chronic heart failure based on ejection fraction as preserved, intermediate, and heart failure reduced ejection fraction [91][101]. Additionally, in pathogenesis, myocardial interstitial fibrosis contributes to left ventricular dysfunction defined by the diffuse, disproportionate accumulation of collagen in the myocardial interstitium and activation of multiple molecular signalling pathways, such as endothelial dysfunction, hypertrophy of cardiomyocytes, and cardiac inflammation [10].2.4.1. Treatment of Chronic Heart Failure Using Approved Drugs
According to the guidelines for the diagnosis and treatment of acute and chronic heart failure, the following pharmacotherapeutic groups are recommended: drugs for the modulation of the renin-angiotensin-aldosterone (RAAS) and sympathetic nervous systems with ACE inhibitors or an angiotensin receptor-neprilysin inhibitor (ARNI), beta-blockers, mineralocorticoid receptor antagonists (MRA), loop and thiazide diuretics, and newly introduced gliflozins (inhibitors of sodium-glucose transport proteins 2) and ivabradine [91][101]. The side effects of high-dose diuretics can lead to low blood pressure, electrolyte disorders, and worsening of heart failure symptoms. Aldosterone antagonists can induce hyperkalaemia (Figure 4).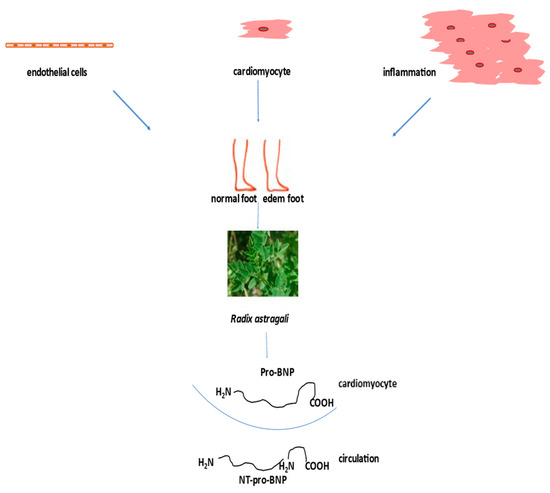
Figure 4. Treatment of heart failure with naturally derived astragaloside IV.
