You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Antonina Karlina.
Microsilica, a by-product of silicon or ferrosilicon production. Widely utilized within the construction industry, microsilica serves as a modifying component in concrete production, leveraging its chemical composition and physical attributes as a highly active pozzolan. Natural additives encompass crushed volcanic and sedimentary rocks, diatomites, volcanic ash, and tuff. Within technogenic additives lie waste or by-products from various industries, such as fly ash, granulated blast furnace slag, and microsilica.
- nanomaterials
- nanosilica
- cementitious materials
- mechanical properties
1. Introduction
Since the 1970s and 1980s, ultrafine forms of silica SiO2, such as microsilica and nanosilica, have been actively integrated into concrete technology. There are four primary types of amorphous silica available in the global market: precipitated silica (white soot), silica gel (dried silica gel), pyrogenic silica, and microsilica. The worldwide consumption of all synthetic silica types amounts to approximately 1 million tonnes [1,2,3,4,5][1][2][3][4][5].
Microsilica, a by-product of silicon or ferrosilicon production, presents as amorphous silica (SiO2) in spherical particle form [1,2,3,4,5,6,7,8,9,10][1][2][3][4][5][6][7][8][9][10]. Widely utilized within the construction industry, microsilica serves as a modifying component in concrete production, leveraging its chemical composition and physical attributes as a highly active pozzolan [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25]. The extensive introduction of various highly effective chemical and mineral additives has led to significant advancements in modern concrete technology.
It is acknowledged [25] that the incorporation of mineral fillers in concrete mixtures stands as a priority for reducing cement consumption and enhancing both its technological and operational properties. Natural additives encompass crushed volcanic and sedimentary rocks, diatomites, volcanic ash, and tuff. Within technogenic additives lie waste or by-products from various industries, such as fly ash, granulated blast furnace slag, and microsilica [25,26,27,28,29][25][26][27][28][29]. Microsilica emerges as one of the most potent and finely dispersed mineral additives. Its substantial specific surface area and amorphous structure endow it with high pozzolanic activity, functioning as an effective microfiller. The utilization of microsilica as a mineral additive proves a promising method for generating concrete with high-performance characteristics [25,26][25][26].
Wastes from ferroalloy production contain an elevated SiO2 content ranging from 85% to 95%, with a reduced content down to 65–75%. The specific surface area of microsilica ranges from 15,000 to 25,000 m2/kg, significantly surpassing the specific surface area of Portland cement, which stands at 300 to 400 m2/kg. Exhibiting high pozzolanic activity, microsilica operates effectively as a microfiller [30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49]. The development of a cementitious structure in the presence of microsilica relies on microfilling and pozzolanic effects. Furthermore, these particles serve as crystallization centers, amplifying the cement’s early-age hydration and increasing the packing density of the hydrate phases.
During cement hydration, the release of calcium hydroxide allows SiO2 to interact due to its high pozzolanic activity. This reaction leads to the formation of highly dispersed, low-basic calcium hydrosilicates C-S-H (I) instead of portlandite crystals Ca(OH)2, significantly enhancing the properties of the cement structure. This transformation reduces the presence of capillary pores, compacting the structure and decreasing cement stone permeability, thereby boosting its resistance to aggressive environments and overall durability [23,34,45,46][23][34][45][46]. Concrete mixtures containing microsilica exhibit increased thixotropy, viscosity, and resistance to delamination due to their high water-holding capacity [10,22,34,50,51][10][22][34][50][51]. However, the use of microsilica, due to its high specific surface area, significantly increases the water demand for concrete mixtures. It is estimated that 1 kg of microsilica raises the water requirement of a concrete mixture by 1 L [50]. Consequently, under conditions of uniform mobility of concrete mixtures, the use of microsilica exhibits an insignificant strength effect and proves ineffective [11,12,13,14,15,16,17][11][12][13][14][15][16][17]. To mitigate this, plasticizing additives are necessary to reduce water demand in concrete mixtures and enhance the efficacy of microsilica as a plasticizer.
Recent reviews [2,3][2][3] have meticulously explored ultrafine mineral compounds with minimal silica content, examining the addition of microsilica to concrete mixtures to advance concrete technologies. Concrete remains a cornerstone material in construction, with its demand escalating annually [4,5][4][5]. Ordinary Portland cement (OPC) is a crucial component contributing significantly to greenhouse gas emissions [10,11,12,13][10][11][12][13]. OPC production accounts for around 5–8% of global CO2 emissions [8,9,10,11][8][9][10][11]. Cement usage surpasses 4000 million tons annually and is projected to reach approximately 6000 million tons by 2060 [12[12][13],13], greatly contributing to climate change through greenhouse gas emissions [13,14,15][13][14][15]. To address this, agricultural and industrial wastes, such as rice husk ash (RHA), sugarcane bagasse ash, and olive oil ash, are utilized to partially substitute OPC in the production of green concrete [16,17,18,19,20,21][16][17][18][19][20][21].
Reviews [22,23][22][23] that analyzed studies involving micro- and nanosilica in construction demonstrate their use alongside fly ash and other microparticles to enhance the compactness, strength, and durability of cementitious materials. A scientometric analysis in [22] evaluated the keywords prevalent in nanostructured (NS)-modified concrete studies, listing the 20 most frequently used terms (Table 1). Nanosilica, microsilica, compressive strength, concrete, and cement emerged as the top five terms consistently employed in this field of research.
Table 1.
| S/N | Keyword | Occurrences |
|---|---|---|
| 1 | Nanosilica | 539 |
| 2 | Silica | 533 |
| 3 | Compressive strength | 393 |
| 4 | Concretes | 343 |
| 5 |
28][41].
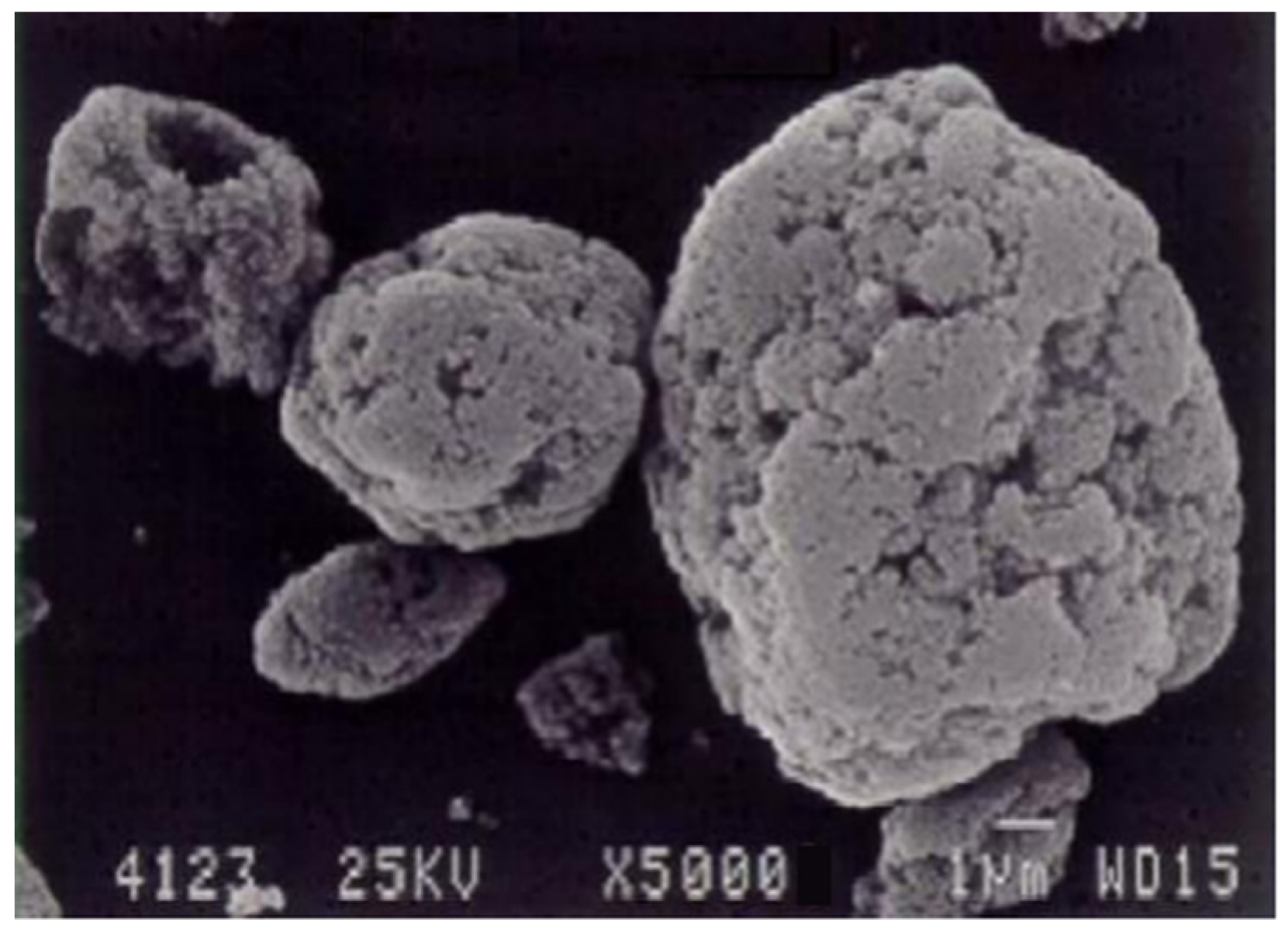
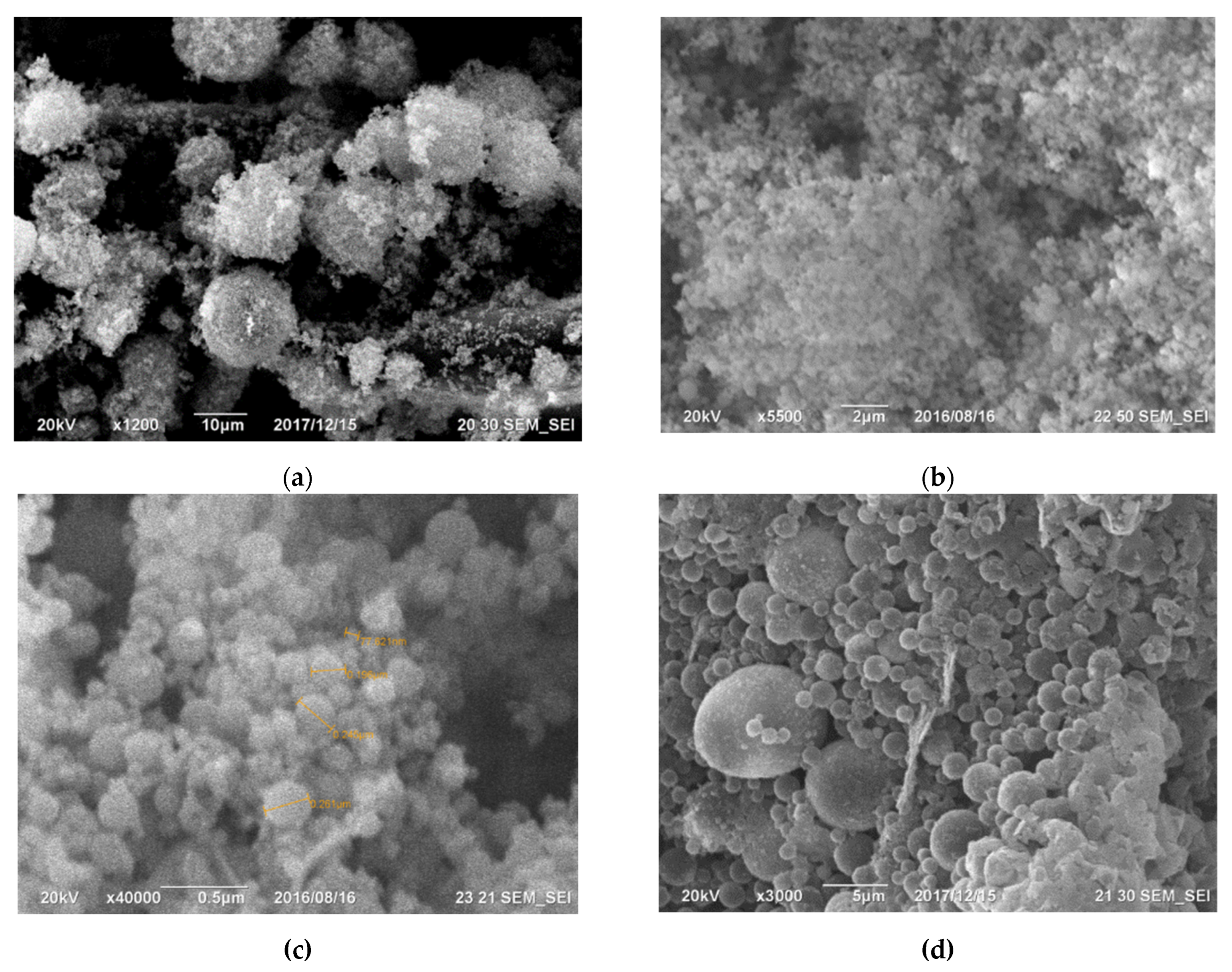
Microsilica, depicted in Figure 1, is a powdery substance composed of ultrafine spherical particles [41]. It emerges as a by-product during the gas purification process in technological furnaces utilized for producing silicon-containing alloys (such as metallic silicon, ferrosilicon, silicomanganese, and ferrosilicochrome). Table 3 illustrates the X-ray spectral analysis, presenting the primary elements found in the cyclone dust [28,41][
The primary components within the initial cyclone dust were nano- and micro-sized SiO2 spheres, as depicted in Figure 1. Figure 1a displays micro- and nanospheres of SiO2 magnified 15,000 times, whereas Figure 1b, magnified at 50,000 times, exhibits a micro-sized sphere enveloped with nano-sized SiO2 spheres. Figure 2 illustrates the differential and indirect particle distribution by dust size within the cyclones at the Bratsk Ferroalloy Plant.
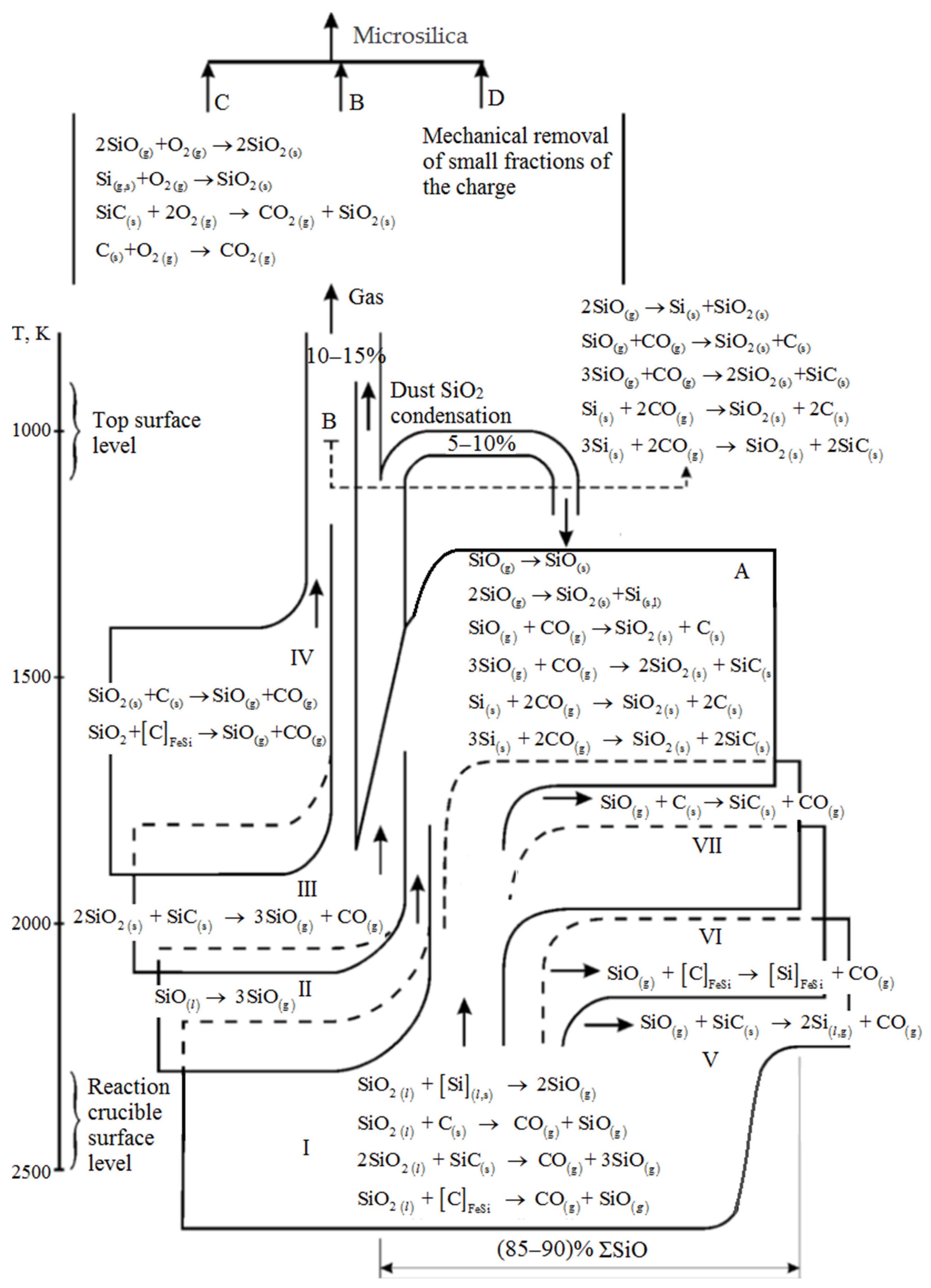
 Protopopov, E.V. et al. [39] present a diagram depicting the silicon monoxide formation balance during silicon production (Figure 3). When a charge containing silicon is melted, silica undergoes sublimation to create silicon monoxide, which then condenses within the colder, upper layers of the charge. A portion of the silicon monoxide is carried by the gas flow to the furnace surface, where it oxidizes to silicon dioxide and is captured by gas cleaning devices [40,41][40][41].
About 10% of the total gas content consists of primary dust, characterized by low dispersion. This dust can be efficiently eliminated from traditional dry and wet cleaning systems due to its granulometric composition. However, the remaining 90% of the dust comprises highly dispersed particles, primarily 2 μm in size. The formation of microsilica is thought to result from the processes outlined in Figure 3, according to the authors of [39]:
Protopopov, E.V. et al. [39] present a diagram depicting the silicon monoxide formation balance during silicon production (Figure 3). When a charge containing silicon is melted, silica undergoes sublimation to create silicon monoxide, which then condenses within the colder, upper layers of the charge. A portion of the silicon monoxide is carried by the gas flow to the furnace surface, where it oxidizes to silicon dioxide and is captured by gas cleaning devices [40,41][40][41].
About 10% of the total gas content consists of primary dust, characterized by low dispersion. This dust can be efficiently eliminated from traditional dry and wet cleaning systems due to its granulometric composition. However, the remaining 90% of the dust comprises highly dispersed particles, primarily 2 μm in size. The formation of microsilica is thought to result from the processes outlined in Figure 3, according to the authors of [39]:
 Aligned with the Si-O-C diagram and based on process conditions and the condensed phase composition, the furnace bath can be classified into three distinct zones: low, medium, and high temperature [40,41,42,43,44,45][40][41][42][43][44][45]. These zones exhibit variations in temperature conditions, condensed phase composition, and, most significantly, the inherent processes involved.
The low-temperature zone, existing under conditions of thermal equilibrium, maintains a temperature not exceeding 1500 °C. The condensed phases in this zone are dictated by the initial charge’s composition and the associated processes.
Moving to the medium temperature zone, this area lacks carbon but features the presence of only silica and silicon carbide in its condensed phases.
The high-temperature zone, surpassing 1817 °C, delineates a space where silicon remains stable solely at elevated temperatures exceeding 1817 °C and at a high concentration of SiO(g). Here, the destruction of silicon carbide and the production of silicon may lead to substantial SiO(g) losses. These losses are mitigated by removing them from the high-temperature zone of the furnace bath, resulting in the predominance of silicon dioxide—a primary component of process dust removed from the furnace along with process gases. This promising raw material is sourced from waste dust in the cyclones within the emission purification system used during catalytic silicon production.
Preliminary research indicates that silicon-containing dust comprises 80%–95.28% SiO2 [39,40,41,42,43][39][40][41][42][43]. The dust consists of spherical particles, ranging from nano-disperse particles up to 100 nm, which have a tendency to aggregate. The study of this waste is essential for extracting silicon dioxide, silicon carbide, and carbon [40,41][40][41].
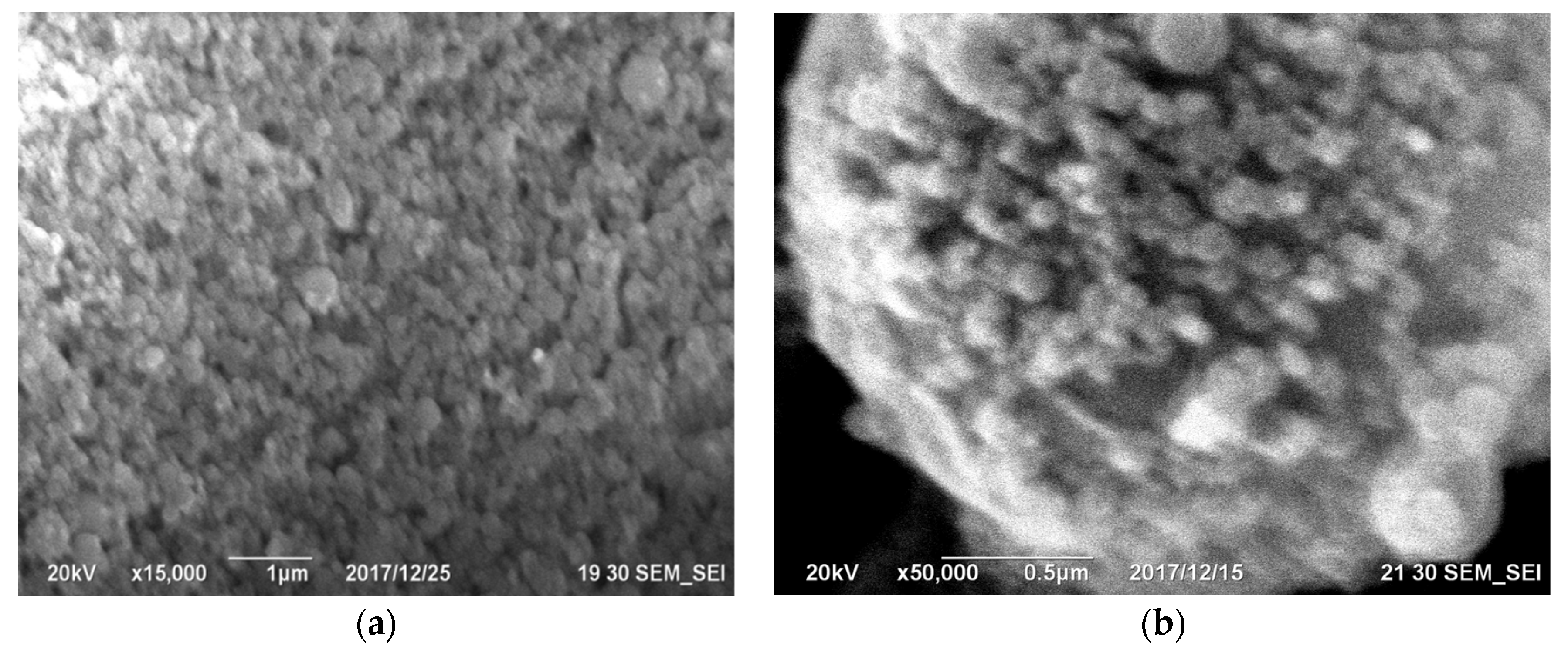
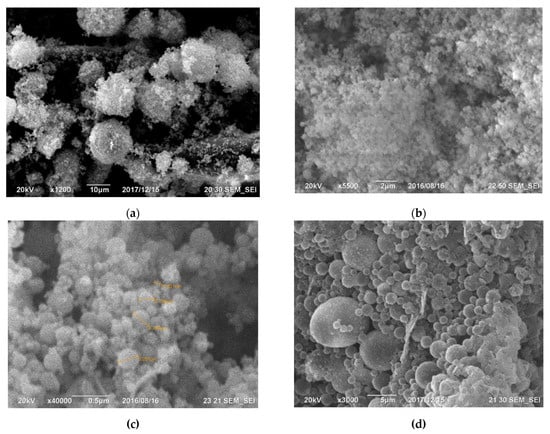
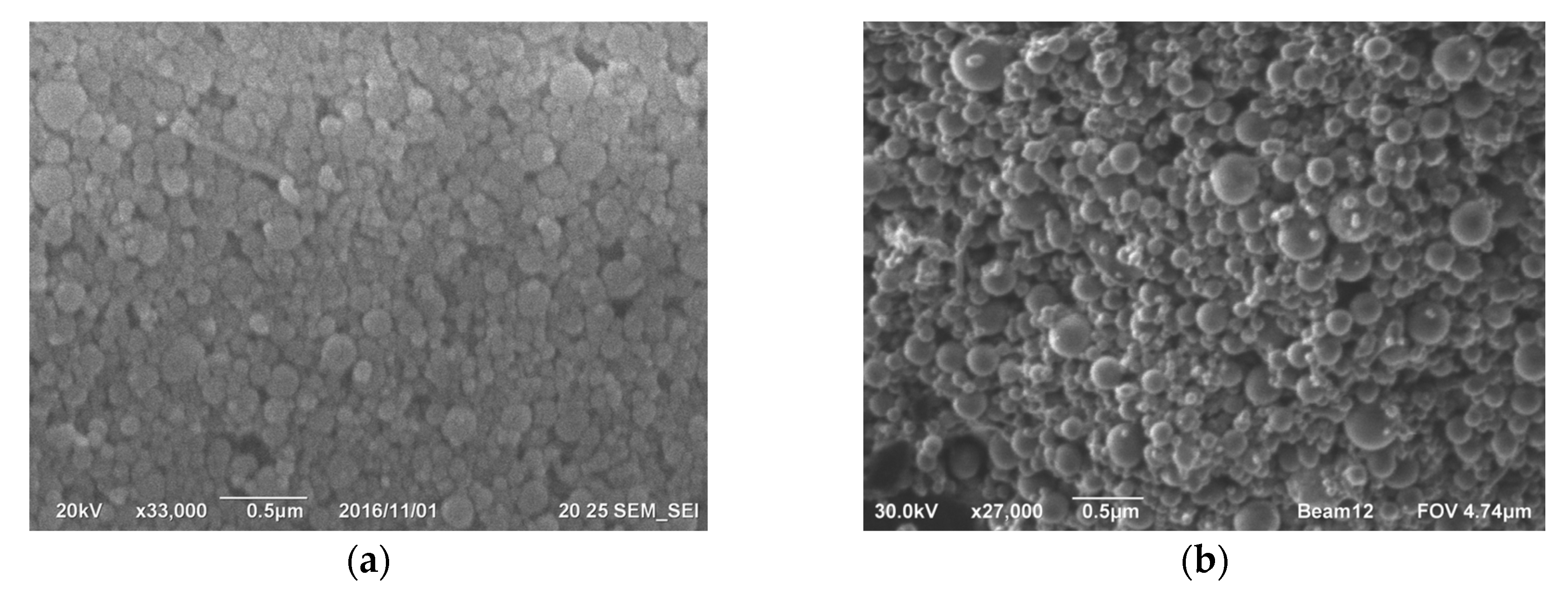
Gas purification dust from silicon production is actively used in construction. However, cyclone dust is unsuitable for this application due to its high carbon content, which violates regulatory documents [47,48,49][47][48][49]. Conversely, microsilica obtained by trapping furnace gases exhibits a minimal bulk density, ranging from 130 to 430 kg/m3. Challenges in utilizing and storing this product have led to its application in a more convenient, compressed form (with a density of 480–720 kg/m3) or as an aqueous suspension with a solid content of 50 wt% (with a density of 1320–1440 kg/m3). The compressed product is produced by passing air through silos containing microsilica over several hours, causing the fusion of clusters into larger aggregates, ranging from 10 to several 100 microns in size (Figure 5).
Aligned with the Si-O-C diagram and based on process conditions and the condensed phase composition, the furnace bath can be classified into three distinct zones: low, medium, and high temperature [40,41,42,43,44,45][40][41][42][43][44][45]. These zones exhibit variations in temperature conditions, condensed phase composition, and, most significantly, the inherent processes involved.
The low-temperature zone, existing under conditions of thermal equilibrium, maintains a temperature not exceeding 1500 °C. The condensed phases in this zone are dictated by the initial charge’s composition and the associated processes.
Moving to the medium temperature zone, this area lacks carbon but features the presence of only silica and silicon carbide in its condensed phases.
The high-temperature zone, surpassing 1817 °C, delineates a space where silicon remains stable solely at elevated temperatures exceeding 1817 °C and at a high concentration of SiO(g). Here, the destruction of silicon carbide and the production of silicon may lead to substantial SiO(g) losses. These losses are mitigated by removing them from the high-temperature zone of the furnace bath, resulting in the predominance of silicon dioxide—a primary component of process dust removed from the furnace along with process gases. This promising raw material is sourced from waste dust in the cyclones within the emission purification system used during catalytic silicon production.
Preliminary research indicates that silicon-containing dust comprises 80%–95.28% SiO2 [39,40,41,42,43][39][40][41][42][43]. The dust consists of spherical particles, ranging from nano-disperse particles up to 100 nm, which have a tendency to aggregate. The study of this waste is essential for extracting silicon dioxide, silicon carbide, and carbon [40,41][40][41].
Gas purification dust from silicon production is actively used in construction. However, cyclone dust is unsuitable for this application due to its high carbon content, which violates regulatory documents [47,48,49][47][48][49]. Conversely, microsilica obtained by trapping furnace gases exhibits a minimal bulk density, ranging from 130 to 430 kg/m3. Challenges in utilizing and storing this product have led to its application in a more convenient, compressed form (with a density of 480–720 kg/m3) or as an aqueous suspension with a solid content of 50 wt% (with a density of 1320–1440 kg/m3). The compressed product is produced by passing air through silos containing microsilica over several hours, causing the fusion of clusters into larger aggregates, ranging from 10 to several 100 microns in size (Figure 5).
 The electric furnace generates soot collected from various collection systems, including baghouses. Instead of disposing of it in slurry fields, this soot is sold as an additive (AS) [41]. One of the significant advantages of using silica fume is its suitability as a mineral admixture in concrete [49]. Essentially, silica consists of amorphous (non-crystalline) silicon dioxide (SiO2). Currently, concrete failure when incorporating microsilica arises from corrosion, leading to increased expenses due to sea salt or icing. Therefore, it is crucial to ensure the end product’s resistance to sulphate, thereby fulfilling a primary objective.
The consensus recognizes that utilizing compressed microsilica necessitates longer and more intense mixing to disperse agglomerates. Despite the technological advancement of microsilica suspensions, they cannot be stored under subzero temperatures and require redispersion after prolonged storage. In the UK, the use of compressed microsilica holds paramount practical importance, while in European countries, both suspended forms and compressed microsilica are commonly employed.
The electric furnace generates soot collected from various collection systems, including baghouses. Instead of disposing of it in slurry fields, this soot is sold as an additive (AS) [41]. One of the significant advantages of using silica fume is its suitability as a mineral admixture in concrete [49]. Essentially, silica consists of amorphous (non-crystalline) silicon dioxide (SiO2). Currently, concrete failure when incorporating microsilica arises from corrosion, leading to increased expenses due to sea salt or icing. Therefore, it is crucial to ensure the end product’s resistance to sulphate, thereby fulfilling a primary objective.
The consensus recognizes that utilizing compressed microsilica necessitates longer and more intense mixing to disperse agglomerates. Despite the technological advancement of microsilica suspensions, they cannot be stored under subzero temperatures and require redispersion after prolonged storage. In the UK, the use of compressed microsilica holds paramount practical importance, while in European countries, both suspended forms and compressed microsilica are commonly employed.

Figure 1.
a
) ×15,000; (
b
) ×50,000.
Table 3.
| Elements | C | O | Na | Mg | Al | Si | K | Ca | Mn | Fe | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Weight, % | 3.98 | 53.78 | 0.37 | 0.62 | 0.34 | 38.54 | 0.79 | ||||
| 3 | |||||||||||
| : 0.4%–0.7%; CaO: 0.4%–0.9%; MgO: 0.8%–1.0%; Na | 2 | O: 0.6%–0.8%; K | 2O: 1.2%–1.4%; C: 0.9%–1.2%; S: 0.2%–0.3% | ||||||||
| Serov Ferroalloy Plant, Ltd. | FS 65, FS 45 | FS 65 (Si: 63%–68%; C: 0.1%; S: 0.02%; P: 0.05%; Al: 2.5%; Mg: 0.4%; Cr: 0.4%); FS 45 (Si: 41%–47%; C: 0.2%; S: 0.02%; P: 0.05%; Al: 2.0%; Mg: 1.0%; Cr: 0.5%) |
|||||||||
| Bratsk Ferroalloy Plant, Ltd. | Additive to concrete, which is widely used in the manufacture of classes of concrete subject to erosive abrasion and possessing improved water resistance | FS 65, FS 75 | FS 65 (Si: 63%–68%; C: 0.1%; S: 0.02%; P: 0.05%; Al: 2.5%; Mg: 0.4%; Cr: 0.4%); FS 75 (Si: 74%–80%; C: 0.1%; S: 0.02%; P: 0.04%; Al: 3.0%; Mg: 0.4%; Cr: 0.3%) |
||||||||
| Kremniy (Shelekhov), RUSAL, Ltd. | Needs of chemical and electrical industry enterprises | No information | Na2O: 0.04%; MgO: 0.13%; Al2O3: 0.14%; SiO2: 98.99%;P2O5: 0.0060%; S: 0.0038%; K2O: 0.28%; CaO: 0.47%; TiO2: <0.001%; MnO: 0.015%; Fe2O3: 0.034% |
| 0.17 | 0.17 | 1.24 | 100.00 |

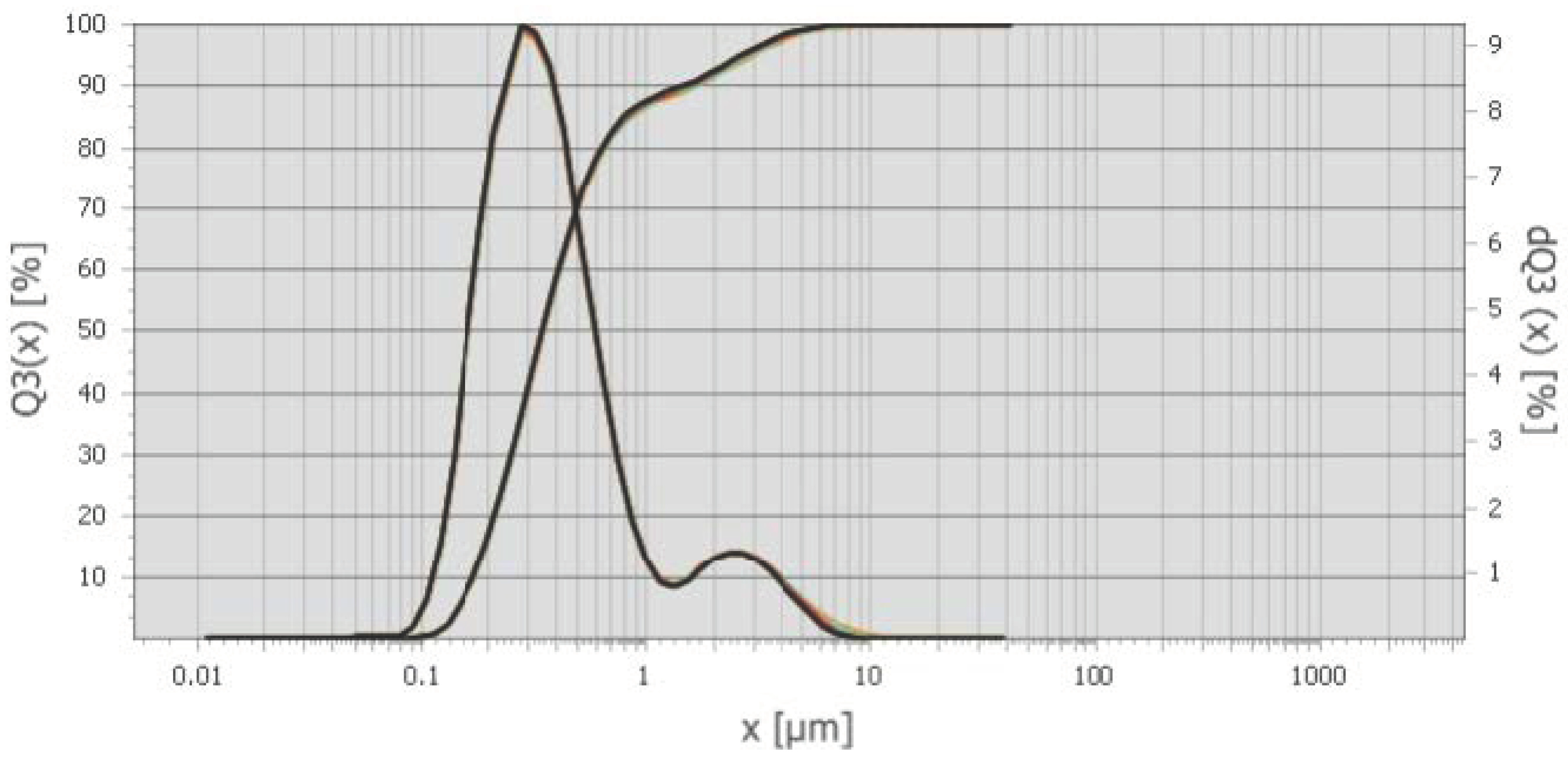
Figure 2. The differential and integral distribution of particles by dust size from the cyclones of the Bratsk Ferroalloy Plant is shown in the graph (Distribution mode is 0.29 μm) [41].
-
Interaction between silicon monoxide and carbon monoxide in a gas furnace at temperatures ranging from 1400 to 1800 K leads to the formation of microsilica.
-
In the low-temperature zones of the furnace, where the gas phase temperature and the equilibrium concentration of SiO sharply decrease, silicon monoxide disproportionation may occur.
-
The very high cooling rates of the gas phase could potentially lead to the direct condensation of silicon monoxide.




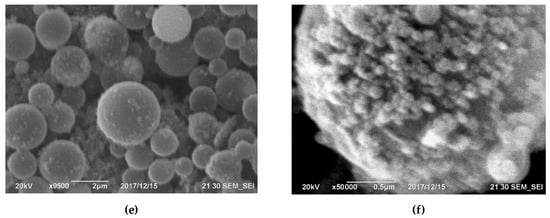
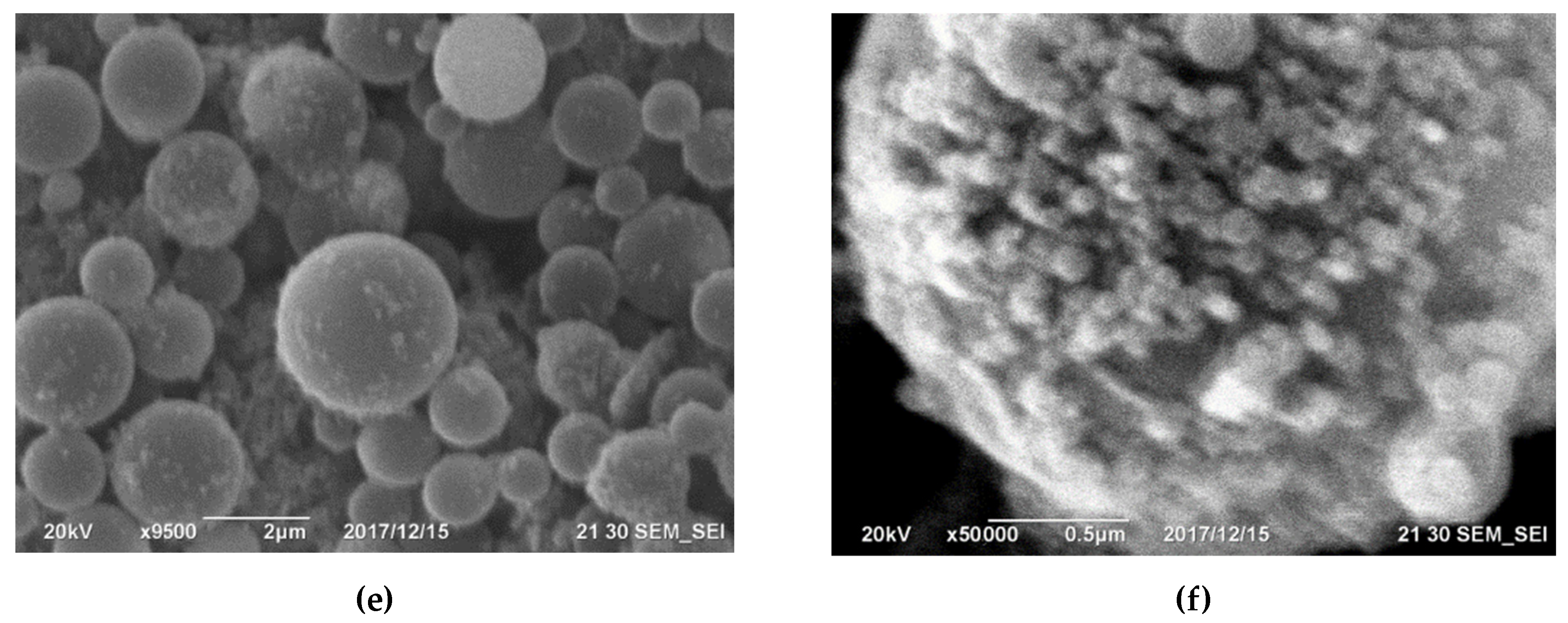
Figure 4. Microphotographs of silicon hose dust surface from the Kamensk-Uralsky branch of OJSC SUAL have been taken, showcasing (a) the top view, (b) the appearance at higher magnification, and (c) surface detailing, with a determination of the diameters of spherical particles of silicon dioxide. Images of the surface of silicon dust after wet cleaning by JSC Kremniy [39]: (d) viewed from above, (e) a closer look at the surface at higher magnification, and (f) a detailed view at 50,000 times magnification, showing spherical particles of silicon dioxide on the surface. [41].

Figure 5.
a
) ×33,000; (
b
) ×27,000.
4. History of the Use of Micro and Nanosilica as a Cement Additive
In 1950, the Fiskaa plant in Kristiansand, Norway, installed the first experimental filters for capturing microsilica [50]. Two years later, initial tests were conducted using MS as an additive to Portland cement concrete, coinciding with the first publication on its concrete application. The commercial sales of microsilica in concrete plants commenced around 1971. In 1974, engineers from the Fiskaa plant (later forming the Elkem organization) significantly redesigned the industrial bag filter. The increased use of microsilica in concrete led to compliance with silica fume standards in cement in Norway between 1976 and 1978, slightly later in concrete. The utilization of MS in Bluetooth and Iceland began in 1981. By 1990, microsilica had gained global recognition as a concrete additive, proving its capability to enhance final product properties. Considerable attention was directed towards ensuring reliability and extended service life. By the year 2000, international standards for the use of Microsilica additives (MS) in concrete technologies became available and were commonly applied in industrial production across most countries. High-strength concrete incorporating MS was used in the construction of significant infrastructure projects, including high-rise buildings in Chicago, the Channel Tunnel, the Northumberland Strait Bridge in Washington, and offshore drilling platforms in the North Sea. In Russia, MS-based construction projects were undertaken, albeit with a delay of several decades. These projects in Russia encompass transport tunnels on Kutuzovsky, Leninsky, and Nakhimovsky prospects, a bridge on Bratislavskaya, an overpass on Oleniy Valkikh, and gravity-type oil platform streets for the Sakhalin-2 project. As previously noted, MS is a byproduct derived from silicon production, processed in electric furnaces [28,39[28][39][40],40], with required raw materials including quartz, coal, or wood chips.5. History of the Use of Micro and Nanosilica as a Cement Additive in Russia
Russia has implemented a standard [49] for an active mineral additive of technogenic origin exhibiting high pozzolanic activity, known as condensed microsilica (hereinafter referred to as microsilica). This is designed to specifically regulate the properties of concrete, mortar, and dry building mixtures, which are made with binders primarily based on Portland cement clinker (Table 4). Microsilica is categorized in Russian documents like “MK” into three types, each identified as follows:Table 4.
| The Name of Indicators | Standard Value of Quality Indicator for Grades of Condensed Microsilica * | ||||
|---|---|---|---|---|---|
| Uncompacted | Compacted | Suspensions (Pastes) | |||
| MK-65 | MK-58 | MKU-65 | MKU-85 | MKS-85 | |
| 1 External appearance | Ultrafine gray powder material | Gray coarse powder material | Dark gray fluid | ||
| 2 Mass fraction of moisture, %, no more | 3 | 3 | 5 | 5 | 60 |
| 3 Mass fraction of silicon oxide (SiO2), %, no less | 65 | 85 | 65 | 85 | 85 ** |
| 4 Mass fraction of loss on ignition, %, no more | 5 | ||||
| 7 Mass fraction of sulfur oxide (SO | |||||
| 3 | |||||
| ), %, no more | 2 | 2 | 2 | 2 | 2 ** |
| 8 Mass fraction of chloride ion (Clˉ), %, no less | 0.1 | ||||
| 70 | |||||
| 95 | |||||
| 70 | 95 | ||||
-
MK: Uncompacted condensed microsilica with a bulk density ranging from 150 to 399 kg/m3.
| 3 | ||
| 5 | ||
| 3 | 5 ** | |
| Cements | 202 | |
| 6 | Durability | 192 |
| 7 | Silica fume | 159 |
| 8 | Fly ash | 154 |
| 9 | Mechanical properties | 152 |
| 10 | Concrete | 141 |
| 11 | Scanning electron microscopy | 137 |
| 12 | Hydration | 123 |
| 13 | Nano-particles | 108 |
| 14 | Tensile strength | 108 |
| 15 | Microstructure | 106 |
| 16 | Mortar | 103 |
| 17 | Concrete mixtures | 99 |
| 18 | Aggregates | 96 |
| 19 | Water absorption | 93 |
| 20 | Portland cement | 87 |
Various minerals and chemicals have been utilized in the production of amorphous silica, with quartz sand standing as the most commonly used raw material containing silica. The methods for processing and obtaining finalized raw materials involve multi-stage and intricate processes employing expensive reagents and complex equipment [30,31,32,33,34,35,36,37,38,40,41][30][31][32][33][34][35][36][37][38][40][41]. The primary industrial source for producing silicon dioxide involves the creation of a silicate block through the fusion of sand with sodium hydroxide at a temperature of 1700 °C. This block is then subjected to boiling in an autoclave under a pressure of 4.8–5.0 atm. while being supplied with steam [30,31][30][31].
Amorphous silicon dioxide with minimal metal impurities (less than 10−3–10−5 wt.%) is formed by sintering quartz foundry sands with ammonium hydrodifluoride (NH4HF2) [33,34,35,36][33][34][35][36]. Additionally, amorphous silicon dioxide (white soot, 99.997% purity) can be derived from processing zirconium concentrate. This involves fluorination with ammonium bifluoride, heat treatment, subsequent condensation, and processing of the resulting halogenation products [30,31][30][31]. A patent search conducted in the library of patents for inventions of the Russian Federation FIPS (Federal Institute of Industrial Property) revealed that most inventions focus on the complex processing of raw materials. Starting materials such as serpentinite, marshalite, diatomite [9[9][10][11],10,11], nepheline, eudialyte, olivine, and fayalite [33,34,35][33][34][35] have been employed.
An established method for producing environmentally friendly precipitated silica involves neutralizing sodium silicate with sulfuric acid [13]. Recently, waste from various industries has piqued researchers’ interest as a source of Si. For instance, amorphous silicon dioxide can be extracted from waste generated in boron production, ash resulting from the combustion of organic fuel, waste from ferroalloy production, and flue dust from the gas purification process in aluminum industry enterprises [28].
Processing ash and slag waste from low-grade ore and non-metallic raw materials can yield foamed X-ray amorphous material (foam silicate) with a consistent chemical composition, forming either thin mineral fibers or spheres based on technological conditions [28,29,30,31,32,33,34,35,36,37,38,39,40][28][29][30][31][32][33][34][35][36][37][38][39][40]. Research [28,38,40,41][28][38][40][41] indicates that the economically efficient disposal of ash and slag waste on a large scale is feasible through the production of building materials and products. In an innovative approach, renewable silicophilic plants like rice, horsetails, feather grass, and oats can serve as an alternative raw material source [23,24][23][24]. Among these, rice production waste, specifically rice husks and rice straw, holds promise due to their high silica content: 14%–20% in rice husks and up to 13.5% in rice straw. The processing methods are straightforward and cost-effective, favoring the extraction of silica from rice straw because of its higher silicon content. Rice husks, concentrated during grain cleaning in mills, offer a more economical processing route [25,26][25][26].
A review [24] explored the use of rice husk ash as a cement substitute in high-strength sustainable concretes. In this context, the focus revolves around micro- and nanosilica derived from industrial waste.
2. General Understanding of the Hydration Processes of Portland Cement
Through the gradual evolution of scientific research methods and the culmination of numerous experimental and theoretical studies, a comprehensive comprehension of the hydration processes of Portland cement has been achieved [25,26,27,28,29][25][26][27][28][29]. The primary mineral within Portland cement clinker, tricalcium silicate 3CaO·SiO2 (alite), comprises 60%–70% of the clinker content. Researchers commonly delineate five principal stages of hydration: the initial, induction, hydration acceleration, hydration deceleration, and hardening stages. In the initial stage, the interaction between tricalcium silicate and water leads to the adsorption of water molecules and partial surface hydration of the binder grain. This phase, lasting no more than 20 min, is marked by heat release. The adsorption of water molecules results in the protonation of the binder surface, forming HnSiO4n+4 hydrosilicate groups and releasing Ca2+ and OH− ions into the liquid phase. Simultaneously, a semi-permeable film of calcium hydrosilicates forms on the surface of the hydrating grains, facilitating the transmission of water to the clinker mineral’s surface and the release of Ca2+ and OH− ions from it [25]. The second stage, known as the induction period, spans from two to six hours. During the fourth and fifth stages, the crystallization of gel-like calcium hydrosilicates occurs, resulting in the development of both an outer layer and an inner layer of hydrosilicate masses [28,29][28][29].3. State of Production of Micro- and Nanosilica in the Russian Federation
Annually, during the production of metallurgical silicon in Russia (RF), approximately 35,000 tons of fine dust are generated, stored in “big bags” weighing 1 ton each, or maintained on slurry fields [27,28,40][27][28][40]. Consequently, this results in an escalation of waste, emphasizing the crucial need for recycling and large-scale utilization from both economic and environmental standpoints [29,30,31,32,33,34,35][29][30][31][32][33][34][35]. The disposal and processing of dust are exclusively executed using extensive metallurgical equipment within sinter production. Only specially prepared waste can be reintegrated into metallurgical processes. Waste agglomeration not only supplies enterprises with supplementary resources but also diminishes their environmental impact [1,2][1][2]. Forecasts indicate a growth trend in microsilica production in Russia by 2025 [1]. The surge in production, reaching 70–80 thousand tons annually, is primarily attributed to the full operation of metallurgical silicon production, driven by a sharp 80% price hike. Notably, the most appealing method of processing microsilica waste, derived from the reduction smelting of quartzite, totaling 30–40 thousand tons per year, is centralized at enterprises such as JSC Kremniy (Irkutsk region) and JSC Ural Silicon (Kamensk-Uralsky) [28,40][28][40]. The selection of a plant relies on its geographical proximity. Further details are outlined in Table 2, offering a comparative assessment, focusing on the fundamental effects of microsilica sourced from various Russian plants and foreign plants [40]. Russia annually produces over 150 thousand tons of microsilica, with production volumes increasing each year. As per table data, it is evident that the chemical composition of Misrosilica (MK) in the presented list is nearly identical.Table 2.
Comparative characteristics of microsilica from various plants.
| Name Plants | Intention | Class | Chemical Composition | ||
|---|---|---|---|---|---|
| Elkem Microsilica Grade 920 ASTM (Norway) | Concrete and construction solutions | Class 920 is available in two forms: unsealed (920 U), bulk density, which is usually 200–350 kg/m3; and pressed (920 D), bulk density—500–700 kg/m3 | SiO2: 85%–90%; SO3: 1%–2%; Cl: 0.1%–0.3%; CaO: 1.0%; Si: 0.2%–0.4%; Na2O: 1%–1.5%; C: 1.5%–2.0% | ||
| Kuznetsk Ferroalloys, Ltd. | Obtaining concretes with special properties: ultrahigh-strength, improved (i) frost, (ii) sulphate, and (iii) corrosion resistance, water-tightness | Unsealed—MS-85, MS-65; compacted—MSC-85, MSC-65; in the form of a suspension—ISS-85 | SiO2: 90%–92%; Al2O3: 0.6%–0.8%; Fe2O3: 0.4%–0.7%; CaO: 0.4%–0.9%; MgO: 0.8%–1.0%; Na2O: 0.6%–0.8%; K2O: 1.2%–1.4%; C: 0.9%–1.2%; S: 0.2%–0.3% | ||
| Chelyabinsk Electrometallurgical Plant, Ltd. | Additive to concrete for improved performance | Unsealed—MS-85, MS-65; compacted—MSC-85, MSC-65; in the form of a suspension—ISS-85 | SiO2: 90%–92%; Al2O3: 0.6%–0.8%; Fe2O | ||
| 5 Mass fraction of free alkalis (in terms of Na2O), %, no more | 2 | 2 | 2 | 2 | 2 ** |
| 6 Mass fraction of calcium oxide (CaO), %, no more | 5 | 3 | 5 | 3 | 2 ** |
| 0.1 | 0.1 | 0.1 | 0.1 ** | ||
| 9 Mass fraction of chromium oxide (in terms of Cr2O3), %, no more | 2.8 | - | |||
| - | |||||
| 13 Bulk density, kg/m3 | 150–399 | 150–399 | 400–600 | ||
- MKU (Microsilica compacted): Compacted fumed microsilica with a bulk density ranging from 400 to 600 kg/m3.
| 2.8 | |||||
| - | |||||
| - | |||||
| 10 Specific surface area of condensed microsilica, m | |||||
| 2 | |||||
| /kg, no less | |||||
| 12,000 | 12,000 | 12,000 | 12,000 | - | |
| 11 Efficiency index, %, no less | 90 | 105 | 90 | 105 | 105 ** |
| 12 Degree of pozzolanic activity, mg/g MS, no less | |||||
| 400–800 | - | ||||
| 14 Density of suspension (paste), kg/m3, no less | - | - | - | - | 1280 |
| 15 Of an aqueous suspension (paste) of microsilica, no less | - | - | - | - | 7 |
* Classification of microsilica by grade is carried out on the basis of the worst quality indicators obtained. ** In paragraphs 3–8, 11, the standards for the suspension (paste) are given in terms of dry matter.
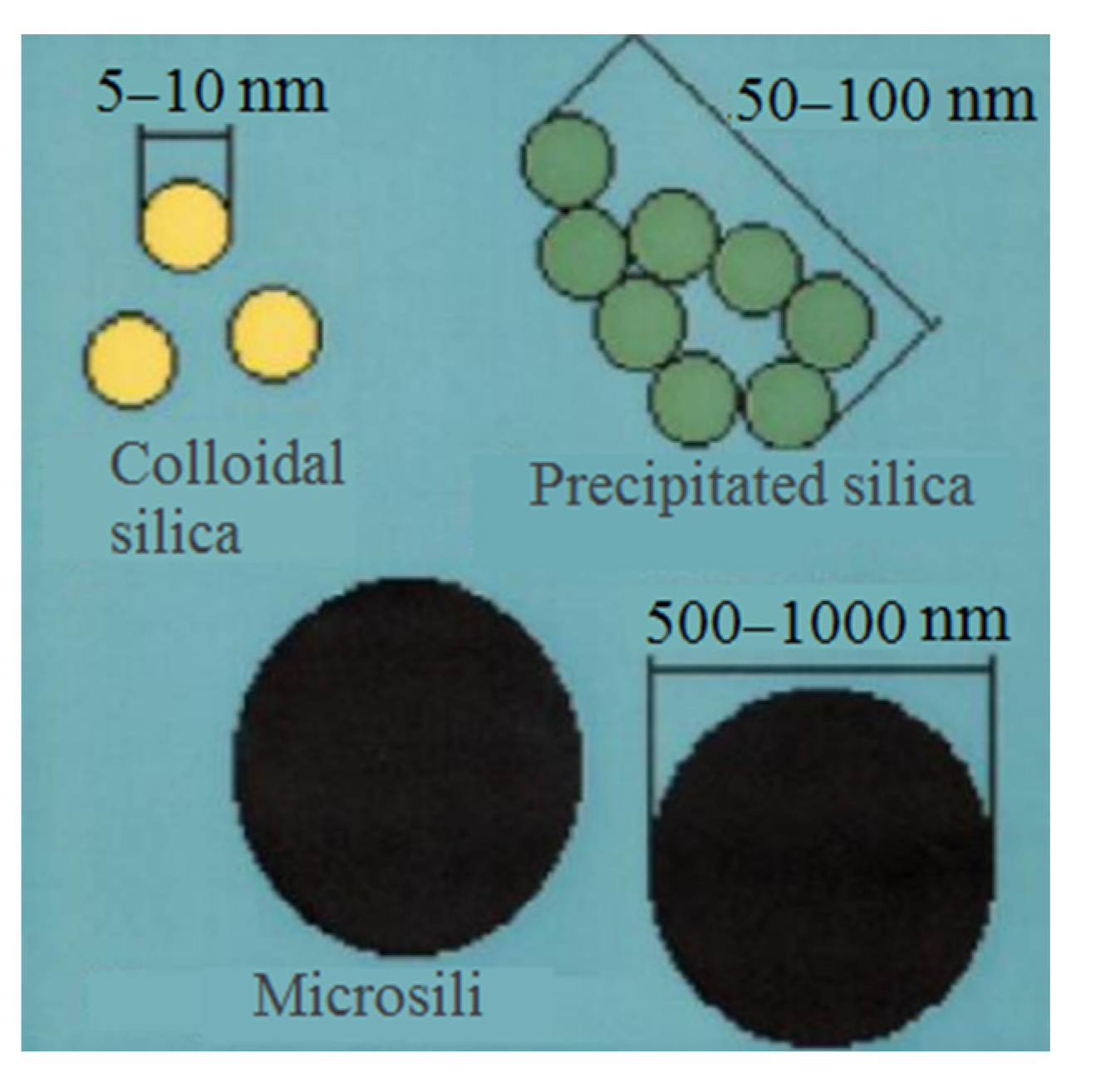
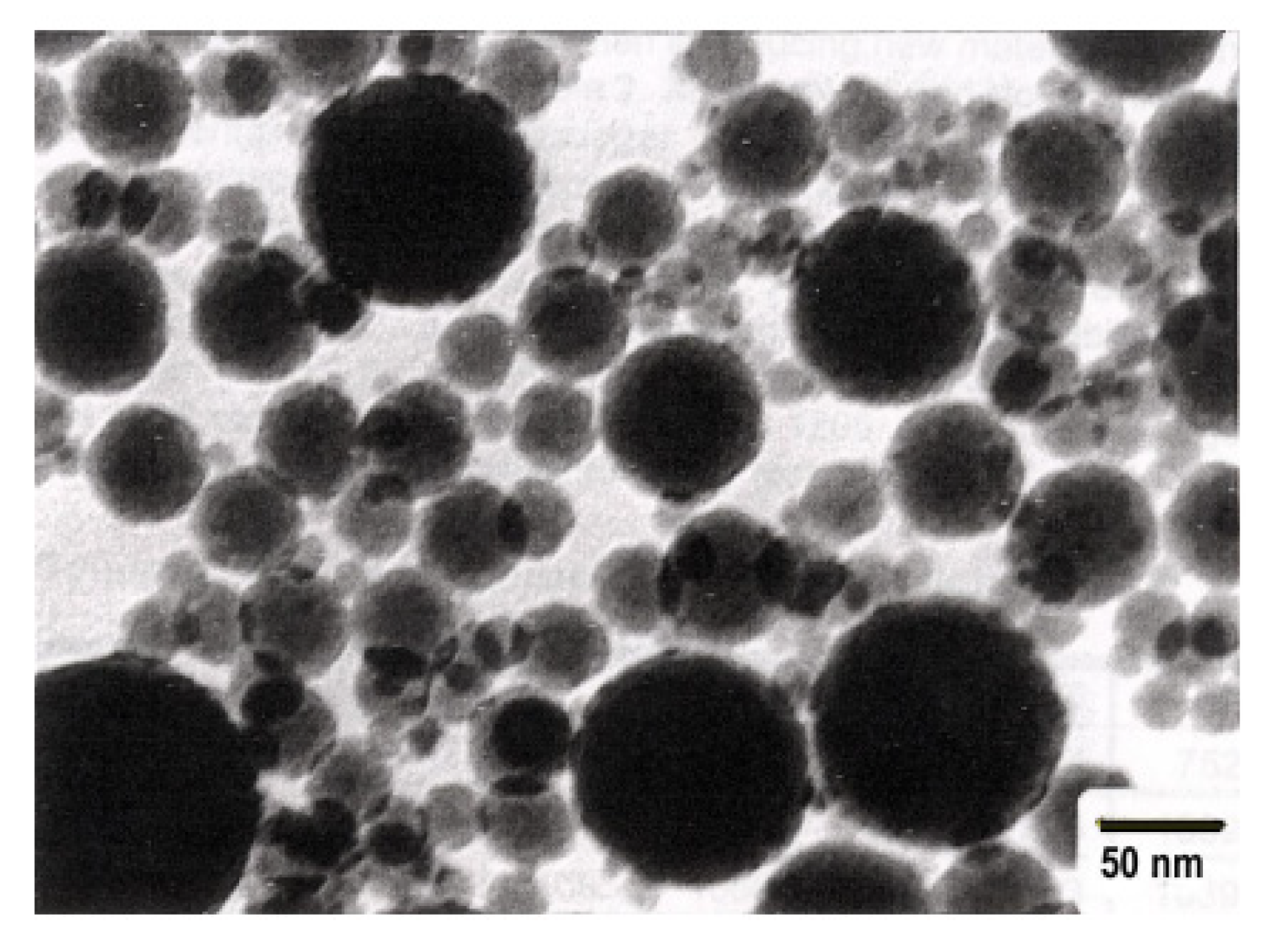
The resulting nanosilica boasts higher dispersion (with particle sizes between 7 and 20 nm) and a specific surface area of approximately 200 m2/g more compared to MS. It also maintains a high degree of purity. Aerosil primarily serves as a filler and curing accelerator in silicone sealants and adhesives. Among all the types of industry-produced ultrafine SiO2, aerosil stands out as the most costly. The raw materials for precipitated and colloidal SiO2, known as hydrochemical silica, originate from aqueous solutions of sodium silicates (liquid glasses). When these solutions undergo acidification, nanodispersed SiO2 particles are initially formed (Figure 7a). The fate of these particles relies on various process conditions such as solution concentration, pH, temperature, the presence of electrolyte salts, and multiply charged cations.
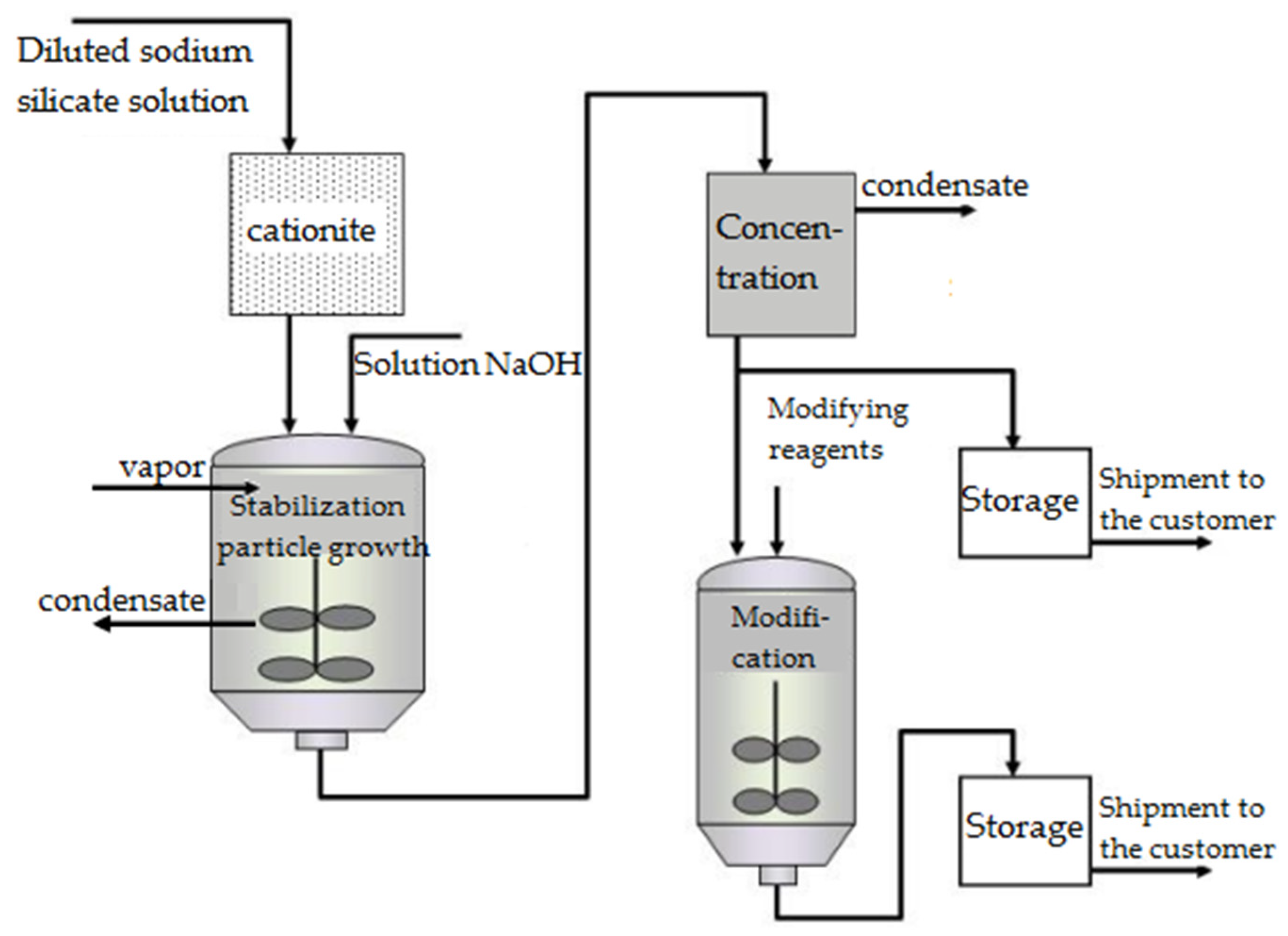
 In the absence of electrolytes and after undergoing specific stabilization in highly diluted conditions, primary SiO2 particles can be enlarged to 10–50 nm by reprecipitating the substance from smaller to larger particles. This process simultaneously raises the SiO2 concentration to 30–50 wt.% while preventing particle aggregation. Under these conditions, stable, concentrated dispersions of colloidal silica, also referred to as silica sol, are produced (Figure 8).
In the absence of electrolytes and after undergoing specific stabilization in highly diluted conditions, primary SiO2 particles can be enlarged to 10–50 nm by reprecipitating the substance from smaller to larger particles. This process simultaneously raises the SiO2 concentration to 30–50 wt.% while preventing particle aggregation. Under these conditions, stable, concentrated dispersions of colloidal silica, also referred to as silica sol, are produced (Figure 8).
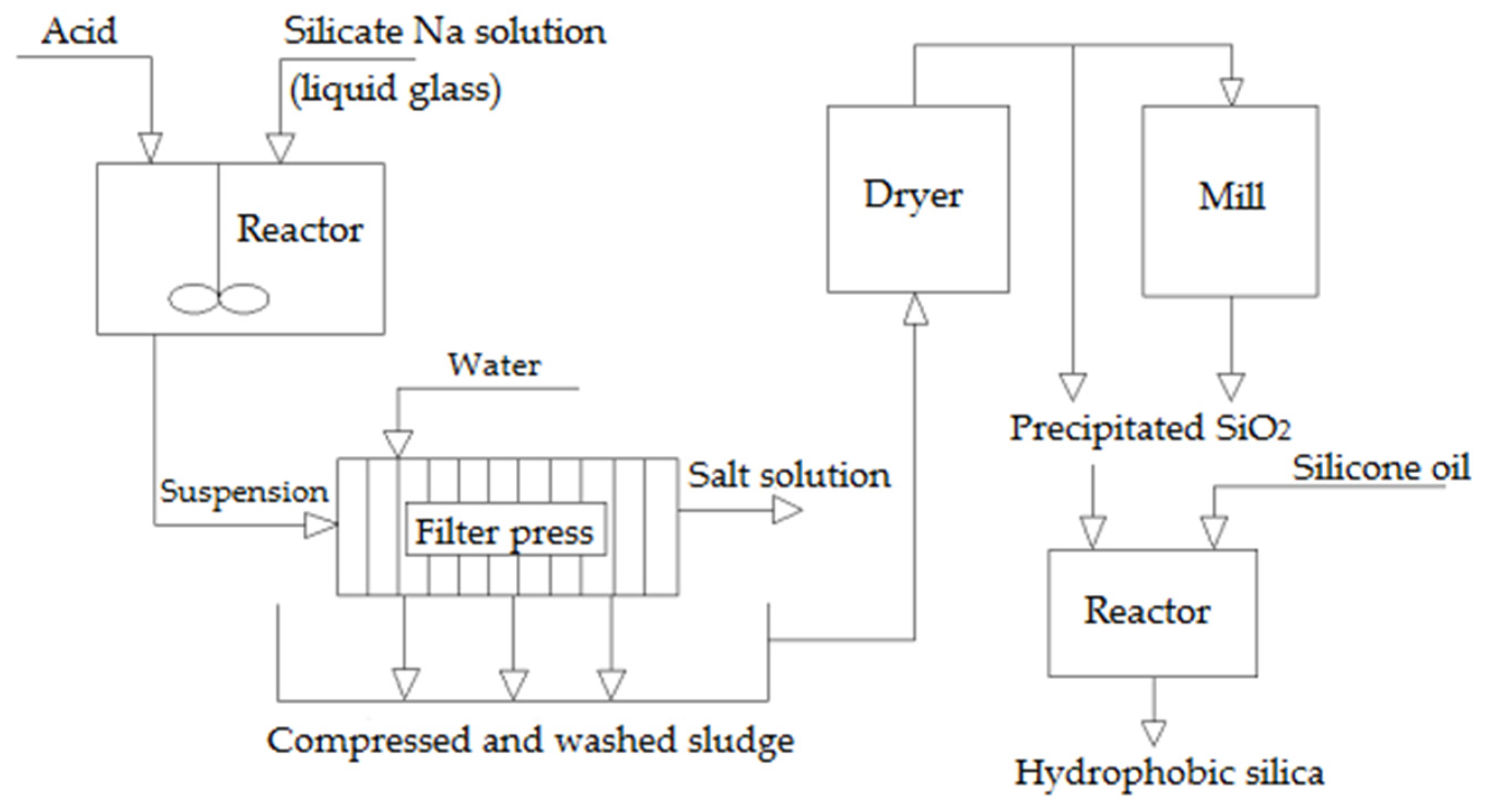
 In industrial processes to produce SiO2 sols, liquid glass is diluted to a SiO2 concentration of 3–5 wt.% and subsequently neutralized by passing it through a column packed with a cation exchanger in the H+ form. A small amount of alkali is added at the outlet to stabilize the particles. The solution is then concentrated by a process involving evaporation combined with sol growth. This operation includes introducing the solution after passing it through the ion exchanger (feeder sol) into the main sol. This allows the introduced silica to reprecipitate onto larger particles (Figure 9). The precipitated silica is filtered and washed with water on filter presses to eliminate salt, and then redissolved and fed as a suspension to a spray dryer (Figure 10). In cases where silicate solutions are acidified to a weakly alkaline medium without removing electrolyte products, for instance, Na2SO4 if the acidification is performed with sulfuric acid, the resulting SiO2 particles grow to 2–5 nm and coagulate to form loose aggregates that lack sintering (Figure 11). This amorphous silica powder, technically known as chemically precipitated SiO2, is an ultra-fine white powder used as a filler in the production of polymeric materials and in the tire, rubber, and light industries. In Russia, it is produced under the brand name “white carbon black”.
For comparison, Table 5 displays the properties of silica produced by well-known global manufacturers, obtained via high-temperature synthetic conditions and hydrochemical methods.
In industrial processes to produce SiO2 sols, liquid glass is diluted to a SiO2 concentration of 3–5 wt.% and subsequently neutralized by passing it through a column packed with a cation exchanger in the H+ form. A small amount of alkali is added at the outlet to stabilize the particles. The solution is then concentrated by a process involving evaporation combined with sol growth. This operation includes introducing the solution after passing it through the ion exchanger (feeder sol) into the main sol. This allows the introduced silica to reprecipitate onto larger particles (Figure 9). The precipitated silica is filtered and washed with water on filter presses to eliminate salt, and then redissolved and fed as a suspension to a spray dryer (Figure 10). In cases where silicate solutions are acidified to a weakly alkaline medium without removing electrolyte products, for instance, Na2SO4 if the acidification is performed with sulfuric acid, the resulting SiO2 particles grow to 2–5 nm and coagulate to form loose aggregates that lack sintering (Figure 11). This amorphous silica powder, technically known as chemically precipitated SiO2, is an ultra-fine white powder used as a filler in the production of polymeric materials and in the tire, rubber, and light industries. In Russia, it is produced under the brand name “white carbon black”.
For comparison, Table 5 displays the properties of silica produced by well-known global manufacturers, obtained via high-temperature synthetic conditions and hydrochemical methods.
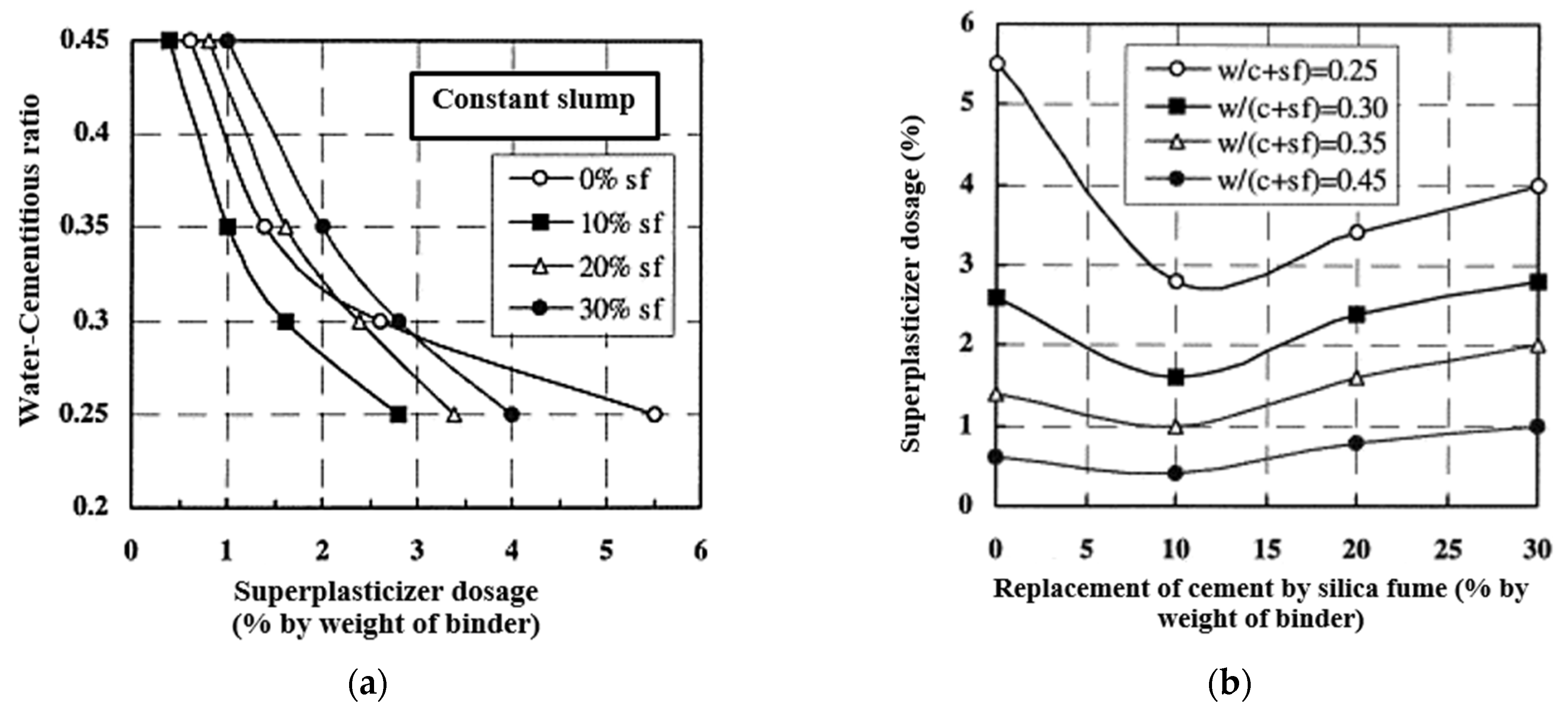 Braulio, M.A.L. et al. and Cheol, MinYoon [51[51][52],52], demonstrated that silica fume concrete exhibited strength comparable to that of concrete made from sulphate-resistant cement. However, no further progress was made in this direction. These studies were conducted amid stringent environmental standards for the metallurgical industry, predominantly concerning industrial gas purification, which incurs substantial costs, especially for multi-stage filtration systems [53,54][53][54].
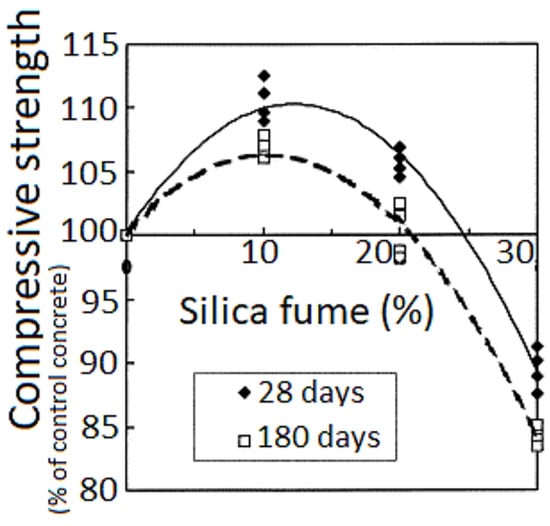
The initial investigation into microsilica’s role as a hardener in concrete mixes took place in Norway [50]. Microsilica was regarded as another essential element in the binary oxide system, including spinel. As indicated in [51[51][55],55], in concrete materials containing Al2O3-MgO, microsilica stabilizes the volumetric expansion linked to spinel formation by creating low-melting-point phases in the Al2O3-CaO-SiO2 system, such as anorthite (CaS2) and helenite (Ca2Al [AlSiO7]). For products composed of a blend of alumina and spinel, silica fume mainly contributes to enhancing the flow coefficient due to its suitable morphology, thereby improving the processing of the final products. Over time, the compressive strength of the control concrete matches that of concrete with 10% and 20% silica fume [46]. Figure 13 illustrates that compressive strength generally increases as the w/(c + sf) ratio declines, emphasizing that the water and cementitious material ratio plays a more significant role than the introduction of microsilica. An analysis of the curves reveals that the optimal cement replacement with microsilica is around 10%–15%.
Braulio, M.A.L. et al. and Cheol, MinYoon [51[51][52],52], demonstrated that silica fume concrete exhibited strength comparable to that of concrete made from sulphate-resistant cement. However, no further progress was made in this direction. These studies were conducted amid stringent environmental standards for the metallurgical industry, predominantly concerning industrial gas purification, which incurs substantial costs, especially for multi-stage filtration systems [53,54][53][54].
The initial investigation into microsilica’s role as a hardener in concrete mixes took place in Norway [50]. Microsilica was regarded as another essential element in the binary oxide system, including spinel. As indicated in [51[51][55],55], in concrete materials containing Al2O3-MgO, microsilica stabilizes the volumetric expansion linked to spinel formation by creating low-melting-point phases in the Al2O3-CaO-SiO2 system, such as anorthite (CaS2) and helenite (Ca2Al [AlSiO7]). For products composed of a blend of alumina and spinel, silica fume mainly contributes to enhancing the flow coefficient due to its suitable morphology, thereby improving the processing of the final products. Over time, the compressive strength of the control concrete matches that of concrete with 10% and 20% silica fume [46]. Figure 13 illustrates that compressive strength generally increases as the w/(c + sf) ratio declines, emphasizing that the water and cementitious material ratio plays a more significant role than the introduction of microsilica. An analysis of the curves reveals that the optimal cement replacement with microsilica is around 10%–15%.
 In their work [53], the authors investigated the impact of introducing microsilica additives into aluminum–magnesium compositions. They noted that an increasing microsilica content led to higher volumetric expansion coefficients of spinel. Additionally, the study highlighted the considerable influence of microsilica on the development of CaO-Al2O3 phases. Compositions with minimal microsilica content (0 and 0.25 wt.%) exhibited notable changes in the sample’s linear dimensions due to the limited interaction between Al2O3 and SiO2. This limited interaction also affected the reaction with CaO, maintaining conditions for CaO to form as a separate phase. Conversely, when microsilica was introduced, interactions among SiO2-Al2O3-CaO led to the formation of low melting point phases, consequently reducing the linear expansion coefficient values. During CaO formation, higher linear dimension growth rates were observed, influenced by a diffusion mechanism limited by the rate of liquid phase formation. Structural analysis revealed the formation of acicular calcium oxide in the sample matrix, which, on the contrary, weakened it.
On the other hand, the absence of microsilica in the mixture resulted in the formation of crystals directed inward toward the alumina filler, leading to a slower increase in the expansion coefficient. The role of microsilica in the formation of needle phases of alumina and spinel was also investigated in [56], considering variations in microsilica content from 0 to 1 wt.%. The obtained results were compared with those from the Al2O3-MgO system in [57]. The findings showed that even small amounts of silica significantly influenced the development of the calcium oxide grain structure in these oxide systems. This underscored the need to consider microstructural changes when developing refractory samples with enhanced properties.
Mermerdaş, K. et al. [58] presented the experimental determination of the effects of steel fiber, granulated ash, lightweight filler, and varying microsilica content on the properties of a high-performance cementitious composite in its green and annealed states was conducted. The mixtures were prepared using 6 mm long steel microfibers at concentrations of 0%, 1%, and 2% of the total volume. Silica fume content ranged from 1% to 25% in the mixes. The results of the experiment indicated that the mechanical properties of HPCC and the shrinkage behavior improved with an increasing volume fraction of steel fibers. This study also demonstrated that the detrimental impact of artificial lightweight filler could be offset by the addition of microsilica. Furthermore, research Kuz’min, M.P. et al. [59] outlined calculations related to the enthalpy and Gibbs free energy in the direct reduction in silica with aluminum, showcasing the potential for producing silumins (Al-Si alloys) using amorphous microsilica. Additionally, this research identified the influence of alloying additions and impurities on the process of silicon reduction in the melt.
A substantial body of scientific literature is dedicated to exploring concrete mixes containing microsilica. For instance, in [60], findings highlighted a significant correlation between the particle surface area increase and internal surface forces, suggesting a boost in concrete’s cohesive ability. This strengthening effect is in line with anticipated shrinkage values during the formation of concrete mix samples, supporting the notion of silica fume serving as a plasticizer. It appears that microsilica from silicon production, given a particular structure and polymorphism, can potentially perform a similar plasticizing function. When an external physical force, such as pumping, vibration, or tamping, is applied to the mix, the spherical microsilica particles act as a transmission mechanism, imparting greater mobility through sliding forces than ordinary concrete with a standard additive.
A comprehensive exploration of this effect is detailed by Xu, W. et al. [61], where the introduction of microsilica leads to a decrease in the viscosity (plasticity) of the material with a slight increase in the shear strength coefficient for a wet concrete mix. An analysis outlined in another scientific paper [63] revealed that the structure and properties of mixes are influenced by the source of the microsilica, specifically the production to which this waste belongs. In general, the formation of products from concrete mixes is also affected by the form of the microsilica additive used, regardless of whether the powder is uncompacted or compacted, and the moisture content in suspension. Fumed silica, with high specific surface areas (typically 15,000–30,000 m2/kg), demands more moisture for mixing, a need that can be partially offset by additional procedures to alter the mix’s composition, such as reducing the fine particle content during screening [53].
Currently, silica fume concrete mixes are frequently developed using the selective particle packing method. This technique involves aggregating ultra-fine spherical particles into a total volume after sorting the mixture by size, thereby achieving a balance between small and large materials and reducing the need for additional water, which preserves the rheological properties of the concrete. Due to its higher degree of cohesion, silica fume-modified concrete is less prone to segregation than conventional concrete [62]. This reduced tendency to segregate is also advantageous in highly fluid mortars or pumpable concrete mixes. The inclusion of a small amount of silica fume into the mix for transportation acts as a pumping aid, enhancing the fluidity of the mix and providing excellent long-distance transport properties [63].
Another outcome of the cohesion effects is that concrete containing silica fume essentially retains moisture within the mix. The absence of water drainage also means that the emergence of flat shapes and structures, such as power fusion, can occur much earlier than with conventional concrete. This occurrence is influenced by increased shear resistance and a tendency toward gelation (solidification without stirring), evident in the higher rates of hardening in the form of a pozzolan. However, this so-called pure pozzolan is initiated by the presence of calcium hydroxide [64]. The necessary amount of calcium hydroxide is obtained via cement hydration, so the pozzolanic reaction is only possible after the cement has started reacting. The curing time for the modified concrete is almost comparable to that of the standard composition concrete. As the concrete solidifies, the chemical effect of the microsilica has a greater influence on the structure and properties of the concrete mix compared to the physical effect. Microsilica primarily reacts with calcium hydroxide to form calcium silicate hydrates (C-S-H). This enhances the binder content, which increases strength but reduces the mix’s permeability by compacting the concrete matrix [66]. Due to its significantly large specific surface area compared to the particle surface area of the primary matrix in the concrete mix, the high silica content determines its reactivity compared to other potential additives like ash, crushed and granulated slag, and alumina [28].
This observation can also be applied to the study of refractory materials. Some researchers have discovered [66,67][66][67] that heightened reactivity initially expedites the cement fraction’s hydration and paves the way for a substantial release of calcium hydroxide, subsequently reducing the interaction rate between mix components by a factor of 2. The study showcased that as microsilica reacts and forms calcium hydrates and silicates, they fill the voids and pores in the concrete, creating a dense packing between the cement grains and filler particles. The combined chemical and physical action on the mixed components results in its uniformity and density throughout the product’s volume. Primarily, this decreases the material’s porosity, which can be instrumental in the development of refractory modification technology using microsilica.
Another study [68] revealed that a relatively porous interface rich in Portland cement encircles the filler particles in standard packaged concrete but is virtually absent when silica fume is introduced into the mix. Modified concrete generates less heat than standard Portland cement within the same curing time. This reduction occurs because the amount of cement within the mix is diminished, consequently reducing the overall heat generated in the initial stages. This is attributed to the fact that silica fume, added at a third of the cement level, begins reacting later than the release of calcium hydroxide and therefore does not generate as much heat as standard cement during its interaction and mixing.
Studies by Glazev, M.V. et al. and Zimina, D.A. et al. [69,70][69][70] have demonstrated the sensitivity of microsilica-modified concrete to temperature fluctuations during its hardening process. Under low temperatures, there is a decrease in the rate of hydration and strength enhancement, whereas elevated temperatures result in a sharp increase in these properties. An analysis of earlier studies [69,70][69][70] unveiled that the addition of microsilica significantly bolsters the strength of a concrete mix. The degree of strength augmentation is contingent on several variables, including the mix type, cement quality, microsilica quantity, water-soluble additive volume, filler characteristics, and curing conditions. Silica fume concrete appears to adhere to the conventional strength-to-water/cement (w/c) ratio relationship. However, the curing process is affected by the inclusion of silica fume, leading to rapid moisture loss in modified concretes, fostering the development of voids and cracks. This phenomenon may result in a decrease in the concrete’s final strength. Conversely, the introduction of ash can reduce shrinkage porosity during solidification [40], showcasing the potential positive impact of silica production in microsilica.
The recycling and utilization of fine waste material from silicon production (microsilica) constitute a crucial avenue for conserving resources [28,40,41][28][40][41]. The comprehensive utilization of waste across various industries empowers companies to allocate additional resources and curtail environmental impacts. For instance, in the oil refining sector, the advancement of technologies and materials for well construction is imperative to meet contemporary standards for well reliability and strength. While the addition of microsilica has been suggested to enhance the properties of cement slurries [69], this fortifying aspect has yet to be wholly proven and substantiated scientifically. Numerous studies have been undertaken to fortify the resistance of the cement ring against dynamic loading. Different methodologies have been explored to address this issue.
Most researchers argue that Portland cement, despite its advantages, is marred by a significant drawback: as the strength of the cement stone increases, its fragility rises, accompanied by low strength [70,71][70][71]. Hence, targeted enhancements in expansive cement mixtures for cementing wells in permafrost regions present considerable interest, offering the potential to modify properties such as strength, frost resistance, expansion, and more [69].
Analysis of the microsilica’s structure demonstrates that the use of silica nanoparticles instigates significant alterations in the material. Notably, it leads to substantial compaction and improvement in the mechanical properties of the cement stone (resulting in a 3–6 fold increase in strength). Moreover, material modification with silica nanoparticles stabilizes key valence interactions such as Ca-Si-H, which is pivotal to concrete cohesion. This modification diminishes calcium leaching and enhances moisture resistance [65,66,67,68,69,70][65][66][67][68][69][70]. By judiciously selecting and developing high-strength refractory concrete mixes, these can be manufactured using standard ready-mix plants. A slight elevation in compressive strength with silica fume in concrete directly yields heightened tensile and flexural strength, following a trend akin to standard concrete [66,67,68][66][67][68].
High-strength concrete often displays brittleness, and silica-modified concrete is no different. In this scenario, the modulus of elasticity does not correlate proportionally to the compressive force. The maximum value of the force causing deformation before failure under uniaxial compression rises with increased strength. However, the stress–strain curve before such failure is typically linear, as observed in [72]. Employing particle packing technology aids in enhancing plasticity by virtue of the particles’ sliding effect, thereby reducing brittleness when altering the filler-to-cement ratio. Consequently, this improves the overall integrity of the refractory concrete mix [73]. Enhanced particle adhesion is achieved through the close contact of small particles between microsilica concrete and the base of the matrix, serving as reinforcement [66,67,68,69,70][66][67][68][69][70]. The shrinkage of microsilica concrete mirrors that of conventional concrete specimens. Yet, due to slower moisture removal, the shrinkage of microsilica concrete is more gradual. For standard tests, this implies that the observed shrinkage in fumed silica concrete is lesser than that in standard concrete specimens [62].
Various studies on refractory mixes [67,68,69][67][68][69] have revealed that modified concretes behave similarly to standard concretes under usual heating conditions. This adaptation allows them to be effectively tailored for their intended use in producing new kinds of refractories for metallurgical furnaces. The present diffusion mechanism in this process appears to resist vapor movement while removing residual moisture. During an intense fire test on the impregnated concrete, the low permeability of silica fume concrete thwarts vapor from escaping to the outer surface. As a consequence, crack spalling (internal fracture) occurs due to excessive vapor pressure within the matrix.
Modified concretes conditioned with high mix hardening rates, as previously described, will not fracture, provided the water/cement ratio is optimally chosen. It is imperative to consider the coefficient of thermal expansion, which impacts the fire resistance of the concrete [61]. Thus, existing technologies suggest that, under equivalent technological conditions, incorporating microsilica into the concrete mix as a modifier establishes a stable structure and enhances the properties of refractory concrete for the sustainable operation of metallurgical units.
It is important to note that microsilica has an ambivalent effect on the mobility of the cement paste. Consequently, the rheological properties of the cement paste change significantly, resulting in increased effective viscosity and plastic strength [69]. It has also been observed that the effect of microsilica on the rheological properties of the cement paste relies on the type and content of the superplasticizer [60,61,62,63,64,65,66,67,68,69,70,71,72][60][61][62][63][64][65][66][67][68][69][70][71][72].
One demonstration of the pozzolanic activity of microsilica is the enhancement of the microstructure of the cement stone and the consolidation of the contact zone between the cement stone and the aggregate. In concrete, the interface primarily forms portlandite and ettringite crystals. Strengthening the contact zone enhances the adhesion of the cement stone to the aggregate, influencing the strength, crack resistance, and increasing resistance to frost and water [61,62][61][62].
In their work [53], the authors investigated the impact of introducing microsilica additives into aluminum–magnesium compositions. They noted that an increasing microsilica content led to higher volumetric expansion coefficients of spinel. Additionally, the study highlighted the considerable influence of microsilica on the development of CaO-Al2O3 phases. Compositions with minimal microsilica content (0 and 0.25 wt.%) exhibited notable changes in the sample’s linear dimensions due to the limited interaction between Al2O3 and SiO2. This limited interaction also affected the reaction with CaO, maintaining conditions for CaO to form as a separate phase. Conversely, when microsilica was introduced, interactions among SiO2-Al2O3-CaO led to the formation of low melting point phases, consequently reducing the linear expansion coefficient values. During CaO formation, higher linear dimension growth rates were observed, influenced by a diffusion mechanism limited by the rate of liquid phase formation. Structural analysis revealed the formation of acicular calcium oxide in the sample matrix, which, on the contrary, weakened it.
On the other hand, the absence of microsilica in the mixture resulted in the formation of crystals directed inward toward the alumina filler, leading to a slower increase in the expansion coefficient. The role of microsilica in the formation of needle phases of alumina and spinel was also investigated in [56], considering variations in microsilica content from 0 to 1 wt.%. The obtained results were compared with those from the Al2O3-MgO system in [57]. The findings showed that even small amounts of silica significantly influenced the development of the calcium oxide grain structure in these oxide systems. This underscored the need to consider microstructural changes when developing refractory samples with enhanced properties.
Mermerdaş, K. et al. [58] presented the experimental determination of the effects of steel fiber, granulated ash, lightweight filler, and varying microsilica content on the properties of a high-performance cementitious composite in its green and annealed states was conducted. The mixtures were prepared using 6 mm long steel microfibers at concentrations of 0%, 1%, and 2% of the total volume. Silica fume content ranged from 1% to 25% in the mixes. The results of the experiment indicated that the mechanical properties of HPCC and the shrinkage behavior improved with an increasing volume fraction of steel fibers. This study also demonstrated that the detrimental impact of artificial lightweight filler could be offset by the addition of microsilica. Furthermore, research Kuz’min, M.P. et al. [59] outlined calculations related to the enthalpy and Gibbs free energy in the direct reduction in silica with aluminum, showcasing the potential for producing silumins (Al-Si alloys) using amorphous microsilica. Additionally, this research identified the influence of alloying additions and impurities on the process of silicon reduction in the melt.
A substantial body of scientific literature is dedicated to exploring concrete mixes containing microsilica. For instance, in [60], findings highlighted a significant correlation between the particle surface area increase and internal surface forces, suggesting a boost in concrete’s cohesive ability. This strengthening effect is in line with anticipated shrinkage values during the formation of concrete mix samples, supporting the notion of silica fume serving as a plasticizer. It appears that microsilica from silicon production, given a particular structure and polymorphism, can potentially perform a similar plasticizing function. When an external physical force, such as pumping, vibration, or tamping, is applied to the mix, the spherical microsilica particles act as a transmission mechanism, imparting greater mobility through sliding forces than ordinary concrete with a standard additive.
A comprehensive exploration of this effect is detailed by Xu, W. et al. [61], where the introduction of microsilica leads to a decrease in the viscosity (plasticity) of the material with a slight increase in the shear strength coefficient for a wet concrete mix. An analysis outlined in another scientific paper [63] revealed that the structure and properties of mixes are influenced by the source of the microsilica, specifically the production to which this waste belongs. In general, the formation of products from concrete mixes is also affected by the form of the microsilica additive used, regardless of whether the powder is uncompacted or compacted, and the moisture content in suspension. Fumed silica, with high specific surface areas (typically 15,000–30,000 m2/kg), demands more moisture for mixing, a need that can be partially offset by additional procedures to alter the mix’s composition, such as reducing the fine particle content during screening [53].
Currently, silica fume concrete mixes are frequently developed using the selective particle packing method. This technique involves aggregating ultra-fine spherical particles into a total volume after sorting the mixture by size, thereby achieving a balance between small and large materials and reducing the need for additional water, which preserves the rheological properties of the concrete. Due to its higher degree of cohesion, silica fume-modified concrete is less prone to segregation than conventional concrete [62]. This reduced tendency to segregate is also advantageous in highly fluid mortars or pumpable concrete mixes. The inclusion of a small amount of silica fume into the mix for transportation acts as a pumping aid, enhancing the fluidity of the mix and providing excellent long-distance transport properties [63].
Another outcome of the cohesion effects is that concrete containing silica fume essentially retains moisture within the mix. The absence of water drainage also means that the emergence of flat shapes and structures, such as power fusion, can occur much earlier than with conventional concrete. This occurrence is influenced by increased shear resistance and a tendency toward gelation (solidification without stirring), evident in the higher rates of hardening in the form of a pozzolan. However, this so-called pure pozzolan is initiated by the presence of calcium hydroxide [64]. The necessary amount of calcium hydroxide is obtained via cement hydration, so the pozzolanic reaction is only possible after the cement has started reacting. The curing time for the modified concrete is almost comparable to that of the standard composition concrete. As the concrete solidifies, the chemical effect of the microsilica has a greater influence on the structure and properties of the concrete mix compared to the physical effect. Microsilica primarily reacts with calcium hydroxide to form calcium silicate hydrates (C-S-H). This enhances the binder content, which increases strength but reduces the mix’s permeability by compacting the concrete matrix [66]. Due to its significantly large specific surface area compared to the particle surface area of the primary matrix in the concrete mix, the high silica content determines its reactivity compared to other potential additives like ash, crushed and granulated slag, and alumina [28].
This observation can also be applied to the study of refractory materials. Some researchers have discovered [66,67][66][67] that heightened reactivity initially expedites the cement fraction’s hydration and paves the way for a substantial release of calcium hydroxide, subsequently reducing the interaction rate between mix components by a factor of 2. The study showcased that as microsilica reacts and forms calcium hydrates and silicates, they fill the voids and pores in the concrete, creating a dense packing between the cement grains and filler particles. The combined chemical and physical action on the mixed components results in its uniformity and density throughout the product’s volume. Primarily, this decreases the material’s porosity, which can be instrumental in the development of refractory modification technology using microsilica.
Another study [68] revealed that a relatively porous interface rich in Portland cement encircles the filler particles in standard packaged concrete but is virtually absent when silica fume is introduced into the mix. Modified concrete generates less heat than standard Portland cement within the same curing time. This reduction occurs because the amount of cement within the mix is diminished, consequently reducing the overall heat generated in the initial stages. This is attributed to the fact that silica fume, added at a third of the cement level, begins reacting later than the release of calcium hydroxide and therefore does not generate as much heat as standard cement during its interaction and mixing.
Studies by Glazev, M.V. et al. and Zimina, D.A. et al. [69,70][69][70] have demonstrated the sensitivity of microsilica-modified concrete to temperature fluctuations during its hardening process. Under low temperatures, there is a decrease in the rate of hydration and strength enhancement, whereas elevated temperatures result in a sharp increase in these properties. An analysis of earlier studies [69,70][69][70] unveiled that the addition of microsilica significantly bolsters the strength of a concrete mix. The degree of strength augmentation is contingent on several variables, including the mix type, cement quality, microsilica quantity, water-soluble additive volume, filler characteristics, and curing conditions. Silica fume concrete appears to adhere to the conventional strength-to-water/cement (w/c) ratio relationship. However, the curing process is affected by the inclusion of silica fume, leading to rapid moisture loss in modified concretes, fostering the development of voids and cracks. This phenomenon may result in a decrease in the concrete’s final strength. Conversely, the introduction of ash can reduce shrinkage porosity during solidification [40], showcasing the potential positive impact of silica production in microsilica.
The recycling and utilization of fine waste material from silicon production (microsilica) constitute a crucial avenue for conserving resources [28,40,41][28][40][41]. The comprehensive utilization of waste across various industries empowers companies to allocate additional resources and curtail environmental impacts. For instance, in the oil refining sector, the advancement of technologies and materials for well construction is imperative to meet contemporary standards for well reliability and strength. While the addition of microsilica has been suggested to enhance the properties of cement slurries [69], this fortifying aspect has yet to be wholly proven and substantiated scientifically. Numerous studies have been undertaken to fortify the resistance of the cement ring against dynamic loading. Different methodologies have been explored to address this issue.
Most researchers argue that Portland cement, despite its advantages, is marred by a significant drawback: as the strength of the cement stone increases, its fragility rises, accompanied by low strength [70,71][70][71]. Hence, targeted enhancements in expansive cement mixtures for cementing wells in permafrost regions present considerable interest, offering the potential to modify properties such as strength, frost resistance, expansion, and more [69].
Analysis of the microsilica’s structure demonstrates that the use of silica nanoparticles instigates significant alterations in the material. Notably, it leads to substantial compaction and improvement in the mechanical properties of the cement stone (resulting in a 3–6 fold increase in strength). Moreover, material modification with silica nanoparticles stabilizes key valence interactions such as Ca-Si-H, which is pivotal to concrete cohesion. This modification diminishes calcium leaching and enhances moisture resistance [65,66,67,68,69,70][65][66][67][68][69][70]. By judiciously selecting and developing high-strength refractory concrete mixes, these can be manufactured using standard ready-mix plants. A slight elevation in compressive strength with silica fume in concrete directly yields heightened tensile and flexural strength, following a trend akin to standard concrete [66,67,68][66][67][68].
High-strength concrete often displays brittleness, and silica-modified concrete is no different. In this scenario, the modulus of elasticity does not correlate proportionally to the compressive force. The maximum value of the force causing deformation before failure under uniaxial compression rises with increased strength. However, the stress–strain curve before such failure is typically linear, as observed in [72]. Employing particle packing technology aids in enhancing plasticity by virtue of the particles’ sliding effect, thereby reducing brittleness when altering the filler-to-cement ratio. Consequently, this improves the overall integrity of the refractory concrete mix [73]. Enhanced particle adhesion is achieved through the close contact of small particles between microsilica concrete and the base of the matrix, serving as reinforcement [66,67,68,69,70][66][67][68][69][70]. The shrinkage of microsilica concrete mirrors that of conventional concrete specimens. Yet, due to slower moisture removal, the shrinkage of microsilica concrete is more gradual. For standard tests, this implies that the observed shrinkage in fumed silica concrete is lesser than that in standard concrete specimens [62].
Various studies on refractory mixes [67,68,69][67][68][69] have revealed that modified concretes behave similarly to standard concretes under usual heating conditions. This adaptation allows them to be effectively tailored for their intended use in producing new kinds of refractories for metallurgical furnaces. The present diffusion mechanism in this process appears to resist vapor movement while removing residual moisture. During an intense fire test on the impregnated concrete, the low permeability of silica fume concrete thwarts vapor from escaping to the outer surface. As a consequence, crack spalling (internal fracture) occurs due to excessive vapor pressure within the matrix.
Modified concretes conditioned with high mix hardening rates, as previously described, will not fracture, provided the water/cement ratio is optimally chosen. It is imperative to consider the coefficient of thermal expansion, which impacts the fire resistance of the concrete [61]. Thus, existing technologies suggest that, under equivalent technological conditions, incorporating microsilica into the concrete mix as a modifier establishes a stable structure and enhances the properties of refractory concrete for the sustainable operation of metallurgical units.
It is important to note that microsilica has an ambivalent effect on the mobility of the cement paste. Consequently, the rheological properties of the cement paste change significantly, resulting in increased effective viscosity and plastic strength [69]. It has also been observed that the effect of microsilica on the rheological properties of the cement paste relies on the type and content of the superplasticizer [60,61,62,63,64,65,66,67,68,69,70,71,72][60][61][62][63][64][65][66][67][68][69][70][71][72].
One demonstration of the pozzolanic activity of microsilica is the enhancement of the microstructure of the cement stone and the consolidation of the contact zone between the cement stone and the aggregate. In concrete, the interface primarily forms portlandite and ettringite crystals. Strengthening the contact zone enhances the adhesion of the cement stone to the aggregate, influencing the strength, crack resistance, and increasing resistance to frost and water [61,62][61][62].
Reches, Y. [82] indicates that nanoparticles serve as highly effective additives for modifying cement products, even at low concentrations (<1%). The principal modifications, as depicted in Figure 14 (displaying typical ranges while acknowledging significant variability), include a reduction in setting time (by 1–2 h) and diffusion coefficient (by 4%–75%), along with an increase in strength (by 5%–25%) and thermal durability (an increase of 0%–30% in residual strength).
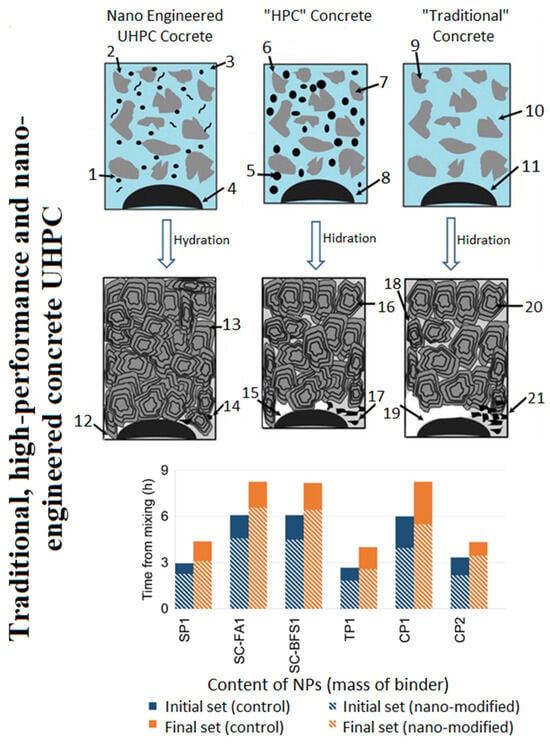 Figure 14 1—nano-particles/fibers; 2,6,9—cement; 3,7,10—water; 5—silica fume/fly ash; 4,8,11—aggregate; 13,16,11—C-S-H phases; 14,17,21—ettringite; 18—capillary pores; 12,15,19—portlandite; (SP1-amorphous silicon dioxide (colloidal) particle size 15 nm; SC-FA1-amorphous silicon dioxide (colloidal) particle size 15 nm plus mineral additive; SC-BFS1—amorphous silicon dioxide (colloidal) particle size 10 nm plus mineral additive; TP1-titanium dioxide (colloidal) particle size 15 nm; CP1-CaCO3 particles 50–120 nm in size; CP2-CaCO3 particles 15–40 nm in size) [46]
Two possible reaction mechanisms exist during cement hydration in the presence of nanosilica [89,90,91,92,93,94,95][89][90][91][92][93][94][95]. Nanosilica addition can accelerate cement hydration. When nanosilica combines with cement grains, it forms H2SiO42− that reacts with existing Ca2+ to produce extra calcium silicate hydrate (C-S-H). These C-S-H particles disperse in the water between the cement particles, acting as seeds for a more compact C-S-H phase. The creation of the C-S-H phase no longer occurs solely at the grain surface, as with pure C3S, but also within the pore space. This high number of nuclei speeds up the initial cement hydration (Figure 14). Nano-SiO2 (nS) altered the characteristics of the fresh solutions.
Reches, Y. [82] illustrated the hydration process schematically in Figure 15. The figure portrays the microstructure of hydration, from the initial to the late stage (left columns), and the temporal evolution of the volume fraction of various cementitious phases (right column).
Figure 14 1—nano-particles/fibers; 2,6,9—cement; 3,7,10—water; 5—silica fume/fly ash; 4,8,11—aggregate; 13,16,11—C-S-H phases; 14,17,21—ettringite; 18—capillary pores; 12,15,19—portlandite; (SP1-amorphous silicon dioxide (colloidal) particle size 15 nm; SC-FA1-amorphous silicon dioxide (colloidal) particle size 15 nm plus mineral additive; SC-BFS1—amorphous silicon dioxide (colloidal) particle size 10 nm plus mineral additive; TP1-titanium dioxide (colloidal) particle size 15 nm; CP1-CaCO3 particles 50–120 nm in size; CP2-CaCO3 particles 15–40 nm in size) [46]
Two possible reaction mechanisms exist during cement hydration in the presence of nanosilica [89,90,91,92,93,94,95][89][90][91][92][93][94][95]. Nanosilica addition can accelerate cement hydration. When nanosilica combines with cement grains, it forms H2SiO42− that reacts with existing Ca2+ to produce extra calcium silicate hydrate (C-S-H). These C-S-H particles disperse in the water between the cement particles, acting as seeds for a more compact C-S-H phase. The creation of the C-S-H phase no longer occurs solely at the grain surface, as with pure C3S, but also within the pore space. This high number of nuclei speeds up the initial cement hydration (Figure 14). Nano-SiO2 (nS) altered the characteristics of the fresh solutions.
Reches, Y. [82] illustrated the hydration process schematically in Figure 15. The figure portrays the microstructure of hydration, from the initial to the late stage (left columns), and the temporal evolution of the volume fraction of various cementitious phases (right column).
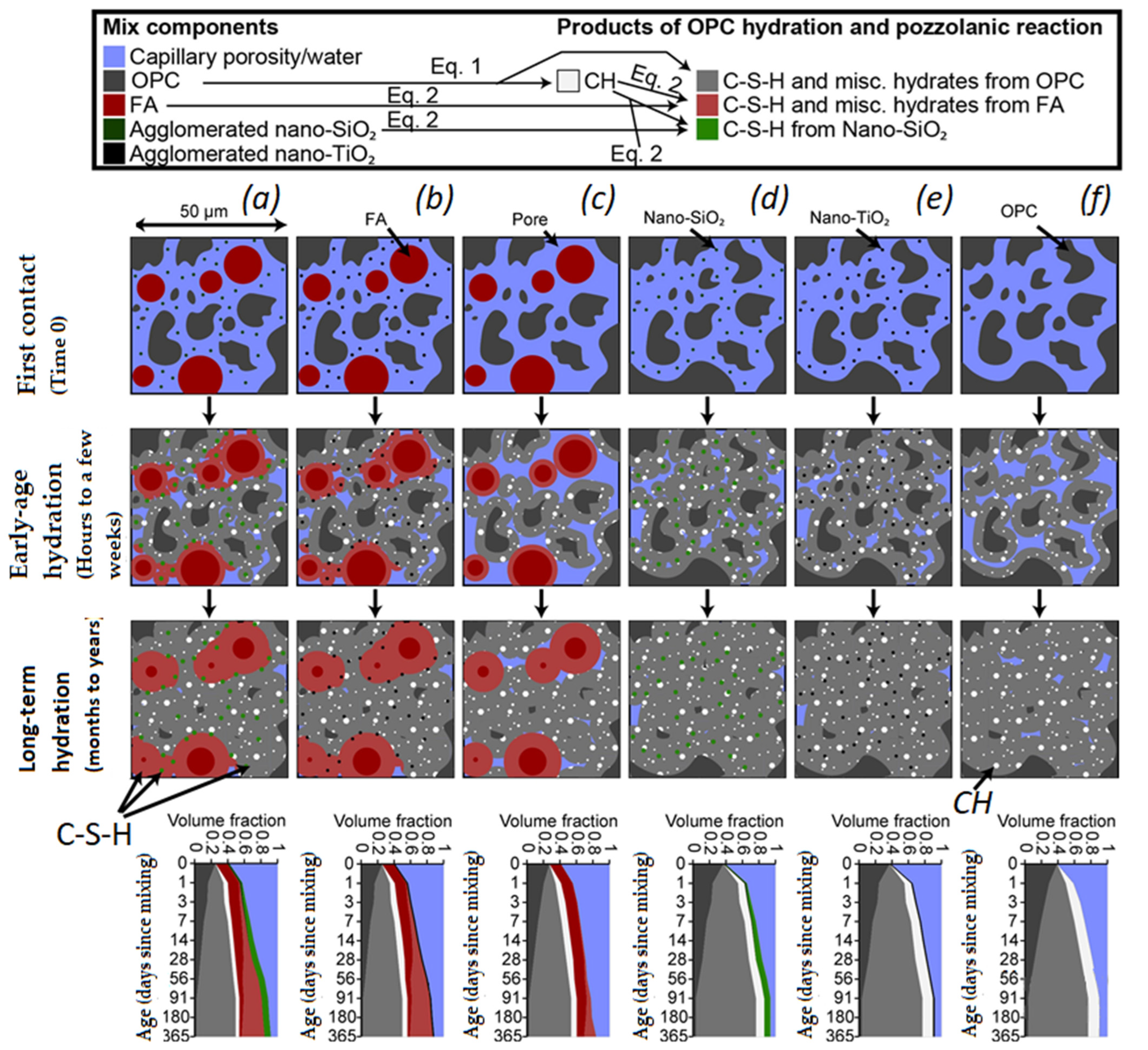 The hydration of OPC (dark grey) to CSH (light grey) and CH (white) results in a decrease in total content and pore size (blue). Figure 15b,e demonstrate non-pozzolanic (TiO2, black) nanomodification of hydration, whereas Figure 15c,f exhibit pozzolanic (SiO2, dark green) nanoparticles. Figure 15d shows the pozzolanic reaction of FA (dark red) to create CSH and other hydrates (light red), whereas the synergistic effect of nanoparticles is depicted in Figure 15e,f.
Nanoparticles, both pozzolanic and non-pozzolanic, serve as nuclei for hydrate precipitation from clinker and impurities (Figure 15b,c,e,f). Pozzolanic nanoparticles prompt additional CSH (light green) through reactions with CH (Figure 15c,f). Reches, Y. [82] then delved into the primary mechanisms of nanomodification. Nanoparticles support clinker hydration and the pozzolanic response of mineral impurities by supplying more surface area for hydrate precipitation from the interstitial solution (Figure 15b,c,e,f). In concrete sans nanoparticles, the concentration of essential ions for hydration reactions (e.g., Ca2+, SiO44−, and SO42−) in the pore water rapidly elevates upon contact with water, OPC, and any impurities. Within the initial ~12 h, the concentration surpasses saturation until reaching a critical nucleation point, which initiates the formation of stable hydrate phases; after this, the ion concentration in the solution gradually diminishes [95]. In contrast, for cement products containing nanoparticle admixtures, researchers have suggested [82,84,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126][82][84][98][99][100][101][102][103][104][105][106][107][108][109][110][111][112][113][114][115][116][117][118][119][120][121][122][123][124][125][126] that the nanoparticles act as nuclei for the precipitation and growth of hydrate phases, starting when the ion concentration surpasses saturation instead of reaching critical nucleation.
In fixed W/B and SP formulations, the presence of nS reduces the available lubricating water in the compound. The most notably impacted rheological parameter, yield strength, experiences a significant increase with the addition of nS to the paste. Conversely, changes in plastic viscosity are less prominent. Additionally, the addition of nS decreases the spreading diameter of these solutions by enhancing the paste’s cohesiveness. The relationship between spreading and yield stress values better elucidates the effects of nS inclusion. For instance, a 2.5% w/w nS inclusion leads to a 19.6% reduction in spreading diameter and a 157% increase in yield stress. In this case, plastic viscosity increases by only 3.6% [89]. The addition of nS enables the anticipation of the setting onset and a reduction in dwell time. Incorporating 2.5 wt.% nS in the compound reduced the setting time by 60% and the time to reach maximum hydration temperature by 51.3%. Consequently, the addition of nS promotes increased CH production at an early age compared to samples without nS. Moreover, the addition of nS decreases the apparent density and increases the air content of these solutions.
An analysis of numerous studies [82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126][82][83][84][85][86][87][88][89][90][91][92][93][94][95][96][97][98][99][100][101][102][103][104][105][106][107][108][109][110][111][112][113][114][115][116][117][118][119][120][121][122][123][124][125][126] highlights the remarkable effectiveness of nanoparticles in modifying cement products, even at low concentrations (<1%). The main modifications include a reduction in setting time (1–2 h) and the diffusion coefficient (4%–75%), along with a strength increase (5%–25%) and enhanced thermal durability (up to 30% increase in residual strength). These changes are attributed to the unique reactivity of nanoparticles, which is linked to their small size and high surface area. Smaller particles boast a higher surface area to volume ratio, and their inclusion leads to an expanded surface area. Consequently, the increased surface area of nanoparticles in the binder can boost reactivity. Typically, nanoparticles minimize the number of nano-sized pores in C-S-H and fortify the nanostructures of cementitious composites. Take nanosilica (SiO2) as an example—each Si atom, chemically bonded to four oxygen atoms, forms a tetrahedron, serving as the basic building block. This structure results in silicate chains composed of oxygen-separated silicate tetrahedra [92,93,94,95,96,97,98][92][93][94][95][96][97][98]. The addition of nanosilica to cementitious materials elevates durability by enhancing C-S-H hardness, rendering it more resistant to calcium leaching [82,83,84,85,86,87,88,89,90,91,92,93,94,95][82][83][84][85][86][87][88][89][90][91][92][93][94][95]. When added to the early stages of fly ash liquor production, nanosilica absorbs most of the Ca(OH)2, leaving a reduced amount available for fly ash hydration [89]. Consequently, the addition of nanosilica may accelerate the pozzolanic reaction in the cementitious composite.
An examination of the literature reveals that the combined effect of microsilica and nanosilica is not entirely comprehended, yet it holds the potential to enhance concrete’s performance (further research is recommended to fully understand the causes of this synergistic effect). The authors of [85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130][85][86][87][88][89][90][91][92][93][94][95][96][97][98][99][100][101][102][103][104][105][106][107][108][109][110][111][112][113][114][115][116][117][118][119][120][121][122][123][124][125][126][127][128][129][130] suggest potential mechanisms for this effect, which demand additional investigation. Firstly, the nucleation effect of nanosilica particles accelerates the cement hydration process, freeing lime for the pozzolanic reaction, thus rendering the silica fume more reactive. Nanosilica demonstrates significantly higher pozzolanic activity than microsilica. Concrete incorporating nanosilica consistently exhibited higher compressive strength than standard concrete across all ages. Furthermore, the addition of nanosilica elevated the flexural and tensile splitting strengths of the concrete. The second observation indicates that while microsilica exhibits a more extensive filling effect than nanosilica due to the larger quantity of material added, silica nanoparticles can supplement microsilica by occupying the voids between the particles to further enhance the packing density of cementitious materials.
Regarding other nanomaterials, few studies have been conducted to assess the morphological attributes of nanoparticles for filling voids between cement grains. Figure 16 clearly illustrates the morphological distinctions of nanoparticles like SiO2, CaCO3, and TiO2. Furthermore, Figure 15 showcases pairs of morphologically different anatase TiO2 nanoparticles: one exhibiting a platelet geometry with a protruding surface {0 0 1} (left) and the other with a tetragonal bipiramidal geometry displaying a protruding surface {1 0 1} (right). The hydrated electron of these surfaces measures 1.6 and 1.0 J/m2, respectively [82]. Such morphological characteristics are scarcely addressed in cement literature.
The hydration of OPC (dark grey) to CSH (light grey) and CH (white) results in a decrease in total content and pore size (blue). Figure 15b,e demonstrate non-pozzolanic (TiO2, black) nanomodification of hydration, whereas Figure 15c,f exhibit pozzolanic (SiO2, dark green) nanoparticles. Figure 15d shows the pozzolanic reaction of FA (dark red) to create CSH and other hydrates (light red), whereas the synergistic effect of nanoparticles is depicted in Figure 15e,f.
Nanoparticles, both pozzolanic and non-pozzolanic, serve as nuclei for hydrate precipitation from clinker and impurities (Figure 15b,c,e,f). Pozzolanic nanoparticles prompt additional CSH (light green) through reactions with CH (Figure 15c,f). Reches, Y. [82] then delved into the primary mechanisms of nanomodification. Nanoparticles support clinker hydration and the pozzolanic response of mineral impurities by supplying more surface area for hydrate precipitation from the interstitial solution (Figure 15b,c,e,f). In concrete sans nanoparticles, the concentration of essential ions for hydration reactions (e.g., Ca2+, SiO44−, and SO42−) in the pore water rapidly elevates upon contact with water, OPC, and any impurities. Within the initial ~12 h, the concentration surpasses saturation until reaching a critical nucleation point, which initiates the formation of stable hydrate phases; after this, the ion concentration in the solution gradually diminishes [95]. In contrast, for cement products containing nanoparticle admixtures, researchers have suggested [82,84,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126][82][84][98][99][100][101][102][103][104][105][106][107][108][109][110][111][112][113][114][115][116][117][118][119][120][121][122][123][124][125][126] that the nanoparticles act as nuclei for the precipitation and growth of hydrate phases, starting when the ion concentration surpasses saturation instead of reaching critical nucleation.
In fixed W/B and SP formulations, the presence of nS reduces the available lubricating water in the compound. The most notably impacted rheological parameter, yield strength, experiences a significant increase with the addition of nS to the paste. Conversely, changes in plastic viscosity are less prominent. Additionally, the addition of nS decreases the spreading diameter of these solutions by enhancing the paste’s cohesiveness. The relationship between spreading and yield stress values better elucidates the effects of nS inclusion. For instance, a 2.5% w/w nS inclusion leads to a 19.6% reduction in spreading diameter and a 157% increase in yield stress. In this case, plastic viscosity increases by only 3.6% [89]. The addition of nS enables the anticipation of the setting onset and a reduction in dwell time. Incorporating 2.5 wt.% nS in the compound reduced the setting time by 60% and the time to reach maximum hydration temperature by 51.3%. Consequently, the addition of nS promotes increased CH production at an early age compared to samples without nS. Moreover, the addition of nS decreases the apparent density and increases the air content of these solutions.
An analysis of numerous studies [82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126][82][83][84][85][86][87][88][89][90][91][92][93][94][95][96][97][98][99][100][101][102][103][104][105][106][107][108][109][110][111][112][113][114][115][116][117][118][119][120][121][122][123][124][125][126] highlights the remarkable effectiveness of nanoparticles in modifying cement products, even at low concentrations (<1%). The main modifications include a reduction in setting time (1–2 h) and the diffusion coefficient (4%–75%), along with a strength increase (5%–25%) and enhanced thermal durability (up to 30% increase in residual strength). These changes are attributed to the unique reactivity of nanoparticles, which is linked to their small size and high surface area. Smaller particles boast a higher surface area to volume ratio, and their inclusion leads to an expanded surface area. Consequently, the increased surface area of nanoparticles in the binder can boost reactivity. Typically, nanoparticles minimize the number of nano-sized pores in C-S-H and fortify the nanostructures of cementitious composites. Take nanosilica (SiO2) as an example—each Si atom, chemically bonded to four oxygen atoms, forms a tetrahedron, serving as the basic building block. This structure results in silicate chains composed of oxygen-separated silicate tetrahedra [92,93,94,95,96,97,98][92][93][94][95][96][97][98]. The addition of nanosilica to cementitious materials elevates durability by enhancing C-S-H hardness, rendering it more resistant to calcium leaching [82,83,84,85,86,87,88,89,90,91,92,93,94,95][82][83][84][85][86][87][88][89][90][91][92][93][94][95]. When added to the early stages of fly ash liquor production, nanosilica absorbs most of the Ca(OH)2, leaving a reduced amount available for fly ash hydration [89]. Consequently, the addition of nanosilica may accelerate the pozzolanic reaction in the cementitious composite.
An examination of the literature reveals that the combined effect of microsilica and nanosilica is not entirely comprehended, yet it holds the potential to enhance concrete’s performance (further research is recommended to fully understand the causes of this synergistic effect). The authors of [85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130][85][86][87][88][89][90][91][92][93][94][95][96][97][98][99][100][101][102][103][104][105][106][107][108][109][110][111][112][113][114][115][116][117][118][119][120][121][122][123][124][125][126][127][128][129][130] suggest potential mechanisms for this effect, which demand additional investigation. Firstly, the nucleation effect of nanosilica particles accelerates the cement hydration process, freeing lime for the pozzolanic reaction, thus rendering the silica fume more reactive. Nanosilica demonstrates significantly higher pozzolanic activity than microsilica. Concrete incorporating nanosilica consistently exhibited higher compressive strength than standard concrete across all ages. Furthermore, the addition of nanosilica elevated the flexural and tensile splitting strengths of the concrete. The second observation indicates that while microsilica exhibits a more extensive filling effect than nanosilica due to the larger quantity of material added, silica nanoparticles can supplement microsilica by occupying the voids between the particles to further enhance the packing density of cementitious materials.
Regarding other nanomaterials, few studies have been conducted to assess the morphological attributes of nanoparticles for filling voids between cement grains. Figure 16 clearly illustrates the morphological distinctions of nanoparticles like SiO2, CaCO3, and TiO2. Furthermore, Figure 15 showcases pairs of morphologically different anatase TiO2 nanoparticles: one exhibiting a platelet geometry with a protruding surface {0 0 1} (left) and the other with a tetragonal bipiramidal geometry displaying a protruding surface {1 0 1} (right). The hydrated electron of these surfaces measures 1.6 and 1.0 J/m2, respectively [82]. Such morphological characteristics are scarcely addressed in cement literature.
- MKS (Microsilica suspension): Suspension (paste) of condensed microsilica.
-
Based on chemical composition and effectiveness, microsilica is further divided into two groups, designated as follows:
-
MK65: Condensed microsilica containing at least 65% silica with an efficiency index of at least 90%.
-
MK85: Condensed microsilica containing a minimum of 85% silica and an efficiency index of at least 105%.
-
High-strength concretes and mortars with reduced permeability and enhanced corrosion resistance are applied in various construction types like industrial, civil, and transportation.
-
Low-cement concretes and mortars that exhibit decreased exotherm.
-
Concrete mixtures with enhanced technological properties, including high mobility and self-compacting features, along with a high degree of resistance to segregation.
6. Preparation of Nanosilica
As per contemporary understanding, large capillary pores serve as stress concentrators in cement stone. The stone’s strength is governed by porosity as long as the pore size surpasses the critical Griffiths crack size. It is a well-established fact that there exists an inverse relationship between porosity and the strength of cement stone. In theory, the maximum strength of cement stone can be achieved when capillary porosity approaches zero [51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68][51][52][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68]. Current trends in concrete technology are geared toward producing concrete with minimal capillary porosity. An essential requirement for this is to not just maintain balanced fractions of fine and coarse aggregates, but also to ensure that all solid components in the concrete mix form a continuous series spanning the entire range of particle sizes—from the smallest nanodispersed components to larger crushed stone pieces. The inclusion of ultrafine silica fillers, featuring particles of various sizes, holds significant importance. While the initial stride toward a new generation of high-quality concrete involved the use of microsilica, the subsequent step should involve integrating ultrafine silica into concrete mixes, characterized by primary particles even smaller than MS (Figure 6). Interest in materials like precipitated and colloidal SiO2 is currently burgeoning and is already being practically applied in concrete technologies. In some instances, precipitated SiO2 is integrated into the composition of RPC concretes alongside microsilica. This inclusion contributes to achieving compressive strength ranging from 200 to 800 MPa and flexural strength of up to 100 MPa [55,56,57,58,59,60,61,62,63,64,65,66,67,68,69][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69]. Aerosil, or fumed silica, is manufactured by burning tetrachlorosilane (SiCl4) in a stream of hydrogen and oxygen:SiCl
4
+ 2H
2
+ O
2
→ SiO
2
+ 4HCl

Figure 7.
Methods of aggregation of colloidal particles: (
a
) sol from colloidal particles; (
b
) gel; (
c
) formation of sediment.

Figure 8.
Colloidal silica particles.
Table 5.
| Indicator Name | Types of Ultra-Dispersed Silica | |||
|---|---|---|---|---|
| High Temperature | Hydrochemical | |||
| Microsilica (Elkem Materials A/S) | Aerosil 200F (Degussa) | Precipitated Silica Catosil NK-3 HS (City Cat Pigments) | Stake SiO2 Ludox HS400 (Grace Davison) | |
| Appearance | Gray powder | White powder | White powder | Opalescent liquid |
| Mass fraction SiO2, wt % | 90.9 | ≥99.8 | 98–99 | 40 |
| Mass fraction Na2O, wt % | - | - | - | 0.4 |
| Density, kg/m3 | 400–600 (bulk) | 50 | 60–80 | 1310 |
| Specific surface, m2/kg | 18 | ≥200 | 150 | 220 |
7. Study of Promising Methods for Processing and Recycling Waste Micro- and Nanosilica for Their Possible Use in Refractory Materials and Concrete Mixtures
The three primary properties of concrete are workability, strength, and durability. While strength and durability are typically associated with hardened concrete, and workability pertains to fresh concrete, the properties of hardened concrete directly link to mix design and fresh concrete characteristics. In essence, the mix design and the properties of fresh concrete serve as the most crucial factors controlling the mechanical performance of hardened concrete. In reference [46], the impact of silica fume dosage on the water requirement, maintaining constant segregation, is illustrated, confirming the efficacy of the superplasticizer as a water reducer regardless of the silica fume content. The outcomes depicted in Figure 12 reveal that replacing up to 10% of the cement can enhance the workability of concrete. When combined with a superplasticizer, silica fume aids in dispersing the flocculated cement particles.
Figure 12. Effect of superplasticizer dosage on the ratio of water-cement materials (a); the effect of replacing cement with microsilica on superplasticizer dosage (b) [46].

Figure 13.
8. Adding Nano Silica to Concrete
According to numerous studies, adding nanosilica to concrete can significantly enhance the material’s compressive strength [74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118][74][75][76][77][78][79][80][81][82][83][84][85][86][87][88][89][90][91][92][93][94][95][96][97][98][99][100][101][102][103][104][105][106][107][108][109][110][111][112][113][114][115][116][117][118]. Moreover, it has been demonstrated that nanosilica can reduce both the initial and final set while accelerating the early strength of concrete. This is attributed to the large specific surface area of nanosilica, which acts as a robust binder between cement and aggregate [22]. Additionally, due to its extremely small particle size, nanosilica exhibits excellent pozzolanic activity [74,75,76,77[74][75][76][77][78][79][80],78,79,80], enabling it to effectively fill all pores and voids in the concrete, including the interfacial transition zone (ITZ), thus augmenting its strength [74,78,82,84][74][78][82][84]. The introduction of nanosilica promotes the formation of calcium silicate hydrate (CSH) gel, a crucial component in concrete strength. The review [22] analyzed the outcomes of various authors, which are presented in Table 6.Table 6.
The previous studies examined the utilization of various nanomaterials and their corresponding substitution ratios [22].
| Reference | Type of Nanomaterial | Type of Concrete | Type of Use | Remarks |
|---|---|---|---|---|
| Amin and Abu el-Hassan (2015) [33] | (Ni ferrite) and (Cu-Zn ferrite) were used in the experiment together with 15 nm nanosilica | High-strength concrete | Weight doses of 1%, 2%, 3%, 4%, and 5% of nanosilica, Ni, and Cu-Zn ferrite were added to cementitious materials | When comparing samples of concrete with nanoferrite to samples of concrete with nanosilica, the latter exhibited superior compressive strength, estimated to be 10% higher |
| Ren et al. (2018) [20] | Nanotitanium dioxide particles 10 nm in diameter and nanosilica particles 20 nm in diameter | Normal concrete | Cement was replaced with nanosilica and nano-TiO2 in different proportions (1%, 3%, and 5%, respectively) | At a mass concentration of 3%, NS and NT can each potentially increase the compressive strength of concrete by up to 16% and 9%, respectively |
| Zhao et al. (2012) [34] | The average nanosilica particle size is around 100 nm | Normal concrete | Nanosilica was used at various weight percentages, ranging from 0% to 20% | The compression and frost resistance capacity increases by 20% when the concentration of nano-SiO2 is at 10%, compared to conventional concrete |
| Shaikh and Supit (2014) [35] | Nano-CaCO3 powder (40 to 50 nm) | Fly ash concrete | Nano-CaCO3 was added to the cement at weight dosages of 1%, 2%, 3%, and 4% | Out of all the concentrations, 1% CaCO3 nanoparticles exhibited the highest compressive strength, surpassing that of the cement mortar by 22% |
| Chithra et al. (2016) [36] | A colloidal dispersion of nanoparticles in water, with a density ranging between 1.3 and 1.32 | High-performance concrete | Nanosilica replaced various weight percentages of cement—0%, 0.5%, 1%, 1.5%, 2%, 2.5%, and 3% | The introduction of nanosilica to cement mortars, substituting 40% copper slag for fine aggregate, increased the compressive strength by 2% |
| Salemi and Behfarnia (2013) [37] | Nanoparticles measuring 20 nm in diameter for silicon and 8 nm in diameter for aluminum oxide | Concrete pavement | NS at 3%, 5%, and 7%, and nano-Al2O3 at 1%, 2%, and 3% were utilized to replace cement at different weight percentages | As per the experimental results, the addition of 5% nanosilica to cementitious materials enhances concrete’s compressive strength by up to 30% and frost resistance by 83% |
| Mohamed (2016) [38] | Nano-silica and nanoclay (NC) | Normal concrete | Substituting cement across a range of weight percentages, spanning from 0.5% to 10% | Both nanosilica and nanoclay distinctly bolster the compressive strength of high-performance concrete, exhibiting an increase of 18% and 11%, respectively |
| Wu et al. (2016) [39] | Nano-CaCO3 elements and nanosilica particles with diameters ranging from 5 to 35 nm and 15 to 105 nm, respectively | High strength concrete | Replacing paste by mass with varying percentages of nano-CaCO3, specifically 0%, 1.6%, 3.2%, 4.8%, and 6.4%, along with nanosilica at 0%, 0.5%, 1.0%, 1.5%, and 2.0% of the cement mass | Nano-SiO2 UHSC blends exhibited a consistent and robust strength increase up to 7 days, while nano-CaCO3 UHSC mixtures showed consistent strength between 3 and 7 days, followed by rapid improvement thereafter |
| Li et al. (2015) [40] | Nanosilica nanoparticles (20 nm) and nanolimestone nanoparticles (15–80 nm) | Ultra-high-performance concrete | Replacing a portion of the cement by mass with nanosilica at 0.5%, 1.0%, 1.5%, and 2.0%, and with nanolimestone at 1.0%, 2.0%, 3.0%, and 4.0% | The increase in the flexural to compressive strengths ratio of the UHPC matrix integrated with 1.0% nanosilica and W/B ratios of 0.16 is 36% |
| Gao et al. (2017) [41] | Nanosilica nanoparticles with an average particle size of 15 nm and nanosilica nanoparticles with a medium grain size of 50 nm | Road flyash concrete | Silica fume and nanosilica were used in quantities of 3%, 2%, and 1% of the cementitious materials’ composition | The concrete containing 2% NS, compared to the reference concrete, exhibited a 124.8% increase in drying shrinkage at 28 days |
| Torabian et al. (2016) [42] | Nanosilica nanoparticles with an average particle size of 20 nm | Normal concrete | Replacing cement with nanosilica in various quantities—0.5%, 1%, and 1.5% | Adding 1.5% NS to concrete with a w/b ratio of 0.65 resulted in a 41% increase in strength |
| Said et al. (2012) [43] | Nanosilica nanoparticles with a medium grain size of 35 nm | Normal concrete | Different quantities of nanosilica nanoparticles, specifically 6% and 12% by weight, were introduced into the cementitious materials | The addition of nanosilica resulted in strength improvements of up to 6% at all curing ages |
| Hosseini et al. (2017) [44] | Nanoclay particles with a density of 1660 kg/m3 | Self-compacting concrete | Varying proportions of cement were replaced with nanoclay, including 0.25%, 0.5%, 0.75%, and 1% of the total weight of the cement | After 56 days, the addition of 0.25% and 0.50% nanoclay increased the compressive strength by 15% and 14%, respectively |

Figure 14. Initial and final setting times of cement products made with or without nanoparticles. (SP1-amorphous silicon dioxide (colloidal) particle size 15 nm; SC-FA1-amorphous silicon dioxide (colloidal) particle size 15 nm plus mineral additive; SC-BFS1—amorphous silicon dioxide (colloidal) particle size 10 nm plus mineral additive; TP1-titanium dioxide (colloidal) particle size 15 nm, CP1-CaCO3 particles 50–120 nm in size; CP2-CaCO3 particles 15–40 nm in size) [46].

9. Patent Research into Methods of Recycling Waste and Its Use in Various Industries as a Strengthening Additive
This section explores patented technical solutions for producing oxide system products by incorporating waste from metallurgical production. The aim is to understand the structure formation mechanism in samples and mass transfer conditions during heating or physical impact [36,38,40,41,42,43,131,132,133,134,135,136,137,138,139,140,141,142][36][38][40][41][42][43][131][132][133][134][135][136][137][138][139][140][141][142]. In a patent by the authors of [131], microsilica was added to the concrete mix as an aqueous suspension, comprising 40%–70% of the total volume. However, this suspension exhibited high viscosity and limited rheological stability over a 90-day period, a drawback addressed by Golotsan A.A. and Dolgopolov A.N. in their work [138]. They reduced the suspension’s viscosity and enhanced its rheological stability, crack resistance, and flowability. The introduction of microsilica in the form of an aqueous suspension prompted considerations regarding the transformation of water-soluble polymorphs of silica upon contact with other matrix materials. Another invention [139] focused on obtaining finely dispersed amorphous microsilica from cost-effective mineral raw materials. This process yields microsilica with high purity and a dispersion ranging between 0.062 and 0.097 microns. Patent [140] describes a production process for finely dispersed amorphous microsilica using the sol-gel method involving diatomaceous earth and sodium hydroxide. Furthermore, patents [131,132][131][132] suggested using microsilica in the production of ceramic-facing materials. Its application was found to reduce water absorption while enhancing frost resistance and strength. However, a raw material mix detailed in [137] containing microsilica exhibited low compressive strength and frost resistance, making it unsuitable for wall panel production. A mix aimed at non-autoclaved aerated concrete [136] incorporated microsilica to bolster material strength but faced challenges due to the high porosity caused by aluminum powder usage. Similarly, ref [135] considered microsilica to reinforce foamed concrete, but difficulty arose due to the fibrous filler content, making homogeneous mixing challenging. The application of microsilica in the refractory industry was reviewed in [134], especially in the production of monolithic linings, to enhance strength and oxidation resistance within the range of 600–1100 °C. Finally, [141,142][141][142] investigated cement slurries utilizing microsilica and calcium oxide, supplementing the solution (12%–16%) alongside microsilica from silicon production waste. Considering the properties and characteristics of microsilica from various types and origins, it can serve as a strengthening additive in concrete mixtures, including refractory compositions. Patent searches have outlined the potential for introducing microsilica into various mixtures, which can be further adapted for use in metallurgical industries, particularly in the production of refractory fireclay products and concrete. This adaptation takes into account the unique properties and structure of microsilica. In summary, studies [56,57,58,59,60,61,62,63,64,65,66,67][56][57][58][59][60][61][62][63][64][65][66][67] explored different microsilica types and indicated that adding this additive to concrete enhances its strength, frost, and chemical resistance. However, the use of superplasticizer C-3 is essential. The authors of [118,119,120,121,122,123,124,125][118][119][120][121][122][123][124][125] suggest that the effective utilization of SiO2 is only feasible in conjunction with a plasticizer, accelerating cement hydration and boosting cement stone strength by 20%. On the other hand, the authors of [110] observe that microsilica extends the setting time of cement mortar due to the small size of its particles. The amorphous nature of nano-SiO2 raises a key challenge: how to meaningfully differentiate between distinct properties of nano-SiO2. Two structural properties can significantly influence the reactivity of nano-SiO2 [82]. Further research should address crucial aspects, starting with the assessment of crystallinity [82]. Considering amorphous SiO2 as akin to defective quartz, the activation energy for the pozzolanic reaction correlates with ideal crystallinity, reaching maximum efficiency. The thermodynamic path of this reaction becomes more favorable as the material moves towards polycrystalline or amorphous structures, which can be evaluated through techniques like X-ray diffraction and 29 Si nuclear magnetic resonance (full width at half maximum). Another essential consideration is the degree of cross-linking [82]. If amorphous SiO2 is viewed as interlinked polymer chains, these connections limit the reactivity of the Si anti-bonding orbital. The pozzolanic reaction replaces Si triple bonds with Si-OH bonds, but this replacement can be hindered by these cross-links. Nano-SiO2 generated through the sol-gel process, when catalyzed by bases, tends to be more cross-linked than when catalyzed by acids. Consequently, acid-catalyzed nano-SiO2 has shown more efficacy in enhancing the compressive strength of mortars compared to base-catalyzed nano-SiO2 [111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130][111][112][113][114][115][116][117][118][119][120][121][122][123][124][125][126][127][128][129][130].References
- Analysis of the Microsilica Market (Chemical Additives for Concrete) in Russia. Available online: https://drgroup.ru/1416-Analiz-rynka-mikrokremnezema-v-Rossii.html (accessed on 27 November 2023).
- Crainic, N.; Marques, A.T. Nanocomposites: A state-of-the-art review. Key Eng. Mater. 2002, 230, 656.
- Norhasri, M.S.M.; Hamidah, M.S.; Fadzil, A.M. Applications of using nano material in concrete: A review. Constr. Build. Mater. 2017, 133, 91–97.
- Khan, M.; Cao, M.; Chaopeng, X.; Ali, M. Experimental and analytical study of hybrid fiber reinforced concrete prepared with basalt fiber under high temperature. Fire Mater. 2021, 46, 205–226.
- Li, L.; Khan, M.; Bai, C.; Shi, K. Uniaxial Tensile Behavior, Flexural Properties, Empirical Calculation and Microstructure of Multi-Scale Fiber Reinforced Cement-Based Material at Elevated Temperature Materials 2021, 14, 1827. Materials 2021, 14, 1827.
- Ahmad, W.; Ahmad, A.; Ostrowski, K.A.; Aslam, F.; Joyklad, P.; Zajdel, P. Sustainable approach of using sugarcane bagasse ash in cement-based composites: A systematic review. Case Stud. Constr. Mater. 2021, 15, e00698.
- Ahmad, A.; Farooq, F.; Niewiadomski, P.; Ostrowski, K.; Akbar, A.; Aslam, F.; Alyousef, R. Prediction of compressive strength of fly ash based concrete using individual and ensemble algorithm. Materials 2021, 14, 794.
- Akhnoukh, A.K. Improving Concrete Infrastructure Project Conditions by Mitigating Alkali–Silica Reactivity of Fine Aggregates. Constr. Mater. 2023, 3, 233–243.
- Mohmmad, S.H.; Shakor, P.; Muhammad, J.H.; Hasan, M.F.; Karakouzian, M. Sustainable Alternatives to Cement: Synthesizing Metakaolin-Based Geopolymer Concrete Using Nano-Silica. Constr. Mater. 2023, 3, 276–286.
- Qattali, S.M.Y.; Pritzel, C.; Kowald, T.; Moni, S.M.F.K.; Killian, M.S.; Trettin, R. Effect of Iron-Doped TiO2 Nanotubes on the Hydration of Tricalcium Silicate. Constr. Mater. 2023, 3, 259–275.
- Irshidat, M.R.; Al-Nuaimi, N. Industrial Waste Utilization of Carbon Dust in Sustainable Cementitious Composites Production. Materials 2020, 13, 3295.
- Nafees, A.; Javed, M.F.; Khan, S.; Nazir, K.; Farooq, F.; Aslam, F.; Musarat, M.A.; Vatin, N.I. Predictive Modeling of Mechanical Properties of Silica Fume-Based Green Concrete Using Artificial Intelligence Approaches: MLPNN, ANFIS, and GEP. Materials 2021, 14, 7531.
- Miller, S.A. Supplementary cementitious materials to mitigate greenhouse gas emissions from concrete: Can there be too much of a good thing? J. Clean. Prod. 2018, 178, 587–598.
- Habert, G.; Miller, S.A.; John, V.M.; Provis, J.L.; Favier, A.; Horvath, A.; Scrivener, K.L. Environmental impacts and decarbonization strategies in the cement and concrete industries. Nat. Rev. Earth Environ. 2020, 1, 559–573.
- Di Filippo, J.; Karpman, J.; DeShazo, J.R. The impacts of policies to reduce CO2 emissions within the concrete supply chain. Cem. Concr. Compos. 2019, 101, 67–82.
- Saidova, Z.; Yakovlev, G.; Orbán, Z.; Grakhov, V.; Urkhanova, L.; Lkhasaranov, S. Cement Compositions Modified with Dispersed Magnesium Silicate Dihydrate- and Carbon-Based Additives. Constr. Mater. 2022, 2, 101–113.
- Agrawal, Y.; Gupta, T.; Sharma, R.; Panwar, N.L.; Siddique, S. A Comprehensive Review on the Performance of Structural Lightweight Aggregate Concrete for Sustainable Construction. Constr. Mater. 2021, 1, 39–62.
- Ayala Valderrama, D.M.; Cuaspud, J.A.G.; Taniolo, N.; Boccaccini, A.R. Glass-Ceramic Materials Obtained by Sintering of Vitreous Powders from Industrial Waste: Production and Properties. Constr. Mater. 2021, 1, 63–79.
- Subpa-asa, P.; Nito, N.; Fujiwara, S.; Date, S. Evaluation of the Prediction and Durability on the Chloride Penetration in Cementitious Materials with Blast Furnace Slag as Cement Addition. Constr. Mater. 2022, 2, 53–69.
- Ahmed, A.; Hyndman, F.; Kamau, J.; Fitriani, H. Rice Husk Ash as a Cement Replacement in High Strength Sustainable Concrete; Trans Tech Publications Ltd.: Bäch, Switzerland, 2020; pp. 90–98.
- Zareei, S.A.; Ameri, F.; Dorostkar, F.; Ahmadi, M. Rice husk ash as a partial replacement of cement in high strength concrete containing micro silica: Evaluating durability and mechanical properties. Case Stud. Constr. Mater. 2017, 7, 73–81.
- AlTawaiha, H.; Alhomaidat, F.; Eljufout, T. A Review of the Effect of Nano-Silica on the Mechanical and Durability Properties of Cementitious Composites. Infrastructures 2023, 8, 132.
- Barbhuiya, G.H.; Moiz, M.A.; Hasan, S.D.; Zaheer, M.M. Effects of the nanosilica addition on cement concrete: A review. Mater. Today Proc. 2020, 32, 560–566.
- Sivtsov, A.V.; Yolkin, K.S.; Yolkin, D.K.; Karlina, A.I. Role of the Silicon Carbide Content in the Certain Workspace Zones of Furnaces Used for Smelting Silicon and High-Silicon Ferroalloys. Metallurgist 2022, 65, 952–959.
- Liu, C.; He, X.; Deng, X.; Wu, Y.; Zheng, Z.; Liu, J.; Hui, D. Application of nanomaterials in ultra-high performance concrete: A review. Nanotechnol. Rev. 2020, 9, 1427–1444.
- Larionova, Z.M.; Nikitina, L.V.; Garashin, V.R. Phase Composition, Microstructure and Strength of Cement Stone and Concrete; Nauka-RAN: Moscow, Russia, 1977; 264p.
- Malinin, Y.S.; Lopatnikova, L.Y.; Guseva, V.N.; Klishanis, N.D. The Problem of Hydration and Hardening of Portland Cement; Nauka-RAN: Moscow, Russia, 1964; pp. 147–164.
- Kondratiev, V.V.; Nemchinova, N.V.; Ivanov, N.A.; Ershov, V.A.; Sysoev, I.A. New technological solutions for processing waste from silicon and aluminum production. Metallurg 2013, 5, 92–95.
- Lothenbach, B.; Wirmefeld, F. The influence of superplasticizers on the hydration of Portland cement. In Proceedings of the 12th International Congress on the Chemistry of Cement, Montréal, QC, Canada, 8–13 July 2007.
- Okamura, H.; Ouchi, M. Self-Compacting Concrete. J. Adv. Concr. Technol. 2003, 1, 5–15.
- Varghese, L.; Rao, V.V.L.K.; Parameswaran, L. Nanosilica-added concrete: Strength and its correlation with time-dependent properties. Proc. Inst. Civ. Eng.-Constr. Mater. 2019, 172, 85–94.
- Ltifi; Guefrech, A.; Mounanga, P.; Khelidj, A. Experimental study of the effect of addition of nano-silica on the behaviour of cement mortars Mounir. Procedia Eng. 2011, 10, 900–905.
- Wu, L.; Lu, Z.; Zhuang, C.; Chen, Y.; Hu, R. Mechanical Properties of Nano SiO2 and Carbon Fiber Reinforced Concrete after Exposure to High Temperatures. Materials 2019, 12, 3773.
- Abna, A.; Mazloom, M. Flexural properties of fiber reinforced concrete containing silica fume and nano-silica. Mater. Lett. 2022, 316, 132003.
- Rong, Z.; Sun, W.; Xiao, H.; Jiang, G. Effects of nano-SiO2 particles on the mechanical and microstructural properties of ultra-high performance cementitious composites. Cem. Concr. Compos. 2015, 56, 25–31.
- Du, H.; Du, S.; Liu, X. Durability performances of concrete with nano-silica. Constr. Build. Mater. 2014, 73, 705–712.
- Lincy, V.; Rao, V.V.L.K.; Lakshmy, P. A study on nanosilica- and microsilica-added concretes under different transport mechanisms. Mag. Concr. Res. 2018, 70, 1205–1216.
- Nemchinova, N.V.; Bychinsky, V.A.; Belsky, S.S.; Klyots, V.E. Basic physico-chemical model of carbothermic smelting of silicon. News Univ. Non-Ferr. Metall. 2008, 4, 56–63.
- Protopopov, E.V.; Galevsky, G.V.; Temlyantsev, M.V. The use of technogenic metallurgical waste in silicon carbide technology. Bull. Kuzbass State Technol. Univ. 2014, 4, 103–110.
- Lokhova, N.A.; Makarova, I.A.; Patramanskaya, S.V. Firing Materials Based on Microsilica; Bratsk State University: Bratsk, Russia, 2002; 163p.
- Karlina, A.I. Technology of Processing Gas Purification Dust from Silicon Production into Modifying Nanoadditives for Cast Iron: Abstract of Dissertation; Candidate of Technical Sciences: 05.16.07; IMET URO RAS: Ekaterinburg, Russia, 2019; 24p.
- Burkat, V.S.; Burkat, T.V.; Lapshin, A.E. Study of the physical and chemical properties of silicon-containing dust of ore-thermal furnaces. Non-Ferr. Met. 2017, 4, 30–34.
- Glazyev, M.V. High-Temperature Phase Interactions during the Disposal of Finely Dispersed Waste from the Production of Metallurgical Silicon, Abstract of Thesis; can. Techn. Sc.: 05.16.02; St. Petersburg Mining University: St. Petersburg, Russia, 2022; 24p.
- Iler, R.K. The Chemistry of Silica: Solubility, Polymerization, Colloid and Surface Properties, and Biochemistry; Wiley: New York, NY, USA, 1979; 866р.
- Iler, R. Chemistry of Silica; 2 parts; Mir: Moscow, Russia, 1982; 1128p.
- Duval, R.; Kadri, E.H. Influence of Silica Fume on the Workability and the Compressive Strength of High-Performance Concretes. Cem. Concr. Res. 1998, 28, 533–547.
- GOST R 57345-2016/EN 206-1:2013; Concrete. General Technical Conditions. GOST-R: Moscow, Russia, 2017; 74p.
- GOST 31384-2017; Protection of Concrete and Reinforced Concrete Structures from Corrosion. GOST-R: Moscow, Russia, 2017; 49p.
- GOST R 58894-2020; Condensed Microsilica for Concrete and Mortars. GOST-R: Moscow, Russia, 2020; 19p.
- Fiskaa, O.; Hansen, H.; Moum, J. Concrete in Alum Slate; Norwegian Geotechnical Institute: Oslo, Norway, 1971; p. 86.
- Braulio, M.A.L.; Brant, P.O.C.; Bittencourt, L.R.M.; Pandolfelli, V.C. Microsilica or MgO grain size: Which one mostly affects the in situ spinel refractory castable expansion? Ceram. Int. 2009, 35, 3327–3334.
- Yoon, C.M.; Min, D.J. Effect of surface properties of MgO on its dissolution and spinel growth in CaO–SiO2–Al2O3 slag. Ceram. Int. 2022, 48, 15017–15025.
- Ershov, V.A.; Karlina, A.I.; Karlina, Y.I. Process Control System for Producing a Silica-Based Modifying Additive. Metallurgist 2021, 65, 446–453.
- Lewis, R.; Fidjestøl, R. Microsilica as an Addition. In Lea’s Chemistry of Cement and Concrete, 5th ed.; Butterworth-Heinemann: Oxford, UK, 2019; pp. 509–535.
- Sako, E.Y.; Braulio, M.A.L.; Pandolfelli, V.C. Microstructural evolution of magnesia-based castables containing microsilica. Ceram. Int. 2012, 38, 6027–6033.
- Sree Manu, K.M.; Sreeraj, K.; Rajan, T.P.D.; Shereema, R.M.; Pai, B.C.; Arun, B. Structure and properties of modified compocast microsilica reinforced aluminum matrix composite. Mater. Des. 2015, 88, 294–301.
- Sako, E.Y.; Braulio, M.A.L.; Milanez, D.H.; Brant, P.O.; Pandolfelli, V.C. Microsilica role in the CA6 formation in cement-bonded spinel refractory castables. J. Mater. Process. Technol. 2009, 209, 555–5557.
- Mermerdaş, K.; İpek, S.; Algn, Z.; Ekmen, Ş.; Güneş, İ. Combined effects of microsilica, steel fibre and artificial lightweight aggregate on the shrinkage and mechanical performance of high strength cementitious composite. Constr. Build. Mater. 2020, 262, 120048.
- Kuz’min, M.P.; Chu, P.K.; Qasim, A.M.; Larionov, L.M.; Kuz’mina, M.Y.; Kuz’min, P.B. Obtaining of Al–Si foundry alloys using amorphous microsilica—Crystalline silicon production waste. J. Alloys Compd. 2019, 806, 806–813.
- Hendi, A.; Rahmani, H.; Mostofinejad, D.; Tavakolinia, A.; Khosravi, M. Simultaneous effects of microsilica and nanosilica on selfconsolidating concrete in a sulfuric acid medium. Constr. Build. Mater. 2017, 152, 192–205.
- Xu, W.T.; Lo, T.Y.; Wang, W.L.; Ouyang, D.; Wang, P.G.; Xing, F. Pozzolanic reactivity of silica fume and ground rice husk ash as reactive silica in a cementitious system: A comparative study. Materials 2016, 9, 146.
- Sandvik, M.; Haug, A.; Hunsbedt, O. Condensed silica fume in high strength concrete for offshore structures–a case record. In Proceedings of the Third International Conference on the Use of Fly Ash Slag, Silica Fume and Natural Pozzolans in Concrete, Trondheim, Norway, 19–26 June 1989.
- Alonso-Domínguez, D.; Álvarez-Serrano, I.; Reyes, E.; Moragues, A. New mortars fabricated by electrostatic dry deposition of nano and microsilica additions: Enhanced properties. Constr. Build. Mater. 2017, 135, 186–193.
- De Rojas, M.I.S.; Rivera, J.; Frais, M. Influence of the microsilica state on pozzolanic reaction rate. Cem. Concr. Res. 1999, 29, 945–949.
- Detwiler, R.; Mehta, P. Chemical and physical effects of silica fume on the mechanical behavior of concrete. ACI Mater. J. 1989, 86, 609–614.
- Udalov, Y.P.; Pozniak, I.V.; Sazavsky, P.; Bakardieva, S.; Tirpekl, V. Erratum to: Physicochemical transformations in multicomponent melts containing uranium, zirconium, and iron oxides and calcium silicates and aluminates. Russ. J. Appl. Chem. 2017, 90, 838–845.
- Ioannou, S.; Chowdhury, M.S.; Badr, A. Rheological, hydration and mechanical characteristics of microsilica fibre reinforced cement combinations with incremental fly ash contents. Constr. Build. Mater. 2018, 191, 423–430.
- Maciej, S. Fractal characterization of thermal cracking patterns and fracture zone in low-alkali cement matrix modified with microsilica. Cem. Concr. Compos. 2020, 103732.
- Glazev, M.V.; Bazhin, V.Y. On the recycling and use of microsilica in the oil industry. E3S Web Conf. 2021, 266, 02010.
- Zimina, D.A.; Dvoinikov, M.V. Development of cement stone with enhanced strength properties. J. Min. Geol. Sci. 2019, 62, 128–132.
- Puzenko, K.N.; Balabanov, V.B.; Munchausen, D. Experience with the use of additives, micro- and nanosilica from waste silicon production in concrete technology proceedings of the universities. Invest. Constr. Realt. 2017, 7, 3.
- Sobolev, K.; Flores-Vivian, I.; Pradoto, R.G.; Kozhukhova, M.; Potapov, V. The effect of natural SiO2 nanoparticles on the performance of portland cement based materials. Bull. Belgorod State Technol. Univ. Named After V. G. Shukhov 2018, 3, 6–16.
- Madania, H.; Bagheria, A.; Parhizkarb, T. The pozzolanic reactivity of monodispersed nanosilica hydrosols and their influence on the hydration characteristics of Portland cement. Cem. Concr. Res. 2012, 42, 1563–1570.
- Tobón, J.I.; Payá, J.; Restrepo, O.J. Study of durability of Portland cement mortars blended with silica nanoparticles. Constr. Build. Mater. 2015, 80, 92–97.
- Güneyisi, E.; Atewi, Y.R.; Hasan, M.F. Fresh and rheological properties of glass fiber reinforced self-compacting concrete with nanosilica and fly ash blended. Constr. Build. Mater. 2019, 211, 349–362.
- Chandra, C.J.; Shadiya, M.; Bipinbal, P.; Narayanankutty, S.K. Effect of Olivine Nanosilica on the Reinforcement of Natural Rubber Nanosilica Composites. Mater. Today Proc. 2019, 9, 127–132.
- Balanovskiy, A.E.; Karlina, A.I.; Kolosov, A.D.; Karlina, Y.I. Study of the effect of nanomodifiers from silicon production wastes on morphological form of gray cast iron graphites. CIS Iron Steel Rev. 2021, 21, 64–69.
- Mebert, A.M.; Baglole, C.J.; Desimone, M.F.; Maysinger, D. Nanoengineered silica: Properties, applications and toxicity. Food Chem. Toxicol. 2017, 109, 753–770.
- Behzadian, R.; Shahrajabian, H. Experimental Study of the Effect of Nano-silica on the Mechanical Properties of Concrete/PET Composites. KSCE J. Civ. Eng. 2019, 23, 3660–3668.
- Li, L.G.; Zheng, J.Y.; Zhu, J.; Kwan, A.K.H. Combined usage of micro-silica and nano-silica in concrete: SP demand, cementing efficiencies and synergistic effect. Constr. Build. Mater. 2018, 168, 622–632.
- Ren, J.; Lai, Y.; Gao, J. Exploring the influence of SiO2 and TiO2 nanoparticles on the mechanical properties of concrete. Constr. Build. Mater. 2018, 175, 277–285.
- Reches, Y. Nanoparticles as concrete additives: Review and perspectives. Constr. Build. Mater. 2018, 175, 483–495.
- Wang, X.; Huang, Y.; Wu, G.; Fang, C.; Li, D.; Han, N.; Xing, F. Effect of nano-SiO2 on strength, shrinkage and cracking sensitivity of lightweight aggregate concrete. Constr. Build. Mater. 2018, 175, 115–125.
- Ying, J.; Zhou, B.; Xiao, J. Pore structure and chloride diffusivity of recycled aggregate concrete with nano-SiO2 and nano-TiO2. Constr. Build. Mater. 2017, 150, 49–55.
- Ardalan, R.B.; Jamshidi, N.; Arabameri, H.; Joshaghani, A.; Mehrinejad, M.; Sharafi, P. Enhancing the permeability and abrasion resistance of concrete using colloidal nano-SiO2 oxide and spraying nanosilicon practices. Constr. Build. Mater. 2017, 146, 128–135.
- Xu, J.; Wang, B.; Zuo, J. Modification effects of nanosilica on the interfacial transition zone in concrete: A multiscale approach. Cem. Concr. Compos. 2017, 81, 1–10.
- Sharkawi, A.M.; Abd-Elaty, M.A.; Khalifa, O.H. Synergistic influence of micro-nano silica mixture on durability performance of cementious materials. Constr. Build. Mater. 2018, 164, 579–588.
- Karlina, A.I.; Kondratev, V.V.; Kolosov, A.D.; Balanovskiy, A.E.; Ivanov, N.A. Production of new nanostructures for modification of steels and cast irons. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2019; p. 012183.
- Bautista-Gutierrez, K.P.; Herrera-May, A.L.; Santamaría-López, J.M.; Honorato-Moreno, A.; Zamora-Castro, S.A. Recent Progress in Nanomaterials for Modern Concrete Infrastructure: Advantages and Challenges. Materials 2019, 12, 3548.
- Singh, L.P.; Karade, S.R.; Bhattacharyya, S.K.; Yousuf, M.M.; Ahalawat, S. Beneficial role of nanosilica in cement based materials—A review. Constr. Build. Mater. 2013, 47, 1069–1077.
- Dingreville, R.; Qu, J.; Cherkaoui, M. Surface free energy and its effect on the elastic behavior of nano-sized particles, wires and films. J. Mech. Phys. Solids 2005, 53, 1827–1854.
- Koutsawa, Y.; Tiem, S.; Yu, W.; Addiego, F.; Giunta, G. A micromechanics approach for effective elastic properties of nano-composites with energetic surfaces/interfaces. Compos. Struct. 2017, 159, 278–287.
- Li, H.; Xiao, H.-G.; Ou, J.-P. A study on mechanical and pressure-sensitive properties of cement mortar with nanophase materials. Cem. Concr. Res. 2004, 34, 435–438.
- Teng, X.; Liu, H.; Huang, C. Effect of Al2O3 particle size on the mechanical properties of alumina-based ceramics. Mater. Sci. Eng. A 2007, 452–453, 545–551.
- Amin, M.; Abu el-Hassan, K. Effect of using different types of nano materials on mechanical properties of high strength concrete. Constr. Build. Mater. 2015, 80, 116–124.
- Zhao, Z.R.; Kong, J.; Yang, H.X. Study on Frost Resistance of Nano SiO2 Cement Concrete. Appl. Mech. Mater. 2012, 198–199, 48–51.
- Shaikh, F.U.; Supit, S.W. Mechanical and durability properties of high volume fly ash (HVFA) concrete containing calcium carbonate (CaCO3) nanoparticles. Constr. Build. Mater. 2014, 70, 309–321.
- Salemi, N.; Behfarnia, K. Effect of nano-particles on durability of fiber-reinforced concrete pavement. Constr. Build. Mater. 2013, 48, 934–941.
- Mohamed, A.M. Influence of nano materials on flexural behavior and compressive strength of concrete. HBRC J. 2016, 12, 212–225.
- Wu, Z.; Shi, C.; Khayat, K.; Wan, S. Effects of different nanomaterials on hardening and performance of ultra-high strength concrete (UHSC). Cem. Concr. Compos. 2016, 70, 24–34.
- Li, W.; Huang, Z.; Cao, F.; Sun, Z.; Shah, S.P. Effects of nano-silica and nano-limestone on flowability and mechanical properties of ultra-high-performance concrete matrix. Constr. Build. Mater. 2015, 95, 366–374.
- Gao, Y.; He, B.; Li, Y.; Tang, J.; Qu, L. Effects of nano-particles on improvement in wear resistance and drying shrinkage of road fly ash concrete. Constr. Build. Mater. 2017, 151, 228–235.
- Torabian Isfahani, F.; Redaelli, E.; Lollini, F.; Li, W.; Bertolini, L. Effects of nanosilica on compressive strength and durability properties of concrete with different water to binder ratios. Adv. Mater. Sci. Eng. 2016, 2016, 8453567.
- Said, A.; Zeidan, M.; Bassuoni, M.; Tian, Y. Properties of concrete incorporating nano-silica. Constr. Build. Mater. 2012, 36, 838–844.
- Hosseini, P.; Afshar, A.; Vafaei, B.; Booshehrian, A.; Raisi, E.M.; Esrafili, A. Effects of nano-clay particles on the short-term properties of self-compacting concrete. Eur. J. Environ. Civ. Eng. 2017, 21, 127–147.
- Wang, J.L.; Meng, L.J. Influence of Carbon Nano-Fiber on Mechanical Property of PALC. Appl. Mech. Mater. 2014, 535, 785–787.
- Saba, N.; Paridah, M.; Abdan, K.; Ibrahim, N. Effect of oil palm nano filler on mechanical and morphological properties of kenaf reinforced epoxy composites. Constr. Build. Mater. 2016, 123, 15–26.
- Oh, S.T.; Yoon, S.J.; Choa, Y.H.; Jeong, Y.K.; Niihara, K. Wear behavior of nano-sized metal particle dispersed Al2O3 nanocomposites. Key Eng. Mater. 2006, 317, 369–372.
- Das, S.; Chandrasekaran, M.; Samanta, S. Comparison of Mechanical properties of AA6061 reinforced with (SiC/B4C) micro/nano ceramic particle reinforcements. Mater. Today Proc. 2018, 5, 18110–18119.
- Schmitz, A.; Schmid, C.; de Boor, J.; Mueller, E. Dispersion of multi-walled carbon nanotubes in skutterudites and its effect on thermoelectric and mechanical properties. J. Nanosci. Nanotechnol. 2017, 17, 1547–1554.
- Shill, S.K.; Al-Deen, S.; Ashraf, M.; Garcez, E.O.; Subhani, M.; Hossain, M.M. Resistance of Geopolymer, Epoxy and Cement Mortar to Hydrocarbon-Based Synthetic Engine Lubricant, Hydraulic Fluid, Jet Fuel and Elevated Temperatures. Constr. Mater. 2022, 2, 15–26.
- Kurniati, E.O.; Kim, H.-J. Utilizing Industrial By-Products for Sustainable Three-Dimensional-Printed Infrastructure Applications: A Comprehensive Review. Infrastructures 2023, 8, 140.
- Sangthongtong, A.; Semvimol, N.; Rungratanaubon, T.; Duangmal, K.; Joyklad, P. Mechanical Properties of Pervious Recycled Aggregate Concrete Reinforced with Sackcloth Fibers (SF). Infrastructures 2023, 8, 38.
- de Abreu, G.B.; Costa, S.M.M.; Gumieri, A.G.; Calixto, J.M.F.; França, F.C.; Silva, C.; Quinõnes, A.D. Mechanical properties and microstructure of high performance concrete containing stabilized nano-silica. Matéria 2017, 22.
- Quercia, G.; Brouwers, H.J.H. Application of nano-silica (nS) in concrete mixtures. In Proceedings of the 8th Fib PhD Symposium in Civil Engineering, Lyngby, Denmark, 20–23 June 2010.
- Senff, L.; Hotza, D.; Repette, W.L.; Ferreira, V.M.; Labrincha, J.A. Mortars with nano-SiO2 and micro-SiO2 investigated by experimental design. Constr. Build. Mater. 2010, 24, 1432–1437.
- Kumar, S.; Kumar, A.; Kujur, J. Influence of nanosilica on mechanical and durability properties of concrete. Struct. Build. 2019, 172, 781–788.
- Khaloo, A.; Mobini, M.H.; Hosseini, P. Influence of different types of nano-SiO2 particles on properties of high-performance concrete. Constr. Build. Mater. 2016, 113, 188–201.
- Khan, K.; Ahmad, W.; Amin, M.N.; Nazar, S. Nano-silica-modified concrete: A bibliographic analysis and comprehensive review of material properties. Nanomaterials 2022, 12, 1989.
- Chu, H.; Zhang, Y.; Wang, F.; Feng, T.; Wang, L.; Wang, D. Effect of graphene oxide on mechanical properties and durability of ultra-high-performance concrete prepared from recycled sand. Nanomaterials 2020, 10, 1718.
- Saleem, H.; Zaidi, S.J.; Alnuaimi, N.A. Recent advancements in the nanomaterial application in concrete and its ecological impact. Materials 2021, 14, 6387.
- Du, S.; Wu, J.; AlShareedah, O.; Shi, X. Nanotechnology in cement-based materials: A review of durability, modeling, and advanced characterization. Nanomaterials 2019, 9, 1213.
- Golewski, G.L. Combined effect of coal fly ash (CFA) and nanosilica (nS) on the strength parameters and microstructural properties of eco-friendly concrete. Energies 2022, 16, 452.
- Golewski, G.L. The role of pozzolanic activity of siliceous fly ash in the formation of the structure of sustainable cementitious composites. Sustain. Chem. 2022, 3, 520–534.
- Abadel, A.A.; Alghamdi, H.; Alharbi, Y.R.; Alamri, M.; Khawaji, M.; Abdulaziz, M.A.M.; Nehdi, M.L. Investigation of alkali-activated slag-based composite incorporating dehydrated cement powder and red mud. Materials 2023, 16, 1551.
- Amin, M.N.; Ahmad, A.; Khan, K.; Ahmad, W.; Ehsan, S.; Alabdullah, A.A. Predicting the rheological properties of super-plasticized concrete using modeling techniques. Materials 2022, 15, 5208.
- Rassokhin, A.; Ponomarev, A.; Shambina, S.; Karlina, A. Different types of basalt fibers for disperse reinforcing of fine-grained concrete. Mag. Civ. Eng. 2022, 109, 10913.
- Rassokhin, A.; Ponomarev, A.; Karlina, A. Nanostructured high-performance concretes based on low-strength aggregates. Mag. Civ. Eng. 2022, 110, 11015.
- Rassokhin, A.S.; Ponomarev, A.N.; Karlina, A.I. High-performance fine-grained nanostructured concrete based on low strength aggregates. Mag. Civ. Eng. 2022, 114, 11413.
- Rassokhin, A.; Ponomarev, A.; Shambina, S.; Karlina, A. High performance lightweight concretes for 3D printing. Mag. Civ. Eng. 2022, 115, 11510.
- Kaprielov, S.S.; Sheinfeld, A.V.; Rudomino, M.V.; Gurevich, M.Z.; Krutikova, N.I.; Kopeiko, E.G. Method for Preparing an Aqueous Suspension. Patent No. 2060242 Russian Federation, C04B40, 20 May 1996. Applicant and Patent Holder Scientific Research, Design and Technological Institute of Concrete and Reinforced Concrete, 3p.
- Tatski, L.N.; Lokhova, N.A.; Gershanovich, G.L.; Senichak, E.B. Raw Mixture for the Manufacture of Wall Ceramic Products. Patent No. 2086517 Russian Federation, C04B35/16, 10 August 1997. Applicant and patent holder State educational institution of higher professional education “Brotherly State Technical University”, 6p.
- Lokhova, N.A.; Maksimova, S.M.; Mamaeva, T.V.; Karnaukhova, A.S.; Ilyin, E.A. Raw Mixture for the Manufacture of Wall Ceramic Products. Patent No. 2191168 Russian Federation, C04B35/16, 24 March 2013. Applicant and patent holder State educational institution of higher professional education “Brotherly State Technical University”, 5p.
- Kamenskikh, V.A.; Kashcheev, I.D.; Gulyaev, A.A. Silicon Carbide Concrete. Patent No. 2257361 Russian Federation, C04B35/56, 27 July 2005. Applicant and patent holder Limited Liability Company “Center for Energy Saving Technologies”, 4p.
- Udachkin, I.B.; Udachkin, V.I.; Smirnov, V.M.; Kolesnikov, V.E. Foam Concrete. Patent No. 2297993 Russian Federation, 27 April 2007. Applicant and patent holder Udachkin, I.B., Udachkin, V.I., Smirnov, V.M., Kolesnikov, V.E., 5p.
- Lupachev, V.N. Raw Mixture for the Preparation of Cellular Concrete. Patent No. 2338723 Russian Federation, C04B38, 20 November 2008. Applicant and patent holder Limited Liability Company “Pioneer”, 4p.
- Timothy, D.; James, M.; James, E. A System Containing Non-Flammable Reinforced Lightweight Panels Made of Cementitious Materials and a Metal Frame, Intended for Fire Walls and Other Fire-Resistant Assemblies. Patent No. 2388874 Russian Federation, 10 May 2010. Applicant and patentee United States Gypsum Company.
- Golotsan, A.A.; Dolgopolov, A.N. Complex Additive to Concrete Mixture in the form of a Stabilized Suspension of Microsilica. Patent No. 2422393 Russian Federation, C04B24/24, 27 June 2011. Applicant and patent holder A.N. Dolgopolov, 4p.
- Molodykh, A.S.; Kosareva, M.A.; Gabdullin, A.N.; Katyshev, S.F.; Vaitner, V.V.; Baykova, L.A.; Nikonenko, E.A. Method for Producing Highly Dispersed Silicon Dioxide. Patent No. 2593861 Russian Federation, C01B33/18, 10 August 2016. Applicant and patent holder A. N. Gabdullin, 5p.
- Selyaev, P.V.; Kupriyashkina, L.I.; Sedova, A.A.; Osipov, A.K.; Selyaev, V.P. Method for Producing Finely Dispersed Amorphous Microsilica by Sol-Gel Method. Patent No. 2625114 Russian Federation, C01B33/193, 11 July 2017. Applicant and patent holder Federal State Budgetary Educational Institution of Higher Education “National Research Mordovian State University named after N.P. Ogarev”, 4p.
- Bazhin, V.Y.; Dvoinikov, M.V.; Savchenkov, S.A.; Glazyev, M.V. Grouting Solution. Patent No. 2707837 Russian Federation, IPC C09K8/467C04B28/02 C04B22/06 C04B22/08 C04B18/14 C04B24/38 C04B111/20, 29 November 2019. Applicant and patent holder: Federal State Budgetary Educational Institution of Higher Education “St. Petersburg Mining University.
- Brichkin, V.N.; Bazhin, V.Y.; Savchenkov, S.A.; Glazyev, M.V. Cement Mixture. Patent No. 2726695 Russian Federation, IPC C09K 8/467, E21B 33/138, 15 July 2020. Applicant and patent holder FSBEI of higher education St. Petersburg Mining University.
More