Cholesterol trafficking is initiated by the endocytic pathway and transported from endo/lysosomes to other intracellular organelles. Deficiencies in cholesterol-sensing and binding proteins NPC1 and NPC2 induce accumulation in lysosomes and the malfunction of trafficking to other organelles. Each organelle possesses regulatory factors to induce cholesterol trafficking. The mutation of NPC1 and NPC2 genes induces Niemann-Pick disease type C (NPDC), which is a hereditary disease and causes progressive neurodegeneration, developmental disability, hypotonia, and ataxia. Oxidative stress induces damage in NPDC-related intracellular organelles. Although studies on the relationship between NPDC and oxidation are relatively rare, several studies have reported the therapeutic potential of antioxidants in treating NPDC. Investigating antioxidant drugs to relieve oxidative stress and cholesterol accumulation is suggested to be a powerful tool for developing treatments for NPDC. Understanding NPDC provides challenging issues in understanding the oxidative stress–lysosome metabolism of the lipid axis.
- cholesterol trafficking
- Niemann-Pick disease type C
- lysosomal storage disorders
- lysosomal proteins
- oxidative stress
1. Introduction
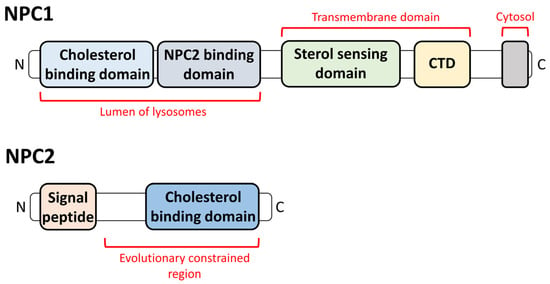
2. The Relationship between NPDC and Lysosomes
2.1. Lysosomal Appearance and the Changes in Lysosomes in NPDC
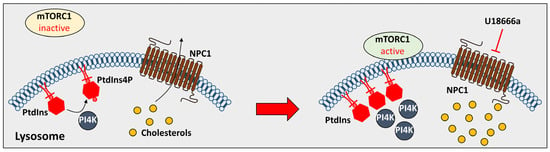
2.2. The Effect of the Lysosomal Proteins on NPDC
3. Current Therapeutic Strategies for NPDC
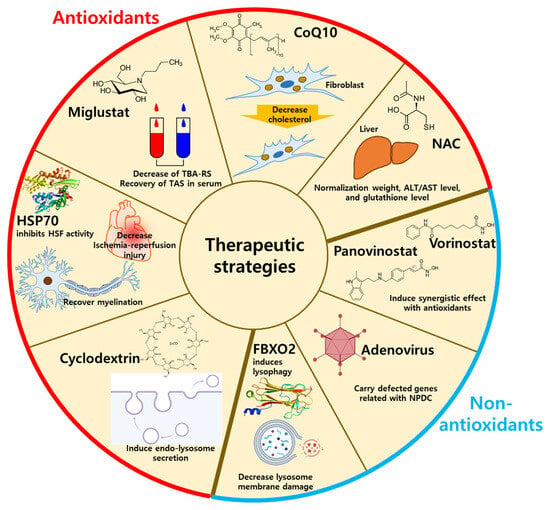
3.1. Antioxidant-Related Drugs for NPDC Treatment
3.1.1. N-Butyl-Deoxynojirimycin (Miglustat)
3.1.2. N-Acetylcysteine and Coenzyme Q10
3.1.3. Heat Shock Factor
3.1.4. Cyclodextrin
3.2. Non-Antioxidant Methods for NPDC Treatment
3.2.1. Lysophagy
3.2.2. Histone Deacetylase Inhibitors
3.2.3. Adenovirus
References
- Vanier, M.T. Complex lipid trafficking in Niemann-Pick disease type C. J. Inherit. Metab. Dis. 2015, 38, 187–199.
- Li, X.; Wang, J.; Coutavas, E.; Shi, H.; Hao, Q.; Blobel, G. Structure of human Niemann-Pick C1 protein. Proc. Natl. Acad. Sci. USA 2016, 113, 8212–8217.
- Winkler, M.B.L.; Kidmose, R.T.; Szomek, M.; Thaysen, K.; Rawson, S.; Muench, S.P.; Wustner, D.; Pedersen, B.P. Structural Insight into Eukaryotic Sterol Transport through Niemann-Pick Type C Proteins. Cell 2019, 179, 485–497.
- Carstea, E.D.; Morris, J.A.; Coleman, K.G.; Loftus, S.K.; Zhang, D.; Cummings, C.; Gu, J.; Rosenfeld, M.A.; Pavan, W.J.; Krizman, D.B.; et al. Niemann-Pick C1 disease gene: Homology to mediators of cholesterol homeostasis. Science 1997, 277, 228–231.
- Kuwabara, P.E.; Labouesse, M. The sterol-sensing domain: Multiple families, a unique role? Trends Genet 2002, 18, 193–201.
- Goldstein, J.L.; DeBose-Boyd, R.A.; Brown, M.S. Protein sensors for membrane sterols. Cell 2006, 124, 35–46.
- Petersen, D.; Reinholdt, P.; Szomek, M.; Hansen, S.K.; Poongavanam, V.; Dupont, A.; Heegaard, C.W.; Krishnan, K.; Fujiwara, H.; Covey, D.F.; et al. Binding and intracellular transport of 25-hydroxycholesterol by Niemann-Pick C2 protein. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183063.
- Brown, M.S.; Goldstein, J.L. A receptor-mediated pathway for cholesterol homeostasis. Science 1986, 232, 34–47.
- Roth, M.G. Clathrin-mediated endocytosis before fluorescent proteins. Nat. Rev. Mol. Cell Biol. 2006, 7, 63–68.
- Kwon, H.J.; Abi-Mosleh, L.; Wang, M.L.; Deisenhofer, J.; Goldstein, J.L.; Brown, M.S.; Infante, R.E. Structure of N-terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol. Cell 2009, 137, 1213–1224.
- Infante, R.E.; Abi-Mosleh, L.; Radhakrishnan, A.; Dale, J.D.; Brown, M.S.; Goldstein, J.L. Purified NPC1 protein. I. Binding of cholesterol and oxysterols to a 1278-amino acid membrane protein. J. Biol. Chem. 2008, 283, 1052–1063.
- Infante, R.E.; Radhakrishnan, A.; Abi-Mosleh, L.; Kinch, L.N.; Wang, M.L.; Grishin, N.V.; Goldstein, J.L.; Brown, M.S. Purified NPC1 protein: II. Localization of sterol binding to a 240-amino acid soluble luminal loop. J. Biol. Chem. 2008, 283, 1064–1075.
- Lee, D.; Hong, J.H. Nanoparticle-Mediated Therapeutic Application for Modulation of Lysosomal Ion Channels and Functions. Pharmaceutics 2020, 12, 217.
- Perera, R.M.; Zoncu, R. The Lysosome as a Regulatory Hub. Annu. Rev. Cell. Dev. Biol. 2016, 32, 223–253.
- Mrschtik, M.; Ryan, K.M. Lysosomal proteins in cell death and autophagy. FEBS J. 2015, 282, 1858–1870.
- Qi, X.; Man, S.M.; Malireddi, R.K.; Karki, R.; Lupfer, C.; Gurung, P.; Neale, G.; Guy, C.S.; Lamkanfi, M.; Kanneganti, T.D. Cathepsin B modulates lysosomal biogenesis and host defense against Francisella novicida infection. J. Exp. Med. 2016, 213, 2081–2097.
- Chen, O.C.W.; Colaco, A.; Davis, L.C.; Kiskin, F.N.; Farhat, N.Y.; Speak, A.O.; Smith, D.A.; Morris, L.; Eden, E.; Tynan, P.; et al. Defective platelet function in Niemann-Pick disease type C1. JIMD Rep. 2020, 56, 46–57.
- Grinan-Ferre, C.; Companys-Alemany, J.; Jarne-Ferrer, J.; Codony, S.; Gonzalez-Castillo, C.; Ortuno-Sahagun, D.; Vilageliu, L.; Grinberg, D.; Vazquez, S.; Pallas, M. Inhibition of Soluble Epoxide Hydrolase Ameliorates Phenotype and Cognitive Abilities in a Murine Model of Niemann Pick Type C Disease. Int. J. Mol. Sci. 2021, 22, 3409.
- Saito, R.; Miyajima, T.; Iwamoto, T.; Wu, C.; Suzuki, K.; Hossain, M.A.; Munakata, M.; Era, T.; Eto, Y. A neuropathological cell model derived from Niemann-Pick disease type C patient-specific iPSCs shows disruption of the p62/SQSTM1-KEAP1-NRF2 Axis and impaired formation of neuronal networks. Mol. Genet. Metab. Rep. 2021, 28, 100784.
- Cawley, N.X.; Lyons, A.T.; Abebe, D.; Luke, R.; Yerger, J.; Telese, R.; Wassif, C.A.; Bailey-Wilson, J.E.; Porter, F.D. Complex N-Linked Glycosylation: A Potential Modifier of Niemann-Pick Disease, Type C1 Pathology. Int. J. Mol. Sci. 2022, 23, 5082.
- Cawley, N.X.; Sojka, C.; Cougnoux, A.; Lyons, A.T.; Nicoli, E.R.; Wassif, C.A.; Porter, F.D. Abnormal LAMP1 glycosylation may play a role in Niemann-Pick disease, type C pathology. PLoS ONE 2020, 15, e0227829.
- Li, P.; Gu, M.X.; Xu, H.X. Lysosomal Ion Channels as Decoders of Cellular Signals. Trends Biochem. Sci. 2019, 44, 110–124.
- Fineran, P.; Lloyd-Evans, E.; Lack, N.A.; Platt, N.; Davis, L.C.; Morgan, A.J.; Hoglinger, D.; Tatituri, R.V.V.; Clark, S.; Williams, I.M.; et al. Pathogenic mycobacteria achieve cellular persistence by inhibiting the Niemann-Pick Type C disease cellular pathway. Wellcome Open Res. 2016, 1, 18.
- Pu, J.; Guardia, C.M.; Keren-Kaplan, T.; Bonifacino, J.S. Mechanisms and functions of lysosome positioning. J. Cell Sci. 2016, 129, 4329–4339.
- Roney, J.C.; Li, S.; Farfel-Becker, T.; Huang, N.; Sun, T.; Xie, Y.; Cheng, X.T.; Lin, M.Y.; Platt, F.M.; Sheng, Z.H. Lipid-mediated impairment of axonal lysosome transport contributing to autophagic stress. Autophagy 2021, 17, 1796–1798.
- Lim, C.Y.; Davis, O.B.; Shin, H.R.; Zhang, J.; Berdan, C.A.; Jiang, X.; Counihan, J.L.; Ory, D.S.; Nomura, D.K.; Zoncu, R. ER-lysosome contacts enable cholesterol sensing by mTORC1 and drive aberrant growth signalling in Niemann-Pick type C. Nat. Cell Biol. 2019, 21, 1206–1218.
- Nguyen, M.K.L.; Jose, J.; Wahba, M.; Bernaus-Esque, M.; Hoy, A.J.; Enrich, C.; Rentero, C.; Grewal, T. Linking Late Endosomal Cholesterol with Cancer Progression and Anticancer Drug Resistance. Int. J. Mol. Sci. 2022, 23, 7206.
- Meneses-Salas, E.; Garcia-Melero, A.; Kanerva, K.; Blanco-Munoz, P.; Morales-Paytuvi, F.; Bonjoch, J.; Casas, J.; Egert, A.; Beevi, S.S.; Jose, J.; et al. Annexin A6 modulates TBC1D15/Rab7/StARD3 axis to control endosomal cholesterol export in NPC1 cells. Cell Mol. Life Sci. 2020, 77, 2839–2857.
- Guardia, C.M.; Farias, G.G.; Jia, R.; Pu, J.; Bonifacino, J.S. BORC Functions Upstream of Kinesins 1 and 3 to Coordinate Regional Movement of Lysosomes along Different Microtubule Tracks. Cell Rep. 2016, 17, 1950–1961.
- Anderson, J.; Walker, G.; Pu, J. BORC-ARL8-HOPS ensemble is required for lysosomal cholesterol egress through NPC2. Mol. Biol. Cell 2022, 33, ar81.
- Kristensen, A.R.; Schandorff, S.; Hoyer-Hansen, M.; Nielsen, M.O.; Jaattela, M.; Dengjel, J.; Andersen, J.S. Ordered Organelle Degradation during Starvation-induced Autophagy. Mol. Cell. Proteom. 2008, 7, 2419–2428.
- Settembre, C.; Ballabio, A. Lysosomal Adaptation: How the Lysosome Responds to External Cues. Csh Perspect. Biol. 2014, 6, a016907.
- Davis, O.B.; Shin, H.R.; Lim, C.Y.; Wu, E.Y.; Kukurugya, M.; Maher, C.F.; Perera, R.M.; Ordonez, M.P.; Zoncu, R. NPC1-mTORC1 Signaling Couples Cholesterol Sensing to Organelle Homeostasis and Is a Targetable Pathway in Niemann-Pick Type C. Dev. Cell 2021, 56, 260–276.e7.
- Arguello, G.; Balboa, E.; Tapia, P.J.; Castro, J.; Yanez, M.J.; Mattar, P.; Pulgar, R.; Zanlungo, S. Genistein Activates Transcription Factor EB and Corrects Niemann-Pick C Phenotype. Int. J. Mol. Sci. 2021, 22, 4220.
- Rosato, A.S.; Krogsaeter, E.K.; Jaslan, D.; Abrahamian, C.; Montefusco, S.; Soldati, C.; Spix, B.; Pizzo, M.T.; Grieco, G.; Bock, J.; et al. TPC2 rescues lysosomal storage in mucolipidosis type IV, Niemann-Pick type C1, and Batten disease. Embo Mol. Med. 2022, 14, e15377.
- Sitarska, D.; Tylki-Szymanska, A.; Lugowska, A. Treatment trials in Niemann-Pick type C disease. Metab. Brain Dis. 2021, 36, 2215–2221.
- Kirkegaard, T.; Gray, J.; Priestman, D.A.; Wallom, K.L.; Atkins, J.; Olsen, O.D.; Klein, A.; Drndarski, S.; Petersen, N.H.T.; Ingemann, L.; et al. Heat shock protein-based therapy as a potential candidate for treating the sphingolipidoses. Sci. Transl. Med. 2016, 8, 355ra118.
- Schultz, M.L.; Fawaz, M.V.; Azaria, R.D.; Hollon, T.C.; Liu, E.A.; Kunkel, T.J.; Halseth, T.A.; Krus, K.L.; Ming, R.; Morin, E.E.; et al. Synthetic high-density lipoprotein nanoparticles for the treatment of Niemann-Pick diseases. BMC Med. 2019, 17, 200.
- Subramanian, K.; Hutt, D.M.; Scott, S.M.; Gupta, V.; Mao, S.; Balch, W.E. Correction of Niemann-Pick type C1 trafficking and activity with the histone deacetylase inhibitor valproic acid. J. Biol. Chem. 2020, 295, 8017–8035.
- Liu, E.A.; Schultz, M.L.; Mochida, C.; Chung, C.; Paulson, H.L.; Lieberman, A.P. Fbxo2 mediates clearance of damaged lysosomes and modifies neurodegeneration in the Niemann-Pick C brain. JCI Insight 2020, 5, e136676.
- Carlin, C.; Manor, D. Adenovirus Reveals New Pathway for Cholesterol Egress from the Endolysosomal System. Int. J. Mol. Sci. 2020, 21, 5808.
- Ribas, G.S.; Pires, R.; Coelho, J.C.; Rodrigues, D.; Mescka, C.P.; Vanzin, C.S.; Biancini, G.B.; Negretto, G.; Wayhs, C.A.; Wajner, M.; et al. Oxidative stress in Niemann-Pick type C patients: A protective role of N-butyl-deoxynojirimycin therapy. Int. J. Dev. Neurosci. 2012, 30, 439–444.
- Hammerschmidt, T.G.; Guerreiro, G.B.; Donida, B.; Raabe, M.; Kessler, R.G.; Ferro, M.B.; Moura, D.J.; Giugliani, R.; Vargas, C.R. Beneficial in vitro effect of N-acetylcysteine and coenzyme Q10 on DNA damage in neurodegenerative Niemann-Pick type C 1 disease: Preliminary results. Naunyn Schmiedebergs Arch. Pharmacol. 2023, 396, 1563–1569.
- Millea, P.J. N-acetylcysteine: Multiple clinical applications. Am. Fam. Physician 2009, 80, 265–269.
- Fu, R.; Wassif, C.A.; Yanjanin, N.M.; Watkins-Chow, D.E.; Baxter, L.L.; Incao, A.; Liscum, L.; Sidhu, R.; Firnkes, S.; Graham, M.; et al. Efficacy of N-acetylcysteine in phenotypic suppression of mouse models of Niemann-Pick disease, type C1. Hum. Mol. Genet 2013, 22, 3508–3523.
- Hargreaves, I.P. Ubiquinone: Cholesterol’s reclusive cousin. Ann. Clin. Biochem. 2003, 40, 207–218.
- Hammerschmidt, T.G.; Donida, B.; Faverzani, J.L.; Moura, A.P.; Dos Reis, B.G.; Machado, A.Z.; Kessler, R.G.; Sebastiao, F.M.; Reinhardt, L.S.; Moura, D.J.; et al. Cytokine profile and cholesterol levels in patients with Niemann-Pick type C disease presenting neurological symptoms: In vivo effect of miglustat and in vitro effect of N-acetylcysteine and coenzyme Q10. Exp. Cell Res. 2022, 416, 113175.
- Verghese, J.; Abrams, J.; Wang, Y.; Morano, K.A. Biology of the heat shock response and protein chaperones: Budding yeast (Saccharomyces cerevisiae) as a model system. Microbiol. Mol. Biol. Rev. 2012, 76, 115–158.
- Hightower, L.E. Heat shock, stress proteins, chaperones, and proteotoxicity. Cell 1991, 66, 191–197.
- Szyller, J.; Bil-Lula, I. Heat Shock Proteins in Oxidative Stress and Ischemia/Reperfusion Injury and Benefits from Physical Exercises: A Review to the Current Knowledge. Oxid. Med. Cell. Longev. 2021, 2021, 6678457.
- Shi, Y.; Mosser, D.D.; Morimoto, R.I. Molecular chaperones as HSF1-specific transcriptional repressors. Genes Dev. 1998, 12, 654–666.
- Gray, J.; Fernandez-Suarez, M.E.; Falah, M.; Smith, D.; Smith, C.; Kaya, E.; Palmer, A.M.; Fog, C.K.; Kirkegaard, T.; Platt, F.M. Heat shock protein amplification improves cerebellar myelination in the Npc1(nih) mouse model. EBioMedicine 2022, 86, 104374.
- Bernardo, A.; De Nuccio, C.; Visentin, S.; Martire, A.; Minghetti, L.; Popoli, P.; Ferrante, A. Myelin Defects in Niemann-Pick Type C Disease: Mechanisms and Possible Therapeutic Perspectives. Int. J. Mol. Sci. 2021, 22, 8858.
- Feltes, M.; Gale, S.E.; Moores, S.; Ory, D.S.; Schaffer, J.E. Monitoring the itinerary of lysosomal cholesterol in Niemann-Pick Type C1-deficient cells after cyclodextrin treatment. J. Lipid Res. 2020, 61, 403–412.
- Donida, B.; Raabe, M.; Tauffner, B.; de Farias, M.A.; Machado, A.Z.; Timm, F.; Kessler, R.G.; Hammerschmidt, T.G.; Reinhardt, L.S.; Brito, V.B.; et al. Nanoparticles containing beta-cyclodextrin potentially useful for the treatment of Niemann-Pick C. J. Inherit. Metab. Dis. 2020, 43, 586–601.
- Lopez-Nicolas, J.M.; Rodriguez-Bonilla, P.; Garcia-Carmona, F. Cyclodextrins and antioxidants. Crit. Rev. Food Sci. Nutr. 2014, 54, 251–276.
- Jo, Y.J.; Cho, H.S.; Chun, J.Y. Antioxidant activity of beta-cyclodextrin inclusion complexes containing trans-cinnamaldehyde by DPPH, ABTS and FRAP. Food Sci. Biotechnol. 2021, 30, 807–814.
- Hoque, S.; Kondo, Y.; Sakata, N.; Yamada, Y.; Fukaura, M.; Higashi, T.; Motoyama, K.; Arima, H.; Higaki, K.; Hayashi, A.; et al. Differential Effects of 2-Hydroxypropyl-Cyclodextrins on Lipid Accumulation in Npc1-Null Cells. Int. J. Mol. Sci. 2020, 21, 898.
- Vacca, F.; Vossio, S.; Mercier, V.; Moreau, D.; Johnson, S.; Scott, C.C.; Montoya, J.P.; Moniatte, M.; Gruenberg, J. Cyclodextrin triggers MCOLN1-dependent endo-lysosome secretion in Niemann-Pick type C cells. J. Lipid Res. 2019, 60, 832–843.
- Singhal, A.; Krystofiak, E.S.; Jerome, W.G.; Song, B. 2-Hydroxypropyl-gamma-cyclodextrin overcomes NPC1 deficiency by enhancing lysosome-ER association and autophagy. Sci. Rep. 2020, 10, 8663.
- Okada, Y.; Kuroiwa, S.; Noi, A.; Tanaka, A.; Nishikawa, J.; Kondo, Y.; Ishitsuka, Y.; Irie, T.; Higaki, K.; Matsuo, M.; et al. Effects of 6-O-a-maltosyl-S cyclodextrin on lipid metabolism in Npc1-deficient Chinese hamster ovary cells. Mol. Genet. Metab. 2022, 137, 239–248.
- Yasmin, N.; Ishitsuka, Y.; Fukaura, M.; Yamada, Y.; Nakahara, S.; Ishii, A.; Kondo, Y.; Takeo, T.; Nakagata, N.; Motoyama, K.; et al. In Vitro and In Vivo Evaluation of 6-O-Maltosyl-Cyclodextrin as a Potential Therapeutic Agent against Niemann-Pick Disease Type C. Int. J. Mol. Sci. 2019, 20, 1152.
- Mizushima, N. The ubiquitin E2 enzyme UBE2QL1 mediates lysophagy. EMBO Rep. 2019, 20, e49104.
- Papadopoulos, C.; Kravic, B.; Meyer, H. Repair or Lysophagy: Dealing with Damaged Lysosomes. J. Mol. Biol. 2020, 432, 231–239.
- Pipalia, N.H.; Subramanian, K.; Mao, S.; Ralph, H.; Hutt, D.M.; Scott, S.M.; Balch, W.E.; Maxfield, F.R. Histone deacetylase inhibitors correct the cholesterol storage defect in most Niemann-Pick C1 mutant cells. J. Lipid Res. 2017, 58, 695–708.
- Cardoso, B.A.; Ramos, T.L.; Belo, H.; Vilas-Boas, F.; Real, C.; Almeida, A.M. Vorinostat synergizes with antioxidant therapy to target myeloproliferative neoplasms. Exp. Hematol. 2019, 72, 60–71.e11.
- Moshref, M.; Questa, M.; Lopez-Cervantes, V.; Sears, T.K.; Greathouse, R.L.; Crawford, C.K.; Kol, A. Panobinostat Effectively Increases Histone Acetylation and Alters Chromatin Accessibility Landscape in Canine Embryonic Fibroblasts but Does Not Enhance Cellular Reprogramming. Front. Vet. Sci. 2021, 8, 716570.
- Luisoni, S.; Suomalainen, M.; Boucke, K.; Tanner, L.B.; Wenk, M.R.; Guan, X.L.; Grzybek, M.; Coskun, U.; Greber, U.F. Co-option of Membrane Wounding Enables Virus Penetration into Cells. Cell Host Microbe 2015, 18, 75–85.
- Suomalainen, M.; Nakano, M.Y.; Boucke, K.; Keller, S.; Greber, U.F. Adenovirus-activated PKA and p38/MAPK pathways boost microtubule-mediated nuclear targeting of virus. Embo J. 2001, 20, 1310–1319.
- Paul, C.A.; Reid, P.C.; Boegle, A.K.; Karten, B.; Zhang, M.; Jiang, Z.G.; Franz, D.; Lin, L.; Chang, T.Y.; Vance, J.E.; et al. Adenovirus expressing an NPC1-GFP fusion gene corrects neuronal and nonneuronal defects associated with Niemann pick type C disease. J. Neurosci. Res. 2005, 81, 706–719.
- Xie, C.; Gong, X.M.; Luo, J.; Li, B.L.; Song, B.L. AAV9-NPC1 significantly ameliorates Purkinje cell death and behavioral abnormalities in mouse NPC disease. J. Lipid Res. 2017, 58, 512–518.
- Zeng, X.; Carlin, C.R. Adenovirus early region 3 RIDalpha protein limits NFkappaB signaling through stress-activated EGF receptors. PLoS Pathog. 2019, 15, e1008017.
- Shah, A.H.; Cianciola, N.L.; Mills, J.L.; Sonnichsen, F.D.; Carlin, C. Adenovirus RIDalpha regulates endosome maturation by mimicking GTP-Rab7. J. Cell Biol. 2007, 179, 965–980.
- Rocha, N.; Kuijl, C.; van der Kant, R.; Janssen, L.; Houben, D.; Janssen, H.; Zwart, W.; Neefjes, J. Cholesterol sensor ORP1L contacts the ER protein VAP to control Rab7-RILP-p150 Glued and late endosome positioning. J. Cell Biol. 2009, 185, 1209–1225.
- Johansson, M.; Rocha, N.; Zwart, W.; Jordens, I.; Janssen, L.; Kuijl, C.; Olkkonen, V.M.; Neefjes, J. Activation of endosomal dynein motors by stepwise assembly of Rab7-RILP-p150Glued, ORP1L, and the receptor betalll spectrin. J. Cell Biol. 2007, 176, 459–471.
- Cianciola, N.L.; Carlin, C.R. Adenovirus RID-alpha activates an autonomous cholesterol regulatory mechanism that rescues defects linked to Niemann-Pick disease type C. J. Cell Biol. 2009, 187, 537–552.
