Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Antonina Karlina and Version 2 by Camila Xu.
Microsilica, a by-product of silicon or ferrosilicon production. Widely utilized within the construction industry, microsilica serves as a modifying component in concrete production, leveraging its chemical composition and physical attributes as a highly active pozzolan. Natural additives encompass crushed volcanic and sedimentary rocks, diatomites, volcanic ash, and tuff. Within technogenic additives lie waste or by-products from various industries, such as fly ash, granulated blast furnace slag, and microsilica.
- nanomaterials
- nanosilica
- cementitious materials
- mechanical properties
1. Introduction
Since the 1970s and 1980s, ultrafine forms of silica SiO2, such as microsilica and nanosilica, have been actively integrated into concrete technology. There are four primary types of amorphous silica available in the global market: precipitated silica (white soot), silica gel (dried silica gel), pyrogenic silica, and microsilica. The worldwide consumption of all synthetic silica types amounts to approximately 1 million tonnes [1][2][3][4][5][1,2,3,4,5].
Microsilica, a by-product of silicon or ferrosilicon production, presents as amorphous silica (SiO2) in spherical particle form [1][2][3][4][5][6][7][8][9][10][1,2,3,4,5,6,7,8,9,10]. Widely utilized within the construction industry, microsilica serves as a modifying component in concrete production, leveraging its chemical composition and physical attributes as a highly active pozzolan [10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25]. The extensive introduction of various highly effective chemical and mineral additives has led to significant advancements in modern concrete technology.
It is acknowledged [25] that the incorporation of mineral fillers in concrete mixtures stands as a priority for reducing cement consumption and enhancing both its technological and operational properties. Natural additives encompass crushed volcanic and sedimentary rocks, diatomites, volcanic ash, and tuff. Within technogenic additives lie waste or by-products from various industries, such as fly ash, granulated blast furnace slag, and microsilica [25][26][27][28][29][25,26,27,28,29]. Microsilica emerges as one of the most potent and finely dispersed mineral additives. Its substantial specific surface area and amorphous structure endow it with high pozzolanic activity, functioning as an effective microfiller. The utilization of microsilica as a mineral additive proves a promising method for generating concrete with high-performance characteristics [25][26][25,26].
Wastes from ferroalloy production contain an elevated SiO2 content ranging from 85% to 95%, with a reduced content down to 65–75%. The specific surface area of microsilica ranges from 15,000 to 25,000 m2/kg, significantly surpassing the specific surface area of Portland cement, which stands at 300 to 400 m2/kg. Exhibiting high pozzolanic activity, microsilica operates effectively as a microfiller [30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49]. The development of a cementitious structure in the presence of microsilica relies on microfilling and pozzolanic effects. Furthermore, these particles serve as crystallization centers, amplifying the cement’s early-age hydration and increasing the packing density of the hydrate phases.
During cement hydration, the release of calcium hydroxide allows SiO2 to interact due to its high pozzolanic activity. This reaction leads to the formation of highly dispersed, low-basic calcium hydrosilicates C-S-H (I) instead of portlandite crystals Ca(OH)2, significantly enhancing the properties of the cement structure. This transformation reduces the presence of capillary pores, compacting the structure and decreasing cement stone permeability, thereby boosting its resistance to aggressive environments and overall durability [23][34][45][46][23,34,45,46]. Concrete mixtures containing microsilica exhibit increased thixotropy, viscosity, and resistance to delamination due to their high water-holding capacity [10][22][34][50][51][10,22,34,50,51]. However, the use of microsilica, due to its high specific surface area, significantly increases the water demand for concrete mixtures. It is estimated that 1 kg of microsilica raises the water requirement of a concrete mixture by 1 L [50]. Consequently, under conditions of uniform mobility of concrete mixtures, the use of microsilica exhibits an insignificant strength effect and proves ineffective [11][12][13][14][15][16][17][11,12,13,14,15,16,17]. To mitigate this, plasticizing additives are necessary to reduce water demand in concrete mixtures and enhance the efficacy of microsilica as a plasticizer.
Recent reviews [2][3][2,3] have meticulously explored ultrafine mineral compounds with minimal silica content, examining the addition of microsilica to concrete mixtures to advance concrete technologies. Concrete remains a cornerstone material in construction, with its demand escalating annually [4][5][4,5]. Ordinary Portland cement (OPC) is a crucial component contributing significantly to greenhouse gas emissions [10][11][12][13][10,11,12,13]. OPC production accounts for around 5–8% of global CO2 emissions [8][9][10][11][8,9,10,11]. Cement usage surpasses 4000 million tons annually and is projected to reach approximately 6000 million tons by 2060 [12][13][12,13], greatly contributing to climate change through greenhouse gas emissions [13][14][15][13,14,15]. To address this, agricultural and industrial wastes, such as rice husk ash (RHA), sugarcane bagasse ash, and olive oil ash, are utilized to partially substitute OPC in the production of green concrete [16][17][18][19][20][21][16,17,18,19,20,21].
Reviews [22][23][22,23] that analyzed studies involving micro- and nanosilica in construction demonstrate their use alongside fly ash and other microparticles to enhance the compactness, strength, and durability of cementitious materials. A scientometric analysis in [22] evaluated the keywords prevalent in nanostructured (NS)-modified concrete studies, listing the 20 most frequently used terms (Table 1). Nanosilica, microsilica, compressive strength, concrete, and cement emerged as the top five terms consistently employed in this field of research.
Table 1.
| S/N | Keyword | Occurrences |
|---|
,41].
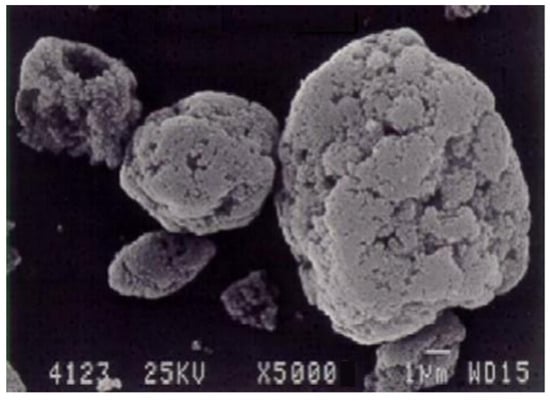
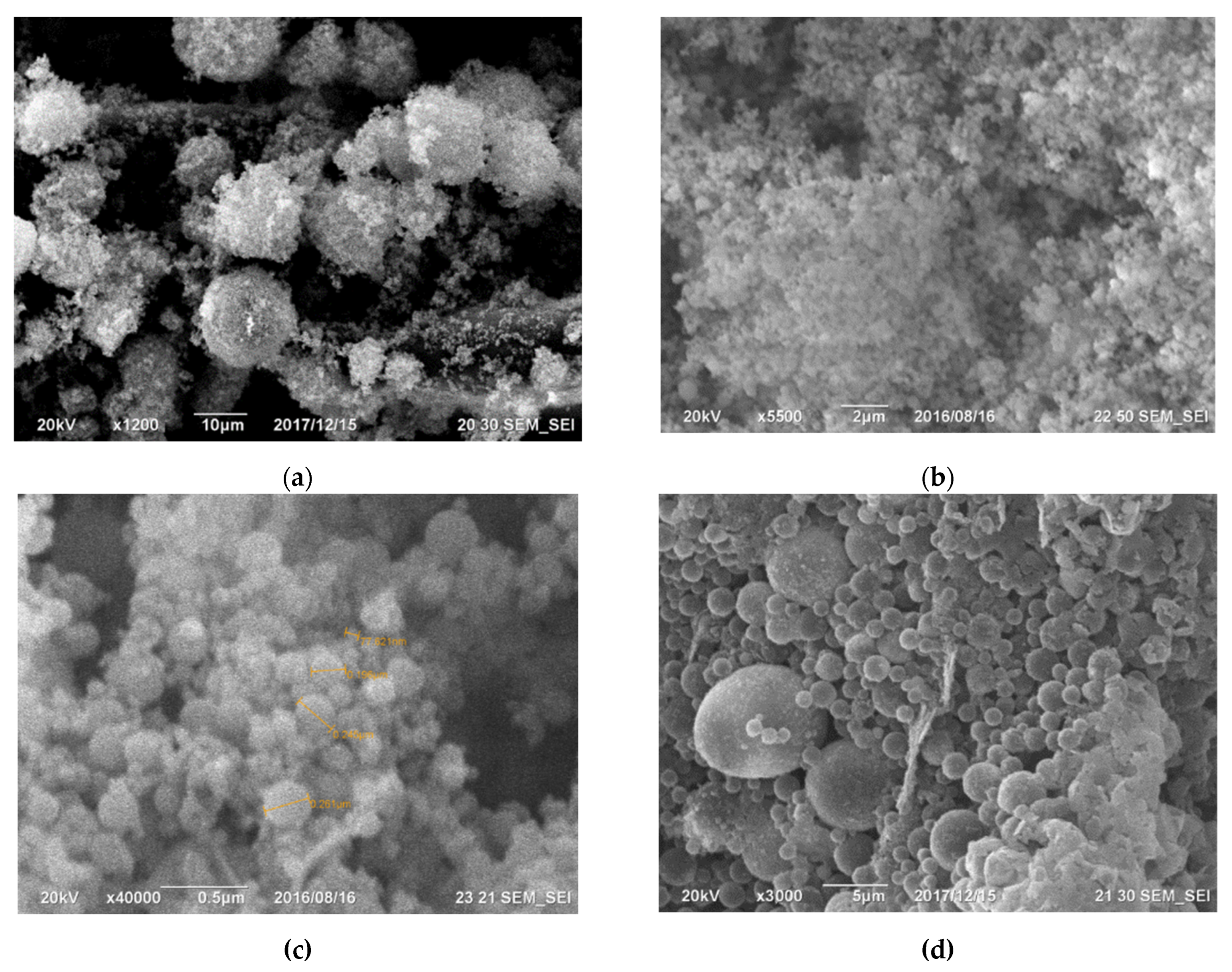
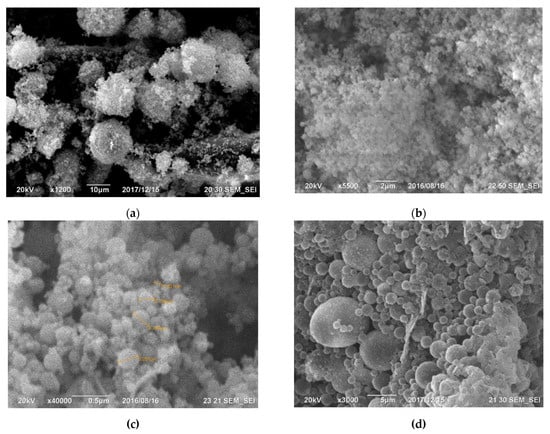
The primary components within the initial cyclone dust were nano- and micro-sized SiO2 spheres, as depicted in Figure 1. Figure 1a displays micro- and nanospheres of SiO2 magnified 15,000 times, whereas Figure 1b, magnified at 50,000 times, exhibits a micro-sized sphere enveloped with nano-sized SiO2 spheres. Figure 2 illustrates the differential and indirect particle distribution by dust size within the cyclones at the Bratsk Ferroalloy Plant.
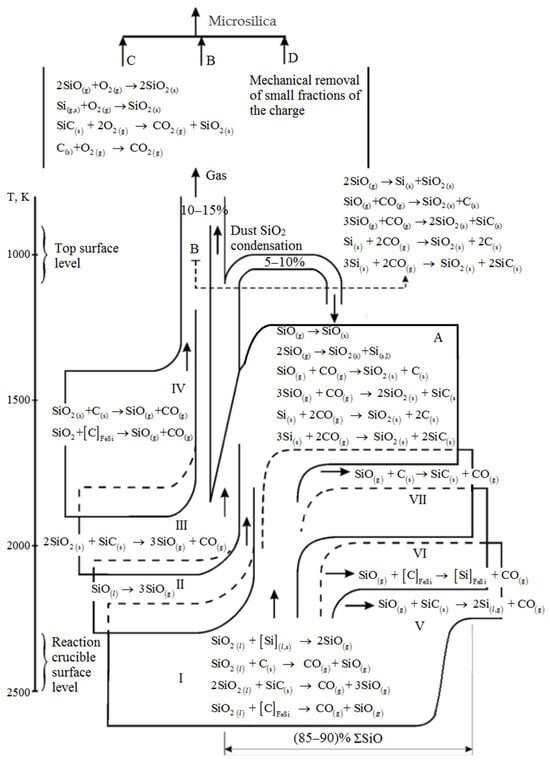
 Protopopov, E.V. et al. [39] present a diagram depicting the silicon monoxide formation balance during silicon production (Figure 3). When a charge containing silicon is melted, silica undergoes sublimation to create silicon monoxide, which then condenses within the colder, upper layers of the charge. A portion of the silicon monoxide is carried by the gas flow to the furnace surface, where it oxidizes to silicon dioxide and is captured by gas cleaning devices [40][41][40,41].
About 10% of the total gas content consists of primary dust, characterized by low dispersion. This dust can be efficiently eliminated from traditional dry and wet cleaning systems due to its granulometric composition. However, the remaining 90% of the dust comprises highly dispersed particles, primarily 2 μm in size. The formation of microsilica is thought to result from the processes outlined in Figure 3, according to the authors of [39]:
Protopopov, E.V. et al. [39] present a diagram depicting the silicon monoxide formation balance during silicon production (Figure 3). When a charge containing silicon is melted, silica undergoes sublimation to create silicon monoxide, which then condenses within the colder, upper layers of the charge. A portion of the silicon monoxide is carried by the gas flow to the furnace surface, where it oxidizes to silicon dioxide and is captured by gas cleaning devices [40][41][40,41].
About 10% of the total gas content consists of primary dust, characterized by low dispersion. This dust can be efficiently eliminated from traditional dry and wet cleaning systems due to its granulometric composition. However, the remaining 90% of the dust comprises highly dispersed particles, primarily 2 μm in size. The formation of microsilica is thought to result from the processes outlined in Figure 3, according to the authors of [39]:
Microsilica, depicted in Figure 1, is a powdery substance composed of ultrafine spherical particles [41]. It emerges as a by-product during the gas purification process in technological furnaces utilized for producing silicon-containing alloys (such as metallic silicon, ferrosilicon, silicomanganese, and ferrosilicochrome). Table 3 illustrates the X-ray spectral analysis, presenting the primary elements found in the cyclone dust [28][41][28
 Aligned with the Si-O-C diagram and based on process conditions and the condensed phase composition, the furnace bath can be classified into three distinct zones: low, medium, and high temperature [40][41][42][43][44][45][40,41,42,43,44,45]. These zones exhibit variations in temperature conditions, condensed phase composition, and, most significantly, the inherent processes involved.
The low-temperature zone, existing under conditions of thermal equilibrium, maintains a temperature not exceeding 1500 °C. The condensed phases in this zone are dictated by the initial charge’s composition and the associated processes.
Moving to the medium temperature zone, this area lacks carbon but features the presence of only silica and silicon carbide in its condensed phases.
The high-temperature zone, surpassing 1817 °C, delineates a space where silicon remains stable solely at elevated temperatures exceeding 1817 °C and at a high concentration of SiO(g). Here, the destruction of silicon carbide and the production of silicon may lead to substantial SiO(g) losses. These losses are mitigated by removing them from the high-temperature zone of the furnace bath, resulting in the predominance of silicon dioxide—a primary component of process dust removed from the furnace along with process gases. This promising raw material is sourced from waste dust in the cyclones within the emission purification system used during catalytic silicon production.
Preliminary research indicates that silicon-containing dust comprises 80%–95.28% SiO2 [39][40][41][42][43][39,40,41,42,43]. The dust consists of spherical particles, ranging from nano-disperse particles up to 100 nm, which have a tendency to aggregate. The study of this waste is essential for extracting silicon dioxide, silicon carbide, and carbon [40][41][40,41].
Gas purification dust from silicon production is actively used in construction. However, cyclone dust is unsuitable for this application due to its high carbon content, which violates regulatory documents [47][48][49][47,48,49]. Conversely, microsilica obtained by trapping furnace gases exhibits a minimal bulk density, ranging from 130 to 430 kg/m3. Challenges in utilizing and storing this product have led to its application in a more convenient, compressed form (with a density of 480–720 kg/m3) or as an aqueous suspension with a solid content of 50 wt% (with a density of 1320–1440 kg/m3). The compressed product is produced by passing air through silos containing microsilica over several hours, causing the fusion of clusters into larger aggregates, ranging from 10 to several 100 microns in size (Figure 5).
Aligned with the Si-O-C diagram and based on process conditions and the condensed phase composition, the furnace bath can be classified into three distinct zones: low, medium, and high temperature [40][41][42][43][44][45][40,41,42,43,44,45]. These zones exhibit variations in temperature conditions, condensed phase composition, and, most significantly, the inherent processes involved.
The low-temperature zone, existing under conditions of thermal equilibrium, maintains a temperature not exceeding 1500 °C. The condensed phases in this zone are dictated by the initial charge’s composition and the associated processes.
Moving to the medium temperature zone, this area lacks carbon but features the presence of only silica and silicon carbide in its condensed phases.
The high-temperature zone, surpassing 1817 °C, delineates a space where silicon remains stable solely at elevated temperatures exceeding 1817 °C and at a high concentration of SiO(g). Here, the destruction of silicon carbide and the production of silicon may lead to substantial SiO(g) losses. These losses are mitigated by removing them from the high-temperature zone of the furnace bath, resulting in the predominance of silicon dioxide—a primary component of process dust removed from the furnace along with process gases. This promising raw material is sourced from waste dust in the cyclones within the emission purification system used during catalytic silicon production.
Preliminary research indicates that silicon-containing dust comprises 80%–95.28% SiO2 [39][40][41][42][43][39,40,41,42,43]. The dust consists of spherical particles, ranging from nano-disperse particles up to 100 nm, which have a tendency to aggregate. The study of this waste is essential for extracting silicon dioxide, silicon carbide, and carbon [40][41][40,41].
Gas purification dust from silicon production is actively used in construction. However, cyclone dust is unsuitable for this application due to its high carbon content, which violates regulatory documents [47][48][49][47,48,49]. Conversely, microsilica obtained by trapping furnace gases exhibits a minimal bulk density, ranging from 130 to 430 kg/m3. Challenges in utilizing and storing this product have led to its application in a more convenient, compressed form (with a density of 480–720 kg/m3) or as an aqueous suspension with a solid content of 50 wt% (with a density of 1320–1440 kg/m3). The compressed product is produced by passing air through silos containing microsilica over several hours, causing the fusion of clusters into larger aggregates, ranging from 10 to several 100 microns in size (Figure 5).
 The electric furnace generates soot collected from various collection systems, including baghouses. Instead of disposing of it in slurry fields, this soot is sold as an additive (AS) [41]. One of the significant advantages of using silica fume is its suitability as a mineral admixture in concrete [49]. Essentially, silica consists of amorphous (non-crystalline) silicon dioxide (SiO2). Currently, concrete failure when incorporating microsilica arises from corrosion, leading to increased expenses due to sea salt or icing. Therefore, it is crucial to ensure the end product’s resistance to sulphate, thereby fulfilling a primary objective.
The consensus recognizes that utilizing compressed microsilica necessitates longer and more intense mixing to disperse agglomerates. Despite the technological advancement of microsilica suspensions, they cannot be stored under subzero temperatures and require redispersion after prolonged storage. In the UK, the use of compressed microsilica holds paramount practical importance, while in European countries, both suspended forms and compressed microsilica are commonly employed.
The electric furnace generates soot collected from various collection systems, including baghouses. Instead of disposing of it in slurry fields, this soot is sold as an additive (AS) [41]. One of the significant advantages of using silica fume is its suitability as a mineral admixture in concrete [49]. Essentially, silica consists of amorphous (non-crystalline) silicon dioxide (SiO2). Currently, concrete failure when incorporating microsilica arises from corrosion, leading to increased expenses due to sea salt or icing. Therefore, it is crucial to ensure the end product’s resistance to sulphate, thereby fulfilling a primary objective.
The consensus recognizes that utilizing compressed microsilica necessitates longer and more intense mixing to disperse agglomerates. Despite the technological advancement of microsilica suspensions, they cannot be stored under subzero temperatures and require redispersion after prolonged storage. In the UK, the use of compressed microsilica holds paramount practical importance, while in European countries, both suspended forms and compressed microsilica are commonly employed.

Figure 1.
a
) ×15,000; (
b
) ×50,000.
Table 3.
| Elements | C | O | Na | Mg | Al | Si | K | Ca | Mn | Fe | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Nanosilica | 539 | |||||||||
| 100.00 | |||||||||||
| 2 | O: 0.6%–0.8%; K | 2 | O: 1.2%–1.4%; C: 0.9%–1.2%; S: 0.2%–0.3% | ||||||||
| Weight, % | 3.98 | 53.78 | 0.37 | 0.62 | 0.34 | ||||||
| 2 | Silica | 533 | |||||||||
| 38.54 | 3 | Compressive strength | 393 |

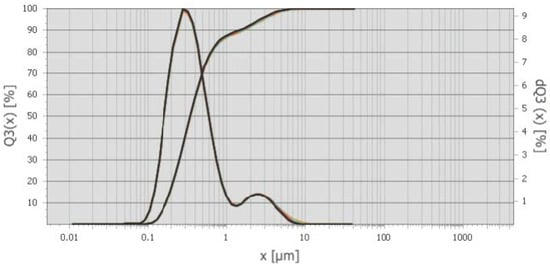
Figure 2. The differential and integral distribution of particles by dust size from the cyclones of the Bratsk Ferroalloy Plant is shown in the graph (Distribution mode is 0.29 μm) [41].
-
Interaction between silicon monoxide and carbon monoxide in a gas furnace at temperatures ranging from 1400 to 1800 K leads to the formation of microsilica.
| 0.79 | 0.17 | 0.17 | 1.24 |
| Serov Ferroalloy Plant, Ltd. | |||
| FS 65, FS 45 | FS 65 (Si: 63%–68%; C: 0.1%; S: 0.02%; P: 0.05%; Al: 2.5%; Mg: 0.4%; Cr: 0.4%); | FS 45 (Si: 41%–47%; C: 0.2%; S: 0.02%; P: 0.05%; Al: 2.0%; Mg: 1.0%; Cr: 0.5%) |
|
| Bratsk Ferroalloy Plant, Ltd. | Additive to concrete, which is widely used in the manufacture of classes of concrete subject to erosive abrasion and possessing improved water resistance | FS 65, FS 75 | FS 65 (Si: 63%–68%; C: 0.1%; S: 0.02%; P: 0.05%; Al: 2.5%; Mg: 0.4%; Cr: 0.4%); FS 75 (Si: 74%–80%; C: 0.1%; S: 0.02%; P: 0.04%; Al: 3.0%; Mg: 0.4%; Cr: 0.3%) |
| Kremniy (Shelekhov), RUSAL, Ltd. | Needs of chemical and electrical industry enterprises | No information | Na2O: 0.04%; MgO: 0.13%; Al2O3: 0.14%; SiO2: 98.99%;P2O5: 0.0060%; S: 0.0038%; K2O: 0.28%; CaO: 0.47%; TiO2: <0.001%; MnO: 0.015%; Fe2O3: 0.034% |
- In the low-temperature zones of the furnace, where the gas phase temperature and the equilibrium concentration of SiO sharply decrease, silicon monoxide disproportionation may occur.
-
The very high cooling rates of the gas phase could potentially lead to the direct condensation of silicon monoxide.




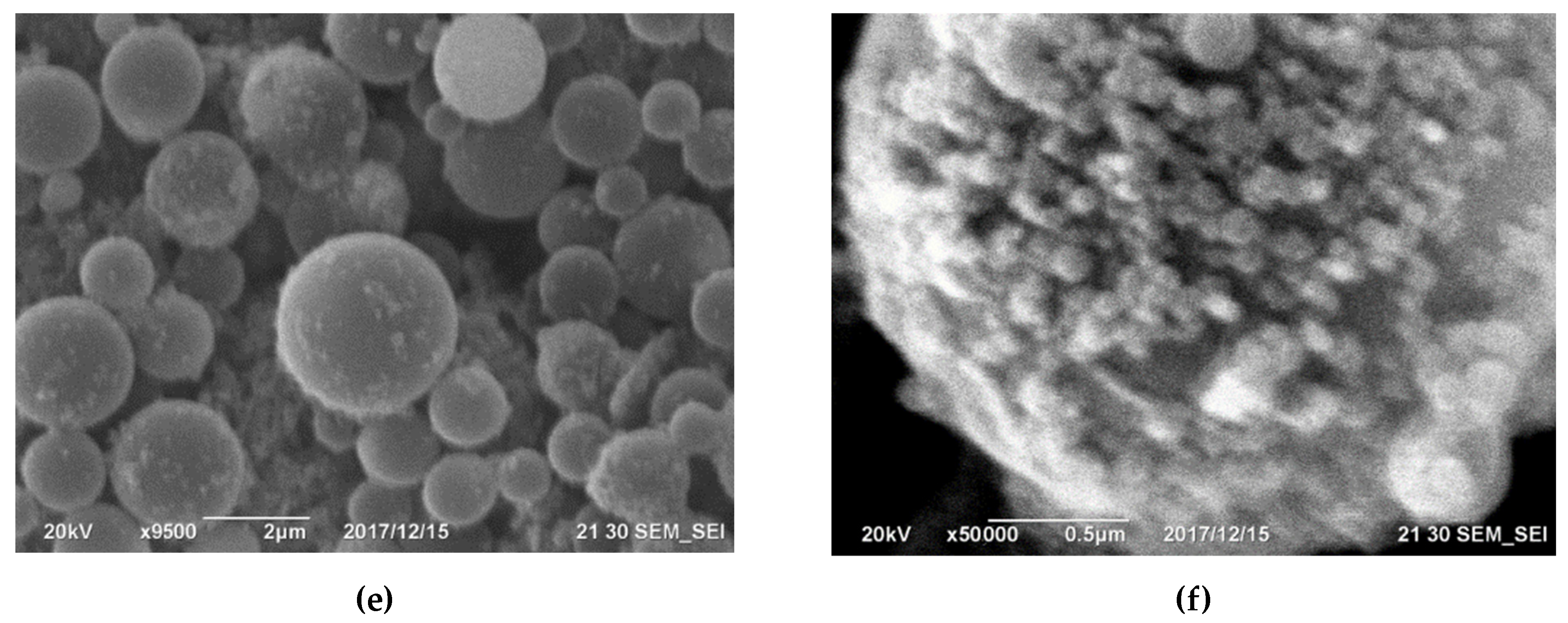
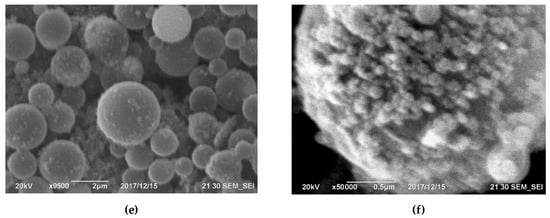
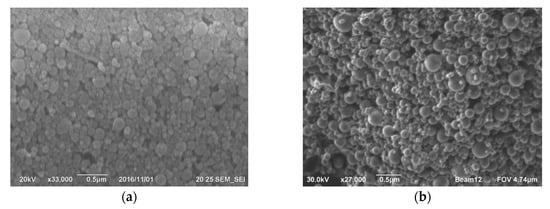
Figure 4. Microphotographs of silicon hose dust surface from the Kamensk-Uralsky branch of OJSC SUAL have been taken, showcasing (a) the top view, (b) the appearance at higher magnification, and (c) surface detailing, with a determination of the diameters of spherical particles of silicon dioxide. Images of the surface of silicon dust after wet cleaning by JSC Kremniy [39]: (d) viewed from above, (e) a closer look at the surface at higher magnification, and (f) a detailed view at 50,000 times magnification, showing spherical particles of silicon dioxide on the surface. [41].

Figure 5.
a
) ×33,000; (
b
) ×27,000.
4. History of the Use of Micro and Nanosilica as a Cement Additive
In 1950, the Fiskaa plant in Kristiansand, Norway, installed the first experimental filters for capturing microsilica [50]. Two years later, initial tests were conducted using MS as an additive to Portland cement concrete, coinciding with the first publication on its concrete application. The commercial sales of microsilica in concrete plants commenced around 1971. In 1974, engineers from the Fiskaa plant (later forming the Elkem organization) significantly redesigned the industrial bag filter. The increased use of microsilica in concrete led to compliance with silica fume standards in cement in Norway between 1976 and 1978, slightly later in concrete. The utilization of MS in Bluetooth and Iceland began in 1981. By 1990, microsilica had gained global recognition as a concrete additive, proving its capability to enhance final product properties. Considerable attention was directed towards ensuring reliability and extended service life. By the year 2000, international standards for the use of Microsilica additives (MS) in concrete technologies became available and were commonly applied in industrial production across most countries. High-strength concrete incorporating MS was used in the construction of significant infrastructure projects, including high-rise buildings in Chicago, the Channel Tunnel, the Northumberland Strait Bridge in Washington, and offshore drilling platforms in the North Sea. In Russia, MS-based construction projects were undertaken, albeit with a delay of several decades. These projects in Russia encompass transport tunnels on Kutuzovsky, Leninsky, and Nakhimovsky prospects, a bridge on Bratislavskaya, an overpass on Oleniy Valkikh, and gravity-type oil platform streets for the Sakhalin-2 project. As previously noted, MS is a byproduct derived from silicon production, processed in electric furnaces [28][39][40][28,39,40], with required raw materials including quartz, coal, or wood chips.5. History of the Use of Micro and Nanosilica as a Cement Additive in Russia
Russia has implemented a standard [49] for an active mineral additive of technogenic origin exhibiting high pozzolanic activity, known as condensed microsilica (hereinafter referred to as microsilica). This is designed to specifically regulate the properties of concrete, mortar, and dry building mixtures, which are made with binders primarily based on Portland cement clinker (Table 4). Microsilica is categorized in Russian documents like “MK” into three types, each identified as follows:Table 4.
| The Name of Indicators | Standard Value of Quality Indicator for Grades of Condensed Microsilica * | ||||
|---|---|---|---|---|---|
| Uncompacted | Compacted | Suspensions (Pastes) | |||
| MK-65 | MK-58 | MKU-65 | MKU-85 | MKS-85 | |
| 1 External appearance | Ultrafine gray powder material | Gray coarse powder material | Dark gray fluid | ||
| 2 Mass fraction of moisture, %, no more | 3 | 3 | |||
| 2 ** | |||||
| 8 Mass fraction of chloride ion (Clˉ), %, no less | |||||
| 0.1 | 0.1 | 0.1 | 0.1 | 0.1 ** | |
| 9 Mass fraction of chromium oxide (in terms of Cr2O3), %, no more | 2.8 | - | 2.8 | - | - |
| 10 Specific surface area of condensed microsilica, m2/kg, no less | |||||
| 13 Bulk density, kg/m | |||||
| 3 | |||||
| 150–399 | 150–399 | 400–600 | 400–800 | - | |
| 7 | |||||
* Classification of microsilica by grade is carried out on the basis of the worst quality indicators obtained. ** In paragraphs 3–8, 11, the standards for the suspension (paste) are given in terms of dry matter.
-
MK: Uncompacted condensed microsilica with a bulk density ranging from 150 to 399 kg/m3.
-
MKU (Microsilica compacted): Compacted fumed microsilica with a bulk density ranging from 400 to 600 kg/m3.
| 5 | |||||
| 5 | 60 | ||||
| 3 Mass fraction of silicon oxide (SiO2), %, no less | 65 | 85 | 65 | 85 | 85 ** |
| 4 | Concretes | 343 | |||
| 5 | Cements | 202 | |||
| 6 | Durability | 192 | |||
| 7 | Silica fume | 159 | |||
| 8 | Fly ash | 154 | |||
| 9 | Mechanical properties | 152 | |||
| 10 | Concrete | 141 | |||
| 11 | Scanning electron microscopy | 137 | |||
| 12 | Hydration | 123 | |||
| 13 | Nano-particles | 108 | |||
| 14 | Tensile strength | 108 | |||
| 15 | Microstructure | 106 | |||
| 16 | Mortar | 103 | |||
| 17 | Concrete mixtures | 99 | |||
| 18 | Aggregates | 96 | |||
| 19 | Water absorption | 93 | |||
| 20 | Portland cement | 87 |
Various minerals and chemicals have been utilized in the production of amorphous silica, with quartz sand standing as the most commonly used raw material containing silica. The methods for processing and obtaining finalized raw materials involve multi-stage and intricate processes employing expensive reagents and complex equipment [30][31][32][33][34][35][36][37][38][40][41][30,31,32,33,34,35,36,37,38,40,41]. The primary industrial source for producing silicon dioxide involves the creation of a silicate block through the fusion of sand with sodium hydroxide at a temperature of 1700 °C. This block is then subjected to boiling in an autoclave under a pressure of 4.8–5.0 atm. while being supplied with steam [30][31][30,31].
Amorphous silicon dioxide with minimal metal impurities (less than 10−3–10−5 wt.%) is formed by sintering quartz foundry sands with ammonium hydrodifluoride (NH4HF2) [33][34][35][36][33,34,35,36]. Additionally, amorphous silicon dioxide (white soot, 99.997% purity) can be derived from processing zirconium concentrate. This involves fluorination with ammonium bifluoride, heat treatment, subsequent condensation, and processing of the resulting halogenation products [30][31][30,31]. A patent search conducted in the library of patents for inventions of the Russian Federation FIPS (Federal Institute of Industrial Property) revealed that most inventions focus on the complex processing of raw materials. Starting materials such as serpentinite, marshalite, diatomite [9][10][11][9,10,11], nepheline, eudialyte, olivine, and fayalite [33][34][35][33,34,35] have been employed.
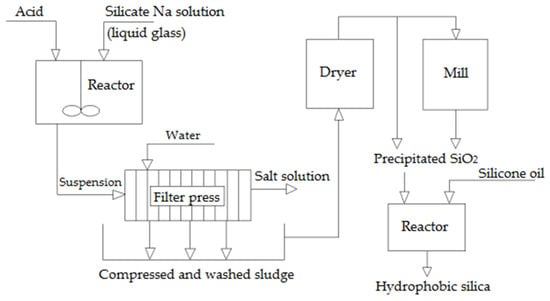
An established method for producing environmentally friendly precipitated silica involves neutralizing sodium silicate with sulfuric acid [13]. Recently, waste from various industries has piqued researchers’ interest as a source of Si. For instance, amorphous silicon dioxide can be extracted from waste generated in boron production, ash resulting from the combustion of organic fuel, waste from ferroalloy production, and flue dust from the gas purification process in aluminum industry enterprises [28].
Processing ash and slag waste from low-grade ore and non-metallic raw materials can yield foamed X-ray amorphous material (foam silicate) with a consistent chemical composition, forming either thin mineral fibers or spheres based on technological conditions [28][29][30][31][32][33][34][35][36][37][38][39][40][28,29,30,31,32,33,34,35,36,37,38,39,40]. Research [28][38][40][41][28,38,40,41] indicates that the economically efficient disposal of ash and slag waste on a large scale is feasible through the production of building materials and products. In an innovative approach, renewable silicophilic plants like rice, horsetails, feather grass, and oats can serve as an alternative raw material source [23][24][23,24]. Among these, rice production waste, specifically rice husks and rice straw, holds promise due to their high silica content: 14%–20% in rice husks and up to 13.5% in rice straw. The processing methods are straightforward and cost-effective, favoring the extraction of silica from rice straw because of its higher silicon content. Rice husks, concentrated during grain cleaning in mills, offer a more economical processing route [25][26][25,26].
A review [24] explored the use of rice husk ash as a cement substitute in high-strength sustainable concretes. In this context, the focus revolves around micro- and nanosilica derived from industrial waste.
2. General Understanding of the Hydration Processes of Portland Cement
Through the gradual evolution of scientific research methods and the culmination of numerous experimental and theoretical studies, a comprehensive comprehension of the hydration processes of Portland cement has been achieved [25][26][27][28][29][25,26,27,28,29]. The primary mineral within Portland cement clinker, tricalcium silicate 3CaO·SiO2 (alite), comprises 60%–70% of the clinker content. Researchers commonly delineate five principal stages of hydration: the initial, induction, hydration acceleration, hydration deceleration, and hardening stages. In the initial stage, the interaction between tricalcium silicate and water leads to the adsorption of water molecules and partial surface hydration of the binder grain. This phase, lasting no more than 20 min, is marked by heat release. The adsorption of water molecules results in the protonation of the binder surface, forming HnSiO4n+4 hydrosilicate groups and releasing Ca2+ and OH− ions into the liquid phase. Simultaneously, a semi-permeable film of calcium hydrosilicates forms on the surface of the hydrating grains, facilitating the transmission of water to the clinker mineral’s surface and the release of Ca2+ and OH− ions from it [25]. The second stage, known as the induction period, spans from two to six hours. During the fourth and fifth stages, the crystallization of gel-like calcium hydrosilicates occurs, resulting in the development of both an outer layer and an inner layer of hydrosilicate masses [28][29][28,29].3. State of Production of Micro- and Nanosilica in the Russian Federation
Annually, during the production of metallurgical silicon in Russia (RF), approximately 35,000 tons of fine dust are generated, stored in “big bags” weighing 1 ton each, or maintained on slurry fields [27][28][40][27,28,40]. Consequently, this results in an escalation of waste, emphasizing the crucial need for recycling and large-scale utilization from both economic and environmental standpoints [29][30][31][32][33][34][35][29,30,31,32,33,34,35]. The disposal and processing of dust are exclusively executed using extensive metallurgical equipment within sinter production. Only specially prepared waste can be reintegrated into metallurgical processes. Waste agglomeration not only supplies enterprises with supplementary resources but also diminishes their environmental impact [1][2][1,2]. Forecasts indicate a growth trend in microsilica production in Russia by 2025 [1]. The surge in production, reaching 70–80 thousand tons annually, is primarily attributed to the full operation of metallurgical silicon production, driven by a sharp 80% price hike. Notably, the most appealing method of processing microsilica waste, derived from the reduction smelting of quartzite, totaling 30–40 thousand tons per year, is centralized at enterprises such as JSC Kremniy (Irkutsk region) and JSC Ural Silicon (Kamensk-Uralsky) [28][40][28,40]. The selection of a plant relies on its geographical proximity. Further details are outlined in Table 2, offering a comparative assessment, focusing on the fundamental effects of microsilica sourced from various Russian plants and foreign plants [40]. Russia annually produces over 150 thousand tons of microsilica, with production volumes increasing each year. As per table data, it is evident that the chemical composition of Misrosilica (MK) in the presented list is nearly identical.Table 2.
Comparative characteristics of microsilica from various plants.
| Name Plants | Intention | Class | Chemical Composition | ||
|---|---|---|---|---|---|
| Elkem Microsilica Grade 920 ASTM (Norway) | Concrete and construction solutions | Class 920 is available in two forms: unsealed (920 U), bulk density, which is usually 200–350 kg/m3; and pressed (920 D), bulk density—500–700 kg/m3 | SiO2: 85%–90%; SO3: 1%–2%; Cl: 0.1%–0.3%; CaO: 1.0%; Si: 0.2%–0.4%; Na2O: 1%–1.5%; C: 1.5%–2.0% | ||
| Kuznetsk Ferroalloys, Ltd. | Obtaining concretes with special properties: ultrahigh-strength, improved (i) frost, (ii) sulphate, and (iii) corrosion resistance, water-tightness | Unsealed—MS-85, MS-65; compacted—MSC-85, MSC-65; in the form of a suspension—ISS-85 | SiO2: 90%–92%; Al2O3: 0.6%–0.8%; Fe2O3: 0.4%–0.7%; CaO: 0.4%–0.9%; MgO: 0.8%–1.0%; Na2O: 0.6%–0.8%; K2O: 1.2%–1.4%; C: 0.9%–1.2%; S: 0.2%–0.3% | ||
| Chelyabinsk Electrometallurgical Plant, Ltd. | Additive to concrete for improved performance | Unsealed—MS-85, MS-65; compacted—MSC-85, MSC-65; in the form of a suspension—ISS-85 | SiO2: 90%–92%; Al2O3: 0.6%–0.8%; Fe2O3: 0.4%–0.7%; CaO: 0.4%–0.9%; MgO: 0.8%–1.0%; Na | ||
| 4 Mass fraction of loss on ignition, %, no more | 5 | 3 | 5 | 3 | 5 ** |
| 5 Mass fraction of free alkalis (in terms of Na2O), %, no more | 2 | 2 | 2 | 2 | 2 ** |
| 6 Mass fraction of calcium oxide (CaO), %, no more | 5 | 3 | 5 | 3 | 2 ** |
| 7 Mass fraction of sulfur oxide (SO3), %, no more | 2 | 2 | 2 | 2 | |
| 12,000 | |||||
| 12,000 | 12,000 | 12,000 | - | ||
| 11 Efficiency index, %, no less | 90 | 105 | 90 | 105 | 105 ** |
| 12 Degree of pozzolanic activity, mg/g MS, no less | 70 | 95 | 70 | 95 | - |
| 14 Density of suspension (paste), kg/m3, no less | - | - | - | - | 1280 |
| 15 Of an aqueous suspension (paste) of microsilica, no less | - | - | - | - |
- MKS (Microsilica suspension): Suspension (paste) of condensed microsilica.
-
Based on chemical composition and effectiveness, microsilica is further divided into two groups, designated as follows:
-
MK65: Condensed microsilica containing at least 65% silica with an efficiency index of at least 90%.
-
MK85: Condensed microsilica containing a minimum of 85% silica and an efficiency index of at least 105%.
-
High-strength concretes and mortars with reduced permeability and enhanced corrosion resistance are applied in various construction types like industrial, civil, and transportation.
-
Low-cement concretes and mortars that exhibit decreased exotherm.
-
Concrete mixtures with enhanced technological properties, including high mobility and self-compacting features, along with a high degree of resistance to segregation.
6. Preparation of Nanosilica
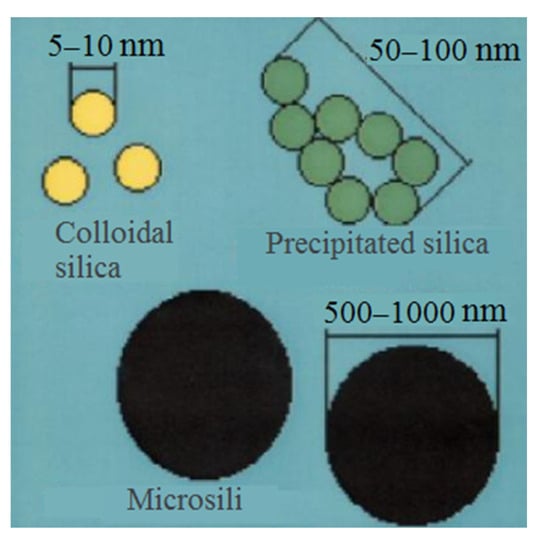
As per contemporary understanding, large capillary pores serve as stress concentrators in cement stone. The stone’s strength is governed by porosity as long as the pore size surpasses the critical Griffiths crack size. It is a well-established fact that there exists an inverse relationship between porosity and the strength of cement stone. In theory, the maximum strength of cement stone can be achieved when capillary porosity approaches zero [51][52][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68]. Current trends in concrete technology are geared toward producing concrete with minimal capillary porosity. An essential requirement for this is to not just maintain balanced fractions of fine and coarse aggregates, but also to ensure that all solid components in the concrete mix form a continuous series spanning the entire range of particle sizes—from the smallest nanodispersed components to larger crushed stone pieces. The inclusion of ultrafine silica fillers, featuring particles of various sizes, holds significant importance. While the initial stride toward a new generation of high-quality concrete involved the use of microsilica, the subsequent step should involve integrating ultrafine silica into concrete mixes, characterized by primary particles even smaller than MS (Figure 6). Interest in materials like precipitated and colloidal SiO2 is currently burgeoning and is already being practically applied in concrete technologies. In some instances, precipitated SiO2 is integrated into the composition of RPC concretes alongside microsilica. This inclusion contributes to achieving compressive strength ranging from 200 to 800 MPa and flexural strength of up to 100 MPa [55][56][57][58][59][60][61][62][63][64][65][66][67][68][69][55,56,57,58,59,60,61,62,63,64,65,66,67,68,69]. Aerosil, or fumed silica, is manufactured by burning tetrachlorosilane (SiCl4) in a stream of hydrogen and oxygen:SiCl
4
+ 2H
2
+ O
2
→ SiO
2
+ 4HCl
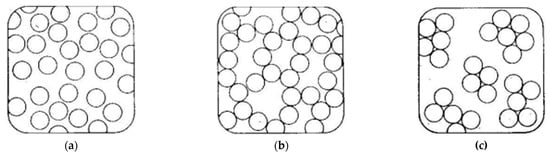
Figure 7.
Methods of aggregation of colloidal particles: (
a
) sol from colloidal particles; (
b
) gel; (
c
) formation of sediment.
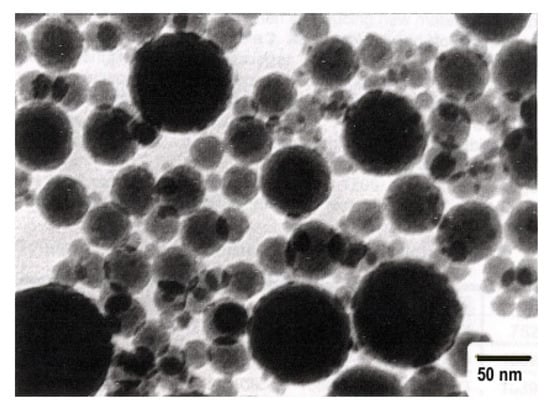
Figure 8.
Colloidal silica particles.
Table 5.
| Indicator Name | Types of Ultra-Dispersed Silica | |||
|---|---|---|---|---|
| High Temperature | Hydrochemical | |||
| Microsilica (Elkem Materials A/S) | Aerosil 200F (Degussa) | Precipitated Silica Catosil NK-3 HS (City Cat Pigments) | Stake SiO2 Ludox HS400 (Grace Davison) | |
| Appearance | Gray powder | White powder | White powder | Opalescent liquid |
| Mass fraction SiO2, wt % | 90.9 | ≥99.8 | 98–99 | 40 |
| Mass fraction Na2O, wt % | - | - | - | 0.4 |
| Density, kg/m3 | 400–600 (bulk) | 50 | 60–80 | 1310 |
| Specific surface, m2/kg | 18 | ≥200 | 150 | 220 |
7. Study of Promising Methods for Processing and Recycling Waste Micro- and Nanosilica for Their Possible Use in Refractory Materials and Concrete Mixtures
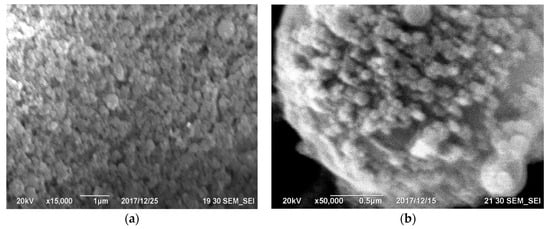
The three primary properties of concrete are workability, strength, and durability. While strength and durability are typically associated with hardened concrete, and workability pertains to fresh concrete, the properties of hardened concrete directly link to mix design and fresh concrete characteristics. In essence, the mix design and the properties of fresh concrete serve as the most crucial factors controlling the mechanical performance of hardened concrete. In reference [46], the impact of silica fume dosage on the water requirement, maintaining constant segregation, is illustrated, confirming the efficacy of the superplasticizer as a water reducer regardless of the silica fume content. The outcomes depicted in Figure 12 reveal that replacing up to 10% of the cement can enhance the workability of concrete. When combined with a superplasticizer, silica fume aids in dispersing the flocculated cement particles.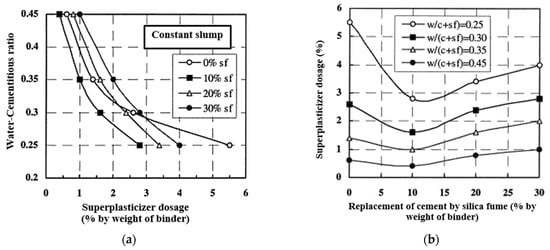
Figure 12. Effect of superplasticizer dosage on the ratio of water-cement materials (a); the effect of replacing cement with microsilica on superplasticizer dosage (b) [46].
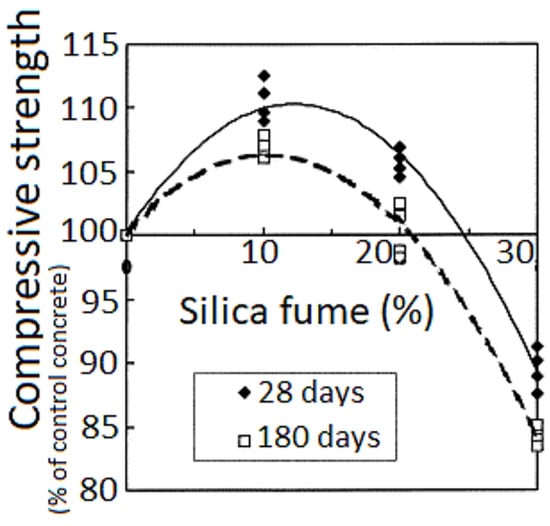
Figure 13.
8. Adding Nano Silica to Concrete
According to numerous studies, adding nanosilica to concrete can significantly enhance the material’s compressive strength [74][75][76][77][78][79][80][81][82][83][84][85][86][87][88][89][90][91][92][93][94][95][96][97][98][99][100][101][102][103][104][105][106][107][108][109][110][111][112][113][114][115][116][117][118][74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118]. Moreover, it has been demonstrated that nanosilica can reduce both the initial and final set while accelerating the early strength of concrete. This is attributed to the large specific surface area of nanosilica, which acts as a robust binder between cement and aggregate [22]. Additionally, due to its extremely small particle size, nanosilica exhibits excellent pozzolanic activity [74][75][76][77][78][79][80][74,75,76,77,78,79,80], enabling it to effectively fill all pores and voids in the concrete, including the interfacial transition zone (ITZ), thus augmenting its strength [74][78][82][84][74,78,82,84]. The introduction of nanosilica promotes the formation of calcium silicate hydrate (CSH) gel, a crucial component in concrete strength. The review [22] analyzed the outcomes of various authors, which are presented in Table 6.Table 6.
The previous studies examined the utilization of various nanomaterials and their corresponding substitution ratios [22].
| Reference | Type of Nanomaterial | Type of Concrete | Type of Use | Remarks |
|---|---|---|---|---|
| Amin and Abu el-Hassan (2015) [33] | (Ni ferrite) and (Cu-Zn ferrite) were used in the experiment together with 15 nm nanosilica | High-strength concrete | Weight doses of 1%, 2%, 3%, 4%, and 5% of nanosilica, Ni, and Cu-Zn ferrite were added to cementitious materials | When comparing samples of concrete with nanoferrite to samples of concrete with nanosilica, the latter exhibited superior compressive strength, estimated to be 10% higher |
| Ren et al. (2018) [20] | Nanotitanium dioxide particles 10 nm in diameter and nanosilica particles 20 nm in diameter | Normal concrete | Cement was replaced with nanosilica and nano-TiO2 in different proportions (1%, 3%, and 5%, respectively) | At a mass concentration of 3%, NS and NT can each potentially increase the compressive strength of concrete by up to 16% and 9%, respectively |
| Zhao et al. (2012) [34] | The average nanosilica particle size is around 100 nm | Normal concrete | Nanosilica was used at various weight percentages, ranging from 0% to 20% | The compression and frost resistance capacity increases by 20% when the concentration of nano-SiO2 is at 10%, compared to conventional concrete |
| Shaikh and Supit (2014) [35] | Nano-CaCO3 powder (40 to 50 nm) | Fly ash concrete | Nano-CaCO3 was added to the cement at weight dosages of 1%, 2%, 3%, and 4% | Out of all the concentrations, 1% CaCO3 nanoparticles exhibited the highest compressive strength, surpassing that of the cement mortar by 22% |
| Chithra et al. (2016) [36] | A colloidal dispersion of nanoparticles in water, with a density ranging between 1.3 and 1.32 | High-performance concrete | Nanosilica replaced various weight percentages of cement—0%, 0.5%, 1%, 1.5%, 2%, 2.5%, and 3% | The introduction of nanosilica to cement mortars, substituting 40% copper slag for fine aggregate, increased the compressive strength by 2% |
| Salemi and Behfarnia (2013) [37] | Nanoparticles measuring 20 nm in diameter for silicon and 8 nm in diameter for aluminum oxide | Concrete pavement | NS at 3%, 5%, and 7%, and nano-Al2O3 at 1%, 2%, and 3% were utilized to replace cement at different weight percentages | As per the experimental results, the addition of 5% nanosilica to cementitious materials enhances concrete’s compressive strength by up to 30% and frost resistance by 83% |
| Mohamed (2016) [38] | Nano-silica and nanoclay (NC) | Normal concrete | Substituting cement across a range of weight percentages, spanning from 0.5% to 10% | Both nanosilica and nanoclay distinctly bolster the compressive strength of high-performance concrete, exhibiting an increase of 18% and 11%, respectively |
| Wu et al. (2016) [39] | Nano-CaCO3 elements and nanosilica particles with diameters ranging from 5 to 35 nm and 15 to 105 nm, respectively | High strength concrete | Replacing paste by mass with varying percentages of nano-CaCO3, specifically 0%, 1.6%, 3.2%, 4.8%, and 6.4%, along with nanosilica at 0%, 0.5%, 1.0%, 1.5%, and 2.0% of the cement mass | Nano-SiO2 UHSC blends exhibited a consistent and robust strength increase up to 7 days, while nano-CaCO3 UHSC mixtures showed consistent strength between 3 and 7 days, followed by rapid improvement thereafter |
| Li et al. (2015) [40] | Nanosilica nanoparticles (20 nm) and nanolimestone nanoparticles (15–80 nm) | Ultra-high-performance concrete | Replacing a portion of the cement by mass with nanosilica at 0.5%, 1.0%, 1.5%, and 2.0%, and with nanolimestone at 1.0%, 2.0%, 3.0%, and 4.0% | The increase in the flexural to compressive strengths ratio of the UHPC matrix integrated with 1.0% nanosilica and W/B ratios of 0.16 is 36% |
| Gao et al. (2017) [41] | Nanosilica nanoparticles with an average particle size of 15 nm and nanosilica nanoparticles with a medium grain size of 50 nm | Road flyash concrete | Silica fume and nanosilica were used in quantities of 3%, 2%, and 1% of the cementitious materials’ composition | The concrete containing 2% NS, compared to the reference concrete, exhibited a 124.8% increase in drying shrinkage at 28 days |
| Torabian et al. (2016) [42] | Nanosilica nanoparticles with an average particle size of 20 nm | Normal concrete | Replacing cement with nanosilica in various quantities—0.5%, 1%, and 1.5% | Adding 1.5% NS to concrete with a w/b ratio of 0.65 resulted in a 41% increase in strength |
| Said et al. (2012) [43] | Nanosilica nanoparticles with a medium grain size of 35 nm | Normal concrete | Different quantities of nanosilica nanoparticles, specifically 6% and 12% by weight, were introduced into the cementitious materials | The addition of nanosilica resulted in strength improvements of up to 6% at all curing ages |
| Hosseini et al. (2017) [44] | Nanoclay particles with a density of 1660 kg/m3 | Self-compacting concrete | Varying proportions of cement were replaced with nanoclay, including 0.25%, 0.5%, 0.75%, and 1% of the total weight of the cement | After 56 days, the addition of 0.25% and 0.50% nanoclay increased the compressive strength by 15% and 14%, respectively |
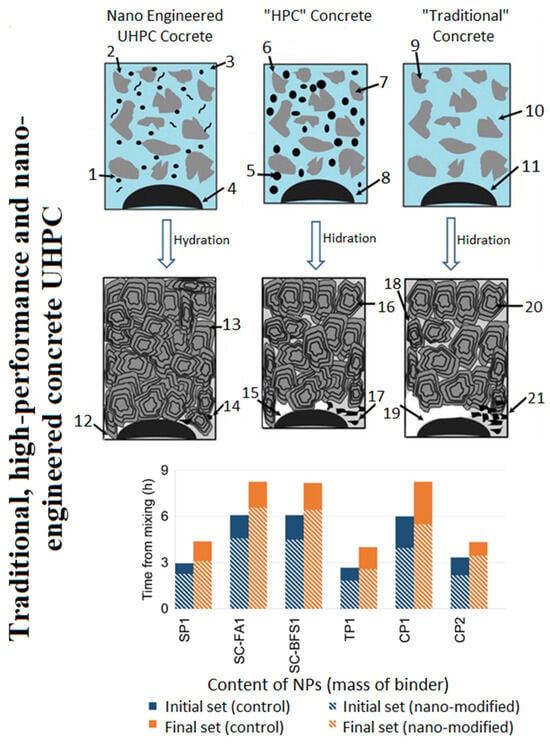
Figure 14. Initial and final setting times of cement products made with or without nanoparticles. (SP1-amorphous silicon dioxide (colloidal) particle size 15 nm; SC-FA1-amorphous silicon dioxide (colloidal) particle size 15 nm plus mineral additive; SC-BFS1—amorphous silicon dioxide (colloidal) particle size 10 nm plus mineral additive; TP1-titanium dioxide (colloidal) particle size 15 nm, CP1-CaCO3 particles 50–120 nm in size; CP2-CaCO3 particles 15–40 nm in size) [46].