1. Introduction
Titanium dioxide (TiO
2) is a semiconductor widely used in photocatalytic and photoelectrochemical processes, since the first works describing reactions involving TiO
2 and UV radiation
[1] and the Fujishima–Honda effect were reported in the 1960s
[2]. In the early 1970s, Fujishima and Honda investigated the behavior of the rutile form of TiO
2 under light irradiation, discussing the similarities between the observed phenomenon and the initial stages of photosynthesis, focusing on studying the mechanism of the latter
[3]. Over the years, the number of studies about TiO
2-based materials combined with light irradiation have multiplied, spreading to different areas of application, such as the oxidation of organic compounds
[4], the degradation of pollutants such as pharmaceuticals and pesticides
[5][6][5,6], the removal of heavy metals from water
[7], bactericidal activity
[8], hydrogen production
[9][10][11][9,10,11], and CO
2 reduction for solar fuel synthesis
[12]. Notably, this last example has contributed to a strengthening of the aspiration for an artificial photosynthesis process, which, similarly to natural photosynthesis, consumes simple products, such as water and carbon dioxide, to produce energetic substances (e.g., H
2, CO, CH
3OH, CH
4) using sunlight, which are also known as solar fuels
[13][14][13,14].
The cited processes are based on TiO
2’s ability to form electron (e
−)/hole (h
+) pairs under UV irradiation. When the energy of the photons absorbed by TiO
2 is more significant than its band gap, the electrons in the valence band (VB) are promoted to the conduction band (CB) of the semiconductor, generating holes in the VB, and electrons in the CB
[15][16][15,16]. The photogenerated e
−/h
+ pairs can recombine inside the photocatalyst particle or migrate to the surface and undergo recombination. If this does not occur, e
−/h
+ pairs at the surface can promote reduction or oxidation reactions of adsorbed species
[15][17][15,17]. This phenomenon is the basis of the various photocatalytic processes. As it can generate highly active radical species, e.g., hydroxyl radicals, photocatalysis can be classified as an advanced oxidation process (AOP)
[16].
Despite its low toxicity, outstanding activity, and considerable chemical stability
[18], titanium dioxide is only active under UV light, restricting TiO
2 applications combined with solar radiation, which includes predominantly visible light and less than 5% of UV radiation
[19]. As one of the possible alternatives, dye sensitization of wide band-gap semiconductors (>3.0 eV), such as TiO
2, has been the subject of different studies for improved solar light harvesting technologies
[20]. In dye sensitization, the dye molecule bonded to the semiconductor surface injects electrons into the conduction band of the semiconductor upon photoexcitation, as described in Equations (1) and (2)
[21][22][21,22].
Based on this principle, one of the third-generation solar cell technologies has gained ground, driven by growing energy demand and the search for renewable energy sources: dye-sensitized solar cells (DSSCs)
[23][24][23,24]. This type of device aims to convert sunlight into electricity, consisting of a substrate made up of a glass conductor, a dye-sensitized semiconductor (metal oxide), and a catalyst counter electrode, separated from the other electrode using an electrolyte solution
[24]. Although this technology still suffers from limitations in terms of efficiency and long-term stability, there is an excellent expectation regarding its development, especially concerning the use of new dyes that improve its performance
[25]. In this context, natural dye extracts have gained attention due to the abundance, low cost, and environmentally friendly nature of the raw material
[25][26][27][28][25,26,27,28].
Among natural dyes, chlorophyll has undoubtedly shown exciting results. As reported by
[29], spinach extract produced the best efficiency results among the different natural extracts used in sensitizing TiO
2 solar cells. Compared to the other natural dyes (black rice, dragon fruit, red cabbage, and blends), the spinach UV-Vis absorption spectrum presented the highest absorption peak at approximately 662 nm
[29], which can be associated with chlorophyll-a. Haghighatzadeh, in a study about phenol photocatalytic degradation under visible light irradiation, observed that TiO
2 nanoparticles sensitized with chlorophyll promoted higher percentages of degradation (85%) than those sensitized with curcumin (75%)
[30]. Thus, TiO
2 sensitized with chlorophyll has gained space in DSSC
[31] and photocatalysis applications, including pollutant degradation
[32], CO
2 reduction
[33], and even artificial photosynthesis processes involving light harvesting and oxygen production
[34].
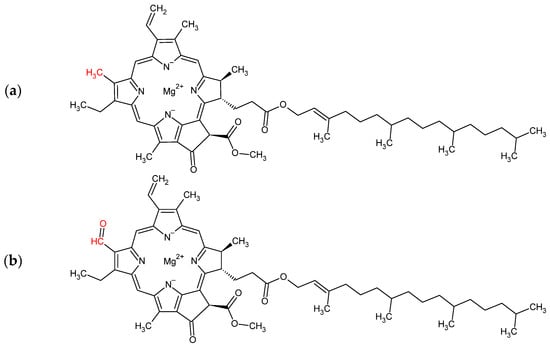
2. Chlorophyll
Chlorophyll is a natural pigment of the porphyrin class, which has a Mg
2+ ion coordinated to the four rings
[35], as shown in
Figure 1. The Mg
2+ ion in the molecule plays a vital role in the light absorption phenomenon, being essential for the excited state of the molecule and affecting the efficiency of the excitation transfer between chlorophyll molecules in the chloroplast
[35][36][37][35,36,37], giving chlorophyll a prominent position in photosynthesis and promoting solar energy conversion into chemical energy
[38]. The green color of chlorophyll pigments is due to their high absorption in the red and blue regions of the light spectrum
[39]. In the absorption spectrum, the ranges of 350 nm to 480 nm can be attributed to the charge transfer transitions of the porphyrin and the Mg ion
[38].
Figure 1.
Chlorophyll-a (
a
) and Chlorophyll-b (
b
).
While chlorophyll-a is a pigment common to all photosynthetic plants, chlorophyll-b is characteristic of algae and vascular plants
[38]. As for the molecular structure, the two pigments are distinguished by the presence of a methyl group (chlorophyll-a,
Figure 1a) or an aldehyde group (chlorophyll-b,
Figure 1b) at position 3
[35]. Green plants usually contain both chlorophyll-a and chlorophyll-b, the former being the major pigment
[35]. There are reports of an increase in the proportion of chlorophyll-b in shade plants, given that this pigment would be more effective in absorbing low-intensity light
[35]. Despite the slight differences between the pigments, it is quite common to use extracts containing both to sensitize TiO
2, whether in extracts obtained from plants
[19] or other sources, such as
Spirulina [40]. Even so, there are reports of the isolated use of chlorophyll-a
[41] and chlorophyll-b
[42] as TiO
2 modifiers.
Chlorophyll-a’s two maximum absorption peaks are at approximately 432 and 670 nm
[35]. However, the chlorophyll absorption spectrum may vary slightly depending on the solvent
[35]. Shen et al., for example, reported a variation in the chlorophyll-a absorption maxima in different solvents, changing from 420 nm and 661 nm in chloroform to 421 nm and 667 nm in ethanol and 440 nm and 673 nm in phosphate-buffered saline (0.01 M, pH 7)
[34]. This type of phenomenon is closely related to the polarity of the solvent, and it is common to observe a red shift as the polarity of the solvent increases
[43][44][43,44].
Through a theoretical study using density functional theory (DFT) and time-dependent DFT (TD-DFT), Faiz et al. concluded that the solvent can also reduce the LUMO–HOMO band gap and affect the light-harvesting energy (LHE)
[45]. Among the results obtained, the authors observed that water could improve chlorophyll’s performance in injecting electrons, even though chlorophyll is not soluble in water
[45]. Similarly, Sabagh et al. reported that the solvent improves the LHE in a comparative study between water and the gas phase
[46].
2.1. Chlorophyll-a and Chlorophyll-b Concentration Estimations
The concentration of chlorophyll-a and chlorophyll-b can be determined directly and simultaneously using spectrophotometry through calculations considering a system of two equations
[35]. The equations may vary slightly since the solvent can affect the absorption spectrum
[35]. Krishnan et al., for example, considered the chlorophyll optical densities (
𝑂𝐷��) in the extract—which were calculated by subtracting the absorbance at 750 nm from absorbances at 647 nm (
𝑂𝐷647��647) and 664 nm (
𝑂𝐷664��664)—to estimate the concentrations of chlorophyll-a and chlorophyll-b in mg L
−1 [19]. Pai et al., in turn, used the absorbance value to estimate the concentration of the two pigments
[39].
2.2. Extraction of Chlorophyll
Natural chlorophyll extracts can be produced from different raw materials including plants
[32][39][32,39] and microorganisms
[22]. Among the plant species,
rwe
searchers can mention leaves of fresh spinach
[19], pandan
[47][48], weeds such as
Chromolaena odorata [39], and parsley
[30]. Among microorganisms, the cyanobacteria stand out, among which
reswe
archers can mention
Spirulina sp.
[22].
The chlorophyll molecule is composed of a hydrophilic part and a hydrophobic part
[39], and its extraction is commonly performed by using organic solvents such as methanol
[22], ethanol
[40], acetone
[19], and petroleum ether
[39], among others. Najihah et al. observed that polar organic solvents tend to promote better chlorophyll extraction (acetone > ethanol > methanol > acetic acid > acetonitrile) than non-polar solvents (hexane)
[48][49]. This result justifies the widespread use of acetone in chlorophyll extraction
[29]. Krishnan and Shriwastav, for example, extracted chlorophyll from ground fresh spinach leaves (after removing their midrib) using a 90% acetone aqueous solution, which was kept in contact with the leaves for 2 h in the dark at 4 °C. The extract was centrifuged for 20 min at 3000 rpm. The authors were able to produce an extract containing 0.39 ± 0.05 mg of chlorophyll per gram of spinach
[19].
Other commonly used solvents are ethanol and methanol. Al-Alwani et al., for example, reported that ethanol showed the best performance in extracting chlorophyll from pandan leaves (
P. amaryllifolius) compared to methanol, chloroform, ethyl ether, and acetonitrile
[47][48]. Kathiravna et al.
[22] extracted chlorophyll from a cyanobacteria
Spirulina sp. using a 90% methanol solution and centrifugation as the separation method.
Regardless of the raw material or solvent used, the extraction process usually follows similar steps under mild conditions and relatively simple procedures to ensure the extraction of chlorophyll by the solvent. The initial steps involved preparing the raw material, generally with the reduction of the sample through crushing and grinding
[19][22][30][48][19,22,30,49]. In some cases, it is also necessary to preliminarily remove the midrib of plant leaves
[19] or carry out a drying step
[47][48]. Once the sample is prepared, it proceeds to the next step, in which the contact between the raw material and the solvent is promoted for a defined time, which can vary from a few hours (1 h
[22] or 2 h
[19]) or days (from 24 h
[29] up to 1 week
[30][47][49][30,48,50]). The sample can be sonicated during this period to promote extraction
[40]. Then, the extract is separated from solid waste using centrifugation
[19][22][19,22] or filtration
[29][30][40][47][48][29,30,40,48,49]. In some cases, it is also possible to increase the extract concentration in a rotary evaporator
[29][47][29,48].
Even though these are the most common procedures, there are alternative processes that can differ significantly from the extraction methods described, such as the procedure followed by Phongamwong et al., who obtained the chlorophyll extract after a short incubation period (2 min) of
Spirulina at 70 °C, at the end of which the mixture was centrifuged and the supernatant collected
[50][51].
Chlorophyll tends to be unstable, suffering from the action of temperature, light, oxygen, or other chemical reactions
[35]. After the extraction, it is essential to take care of the extract’s storage conditions to avoid its degradation, whether that be by keeping it at low temperatures (4 °C
[19][22][30][19,22,30]) or by protecting it from exposure to atmospheric air
[47][48] or light
[22][29][39][40][47][22,29,39,40,48] to prevent autoxidation
[40]. Furthermore, it is necessary to consider that the extract obtained from plants and microorganisms may contain other organic compounds, such as sugars and amino acids, which may lead to chlorophyll degradation during storage or even the detachment of the dye from the TiO
2 surface
[48][49].
Phongamwong et al. did not perform the chlorophyll extraction. Still, they incorporated
Spirulina directly into the TiO
2 using the incipient wetness impregnation method, using deionized water to disperse the ground
Spirulina and then adding it to the N-TiO
2, which was kept under constant stirring at 40 °C until the complete evaporation of water
[33]. In a later work, the authors compared the incorporation of
Spirulina (Sp) and chlorophyll (Chl) to P25 using the incipient wetness impregnation method. They observed that, although both contributed to a significant improvement in P25 performance, the incorporation of extracted chlorophyll led to superior results. While P25 presented a rate constant of k = 8.05 ± 0.23 (10
−3 min
−1) in the degradation of Rhodamine B, the modified catalysts 0.5Sp/P25 and 0.5Chl/P25 presented rate constants equal to k = 23.53 ± 0.91 (10
−3 min
−1) and k = 60.80 ± 2.21 (10
−3 min
−1), respectively
[50][51].