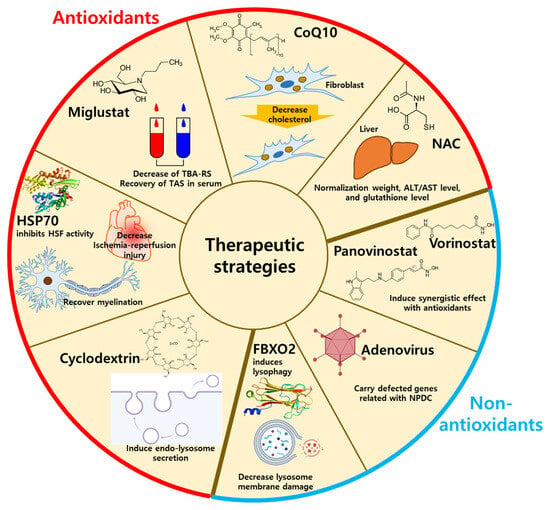
Figure 3. The therapeutic strategies using antioxidants or non-antioxidants. The antioxidants-related NPDC treatment includes HSP70, miglustat, CoQ10, cyclodextrin, and NAC by decreasing oxidative stresses. The non-antioxidant-related NPDC treatment includes lysophagy, panovinostat, vorinostat, and adenovirus by supplementation of antioxidants. HSP70: heat shock protein 70; CoQ10: coenzyme Q10, NAC:
N-acetylcysteine; FBXO2: F-Box protein 2.
3.1. Antioxidant-Related Drugs for NPDC Treatment
3.1.1. N-Butyl-Deoxynojirimycin (Miglustat)
As mentioned in the Introduction, oxidative stress is an outcome of NPDC. For example, thiobarbituric acid-reactive species (TBARS), the product of lipid peroxidation, was found to be increased, and the total antioxidant status (TAS) was decreased in the plasma of NPC patients
[42][96]. Although the damaging effects of miglustat, the inhibitor of glycosphingolipid synthesis, on the neuronal system in NPDC patients have not been demonstrated, treatment with miglustat attenuated TBARS levels and recovered TAS levels in the serum of patients with NPDC
[42][96].
3.1.2. N-Acetylcysteine and Coenzyme Q10
Antioxidants and antioxidation-related compounds, including
N-acetylcysteine (NAC) and coenzyme Q10 (CoQ10), have therapeutic effects on NPDC
[43][97]. The ROS scavenger NAC has been suggested as a clinical application to manage oxidative stress
[44][98]. Treatment with NAC recovered liver pathology in NPDC mice
[45][99]. NAC normalized liver weight, plasma levels of alanine aminotransferase (ALT)/aspartate aminotransferase (AST), and liver glutathione levels in NPDC mice
[45][99]. CoQ10 is a component of the electron transport chain and acts as a lipophilic antioxidant
[46][100]. Treatment with CoQ10 decreased cholesterol levels in NPDC patient dermal fibroblasts and cytokine levels in NPDC patient fibroblast-culture media
[47][101].
3.1.3. Heat Shock Factor
Heat shock factors (HSFs) are metabolic proteins that induce protein folding, inhibit protein misfolding and aggregation, and prevent apoptosis
[48][49][102,103] and oxidative stress
[50][104]. For instance, heat shock protein 70 (HSP70) inhibited the activity of HSF, which induced the antioxidant reaction and reduced oxidative stress and ischemia/reperfusion injury
[50][51][104,105]. HSP70 also recovered myelination in
NPC knock-out mice
[37][52][91,106]. In NPDC, myelin expression is defective because cholesterol is a component of myelination
[53][107]. Thus, myelin defects are considered a marker of NPDC, and HSP70 is suggested for NPDC therapy.
3.1.4. Cyclodextrin
The β-cyclodextrin (CD) is the drug most frequently used for NPDC to decrease lysosomal cholesterol levels
[54][55][108,109]. β-CD has been suggested as a secondary antioxidant, which enhances traditional antioxidants, such as resveratrol, pterostilbene, pinosylvin, oxyresveratrol, and astaxanthin
[56][110], and β-CD shows antioxidant activity, although its efficacy is lower than that of traditional antioxidants
[57][111]. Although the specific mechanisms of β-CD on lysosomes have not been demonstrated, derivatives of β-CD, such as 2-hydroxypropyl-β-CD (HP-β-CD) and 6-O-α-maltosyl-β-CD (Mal-β-CD), modulate lysosomes. HP-β-CD decreased lysosomal accumulation with decreases in lysosomal sphingolipid in
NPC1-deleted CHO cells
[58][112]. In HeLa cells, HP-β-CD induced endolysosomal secretion by activating transient receptor potential mucoplin-1 Ca
2+ channels to reduce accumulated cholesterol
[59][113]. Treatment with HP-β-CD induces lysosome-ER connections to trigger cholesterol trafficking
[60][114]. In the same way, Mal-β-CD decreased the accumulation of lysosomes by reducing lysosomal expansion in
NPC1-deficient CHO cells
[61][62][115,116].
3.2. Non-Antioxidant Methods for NPDC Treatment
3.2.1. Lysophagy
Lysosomal membrane impairment induces oxidative stress
[63][64][117,118]. Deletion of the
Fbxo2 gene decreased lysophagy in mouse cortical neurons
[40][94]. Fbxo2 treatment for NPDC decreased lipid-mediated lysosomal membrane damage
[40][94]. These results suggest that oxidative stress is associated with cholesterol trafficking, and thus, lysophagy might help reduce cholesterol accumulation. Although non-antioxidant mechanisms are not the same as those of antioxidants, non-antioxidants effectively assist antioxidant-related drugs through synergistic effects with the development of endosomal and lysosomal trafficking.
3.2.2. Histone Deacetylase Inhibitors
The histone deacetylase inhibitors vorinostat and panobinostat recovered mutated
NPC1I1061T localization
[65][119].
NPC1I1061T mutation separated NPC1 from lysosomes, and treatment with vorinostat and panobinostat induced the movement of the
NPC1 I1061T mutant type to lysosomes
[65][119]. Although vorinostat and panobinostat are not antioxidants, the combination of these and antioxidants, such as vitamin C and naringenin, showed synergistic effects with vorinostat and panobinostat
[66][67][120,121]. Antioxidant-related molecules, including β-CD, vorinostat, and panobinostat, have therapeutic potential for NPDC, but the relationship between these molecules and oxidation has not been fully demonstrated. The Food and Drug Administration (FDA) approved another histone deacetylase, valproic acid (VPA), to reduce cholesterol accumulation in NPDC patient-derived fibroblasts
[39][93]. The combined administration of VPA with another CD-type of M-β-CD induced NPC1 trafficking from endosomes to the ER or Golgi in
NPC1I1061T-mutated human fibroblasts
[39][93], providing evidence of the synergistic effect with antioxidants of lysosomal trafficking.
3.2.3. Adenovirus
Adenovirus has been used as a gene delivery vector to easily penetrate cellular membranes and transfer to the nucleus
[68][69][122,123]. The transduction of adenovirus cloning with
NPC1 genes induced the stable expression of NPC1 proteins in
NPC1 knock-out mice
[70][124]. Injection with
NPC1-inserted adenovirus increased the survival rate of Purkinje cells in
NPC1 knock-out mice
[71][125]. Recovery of Purkinje cells by adenovirus injection ameliorated impaired behavior and the survival rates of
NPC1 knock-out mice
[71][125]. Adenovirus early region 3 (RIDα) mimics the role of rab7 protein in the translocation of endosomes and lysosomes
[72][73][126,127]. Rab7 interacts with lysosomal motor dynein and OSBP family ORP1L, which senses cholesterol levels in lysosomes for the translocation of lysosomes
[74][75][128,129]. RIDα is substituted for rab7 in rab7-depleted cells to translocate cholesterol
[72][73][126,127]. RIDα also induced the repositioning of lysosomal and autophagic vesicles to juxtanuclear regions to trigger autophagic flux and cholesterol trafficking in NPDC fibroblasts
[76][130].