Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Ye Chen and Version 2 by Mona Zou.
Flotation reagents are significant for modifying the interfacial characteristics of mineral grains to achieve the effective separation of minerals. Since the 1960s, when quantum chemistry was first introduced into the study of flotation reagents, many achievements have been made, although some controversial topics remain.
- quantum chemistry
- density functional theory (DFT)
- flotation reagents
1. Non-Ferrous Metals
In general, non-ferrous metals are metals that do not contain Fe., i.e., all pure metals are non-ferrous. Among them, quantum chemical studies of flotation reagents have been performed for Cu-, Pb-, and Zn-bearing minerals.
1.1. Non-Ferrous Oxide Minerals
1.1.1. Copper Oxide Minerals
Common copper oxide minerals include malachite, Cu2(CO3)(OH)2; azurite, Cu3(CO3)2(OH)2; chrysocolla, (Cu2−xAlx)H2−xSi2O5(OH)4·nH2O; and cuprite, Cu2O. Most quantum chemical studies of copper oxide minerals have been focused on malachite. Yang et al. [1][66] investigated the reactivity of aliphatic oxime derivatives including C7H15CX=NOH (X = H, CH3, NH2 or OH, including octanaldoxime, n-octanohydroxamic acid, n-hydroxyoctanimidamide, and methyl n-heptyl ketoxime) as copper flotation collectors towards malachite using the DFT method. The structure–reactivity relations established in this study provided an understanding of the structural demand for aliphatic oximes to recover copper oxide minerals at the atomic scale. Using the same methodology, Yang et al. [2][67] also studied the chemical reactivity of azolethione derivatives’ (including 1,3,4-oxadiazole-2-thione, 4-amino-5-heptyl-1,2,4-triazole-3-thione, 5-heptyl-1,2,4-triazole-3-thione, 5-heptyl-1,3,4-thiadiazole-2-thione, and 6-heptyl-1,2,4,5-tetrazine-3-thione) collectors on malachite surfaces, which provided an atomic-level understanding of the structure–property relations of azolethione derivatives as chelating reagents for copper mineral flotation. Lu et al. [3][68] designed a series of amide collectors (including Nhydroxy-N-benzyl butyramide (NHNBB), N-hydroxy-N-benzyl acetamide, N-hydroxy-N-phenyl acetamide and N-hydroxy-Nphenyl butyramide) and used them to achieve the efficient separation of malachite from calcite and quartz in the absence of a frother and activator. By combining quantum chemical calculations, solution chemical analyses, X-ray photoelectron spectroscopy (XPS), and Fourier-transform infrared spectroscopy (FTIR), the results suggested that, in addition to electrostatic attraction, NHNBB could interact with malachite through chemisorption to generate a five-membered ring complex on a malachite surface, thus exhibiting an efficient collection power for malachite.
Furthermore, Chen et al. [4][69] reported the promotional effect of ammonium sulphate ((NH4)2SO4) as a modifier in malachite sulfidization flotation with butyl xanthate (BX) as a collector. The DFT calculation results indicated that the adsorption energy of HS− produced by the ionization of sodium sulfide (Na2S, a sulfidizing reagent) in water was reduced by the addition of (NH4)2SO4, which demonstrated that (NH4)2SO4 could improve the adsorption stability of HS− on a malachite surface. However, except for malachite, quantum chemistry studies of flotation reagents for azurite, chrysocolla, and cuprite have been rarely reported.
1.1.2. Lead Oxide Minerals
There are many lead oxide minerals, including cerussite, PbCO3; anglesite, PbSO4; wulfenite, PbMoO4; vanadinite, Pb[Cl(VO4)3; pyromorphite, Pb5(PO4)3Cl; mimetite, Pb5(AsO4)3Cl; plumbojarosite, PbFe6[(OH)6(SO4)2; and so forth. In particular, quantum chemical studies on the sulfidization mechanisms of cerussite are of paramount importance. Since lead oxide minerals are more prone to have higher solubility and stronger surface hydration than lead sulfide minerals, conventional sulfhydryl-based reagents are less effective in their flotation. A sulfidization treatment is able to enhance the hydrophobicity of oxide minerals, thus enabling them to be better recovered by the collector. Therefore, the sulfidization–xanthate method is employed as one of the most effective methods for cerussite flotation in industry, with sodium hydrosulfide (NaSH)/sodium sulfide (Na2S) being added as the sulfidizing reagents [5][6][7][70,71,72].
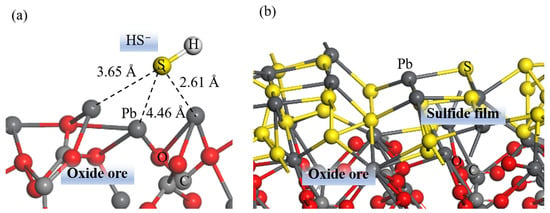
It is worth noting that the existing literature on DFT studies of cerussite primarily concentrates on HS− adsorption. For instance, Feng et al. [8][73], employing XPS and DFT, investigated the sulfidization mechanism of cerussite by adsorbing a main hydrolyzate of sulfidizing reagents, a single HS−, on a cerussite (110) surface (see Figure 12a). Nevertheless, the experimental results confirmed that a PbS film formed on the cerussite surface, leading to changes in the coordination structure of the Pb atoms on the surface [9][10][11][12][13][74,75,76,77,78]. Therefore, to better reflect the actual surface structure of sulfidized cerussite, Tang et al. [14][79] employed the density functional based tight binding (DFTB+) method to study the microscopic mechanism of cerussite flotation using the sulfidization–xanthate method. As shown in Figure 12b, a sulfidization model of a PbS film covering the PbCO3 (001) surface was established, resulting in a change in the ligand type of the surface Pb atoms from the O ligand to the S ligand. The results demonstrated that the adsorption energies of a single water molecule or multiple water molecules adsorbed on a sulfidized cerussite (sul-PbCO3) surface become less negative, whereas the formation energy of BX and water co-adsorbed on a sul-PbCO3 surface become more negative. As predicted using the HSAB theory, after sulfidization, Pb2+ changed its behavior from a hard acid in PbCO3 to a soft acid in PbS, facilitating its interaction with BX (soft base), but weakening its interaction with water (hard base). For the periodic system calculations, the selection of appropriate crystal planes is a prerequisite for the subsequent calculations of reagent adsorption and molecular dynamics. A detailed protocol for selecting crystal planes can be found in Section S3 of the Supplementary Materials.
1.1.3. Zinc Oxide Minerals
The main zinc oxide minerals are smithsonite, ZnCO3; hemimorphite, Zn4(Si2O7)(OH)2·H2O; zincite, ZnO; willemite, Zn2SiO4; and hydrozincite, 3Zn(OH)2·2ZnCO3. Similar to cerussite, quantum chemical studies on smithsonite have mainly focused on the discussion of its sulfidization mechanism. For example, Chen et al. [15][80] developed a model of a smithsonite (101) surface using the DFTB+ method and simulated the adsorption of a monolayer of S2−, HS−, and OH− ions on the surface to investigate the influences of the sulfidization effect and hydration effect on the adsorption on a smithsonite surface. At low concentrations of sodium sulfide, HS− ions formed a Zn−SH−SH structure on a smithsonite surface, which prevented dodecylamine (DDA) from interacting with the Zn atoms on the smithsonite surface. This was consistent with the flotation practice of smithsonite, which required large quantities of sodium sulfide. However, at high Na2S concentrations, a ZnS film formed on the smithsonite surface, which facilitated the adsorption of DDA. DFT calculations were also conducted by Zhao et al. [16][81] to investigate the adsorption mechanism of HS− on a smithsonite (101) surface. The results manifested that HS− ions could spontaneously interact with the top-, bottom-, and bridge-site Zn atoms on a smithsonite surface, resulting in the formation of a stable Zn−S structure on the surface. Meanwhile, at the atomic level, a slight oxidation of smithsonite was also found to have occurred during the sulfidization of smithsonite.
Liu et al. [17][82] combined theoretical and experimental methods to discuss the influences of six different carboxyl collectors (including linoleic acid (CH3(CH2)4(CH)2CH2(CH)2(CH)7COOH), oleic acid (CH3(CH2)7(CH)2(CH2)7COOH), lauric acid (CH3(CH2)10COOH), stearic acid (CH3(CH2)16COOH), palmitic acid (CH3(CH2)14COOH), and naphthenic acid (C6H11COOH)) on smithsonite flotation. The DFT calculations indicated that unsaturated carboxyl collectors exhibit large electrophilicity, whereas benzenoid and saturated carboxyl collectors possess small electrophilicity. Only linoleic acid and oleic acid were considered effective collectors towards smithsonite in the presence of water molecules. It was also found that the adsorption of water molecules weakened the hybridization of the 3s and 3p orbitals and the electrophilicity of the surface Zn atoms, which was detrimental to the adsorption of carboxyl collectors with small electrophilicity.
For hemimorphite (Zn4Si2O7(OH)2⋅H2O), Zhao et al. [18][83] employed the DFT method and the bond valence model to evaluate the correlation between complex stability constants and the bonding strength of the polar groups of flotation collectors (including N-dodecanoylglycine, N-lauroylsarcosine, N-laurylaminoacetic acid, and lauric acid). Through the results, it was believed that the bonding strengths of the polar groups controlled the collecting power for hemimorphite flotation. Jia et al. [19][84] combined a series of experimental methodologies and DFT calculations to study the adsorption mechanism of a green biosurfactant sodium N-lauroylsarcosinate collector on hemimorphite and quartz surfaces. The DFT results showed that the two O atoms of the carboxyl group in sodium N-lauroylsarcosinate were bonded with Zn and H atoms and adsorbed on a hemimorphite surface in a bidentate bonding structure, indicating that sodium N-lauroylsarcosinate has excellent selectivity for hemimorphite and has a great potential for industrial application in hemimorphite–quartz flotation separation. Zuo et al. [20][85], employing the DFT method, studied the effects of Na2S sulfidization and sodium sulfosalicylate (C7H5NaO6S⋅2H2O) activation on hemimorphite flotation. The calculation results confirmed that the Zn atoms on the hemimorphite (110) surface were separated from the surface O atoms, which resulted in the formation of many vacancies, thereby greatly reducing the steric hindrance effect during sulfidization. Zn(SSA)22+, formed via the complexation of C7H5NaO6S⋅2H2O with Zn2+, dissolved from the hemimorphite surface and participated in sulfidization reactions, i.e., Zn4Si2O7(OH)2⋅H2O→Zn2+→Zn(SSA)22+→ZnS, making the sulfidization process simple and efficient.
1.1.4. Other Non-Ferrous Oxide Minerals
In addition to Cu, Pb, and Zn oxide minerals, the quantum chemistry of flotation for Mg- and Na-containing oxide minerals has been studied in detail. The main magnesium resources utilized globally are magnesite, dolomite, brucite, carnallite, and olivine. The quantum chemical studies of the interactions of flotation reagents with mineral surfaces have been focused on magnesite (MgCO3) and dolomite (CaMg(CO3)2). The research was conducted in two main categories: collectors and depressants. Through DFT calculations, Zhong et al. [21][86] and Tang et al. [22][87] investigated the adsorption mechanisms of α-chloro-oleate acid and cetyl phosphate, respectively, as collectors on a magnesite surface. Li et al. [23][88] compared the effects of dodecylamine and n-octanol as collectors on the removal of impurities in the reverse flotation of magnesite by combining experimental studies and computational simulations. It was shown that the dissolved Ca2+ ions in dolomite weaken the selectivity of anion flotation, which is one of the reasons for the difficulty in separating dolomite and magnesite. Whereafter, Liu et al. [24][89] further employed sodium fatty alcohol polyoxyethylene ether sulfonate (AESNa) as an ion-tolerance collector to understand the reason why AESNa reduces or eliminates the adverse effect of Ca2+ ions. The results demonstrated that the EO groups of AESNa were complexed with Ca2+ ions via electrostatic interactions, making it difficult for Ca2+ ions to access the polar head groups of the collectors, hence leaving magnesite flotation unaffected. For depressants, Sun et al. [25][90] first introduced ethylenediamine tetra (methylene phosphonic acid) sodium (EDTMPS) as a chelation depressant to improve the separation efficiency of magnesite from quartz with DDA used as a collector in this system. The DFT calculations revealed that the pre-adsorption of EDTMPS weakened the DDA-magnesite interaction; this observation was confirmed by the expansion of the distance between the magnesite surface and the DDA N atoms (from approximately 2.64 Å to 8.00 Å) in the presence of EDTMPS.
Magnesite and dolomite are Mg-bearing minerals with similar crystal structures, which makes their separation a challenge in production. Reverse flotation reagents for magnesite and dolomite are important subjects of quantum chemical studies directed at dolomite. Sun et al. [26][91] adopted dodecane-1,2-diyl bis (dihydrogen phosphate) (DBDP), and Zhao et al. [27][92] introduced dimethylaminopropyl lauramide (DPLA) as dolomite collectors in magnesite–dolomite reverse flotation. The DFT simulations, micro-flotation tests, contact angle and zeta potential measurements, and FTIR and atomic force microscopy (AFM) analyses verified their potential for industrial applications. Yao et al. [28][93] enhanced the flotation separation of dolomite and magnesite using DDA as a collector and using sodium dihydrogen phosphate (SDP) as an activator for dolomite. The DFT calculations suggested that the strength of spontaneous adsorption between dolomite and SDP was much greater than that between magnesite and SDP, indicating that SDP posed a selective effect on the flotation separation of magnesite and dolomite.
Moreover, phosphate flotation plants also prefer to separate apatite and dolomite via reverse flotation, with sulfuric acid (H2SO4) being commonly used as a specific apatite depressor. Nevertheless, this inorganic acid is unable to prevent the flotation of dolomite with fatty acid collectors, such as oleate anions. The depression mechanism of H2SO4 on apatite flotation is well understood, whereas that on dolomite is unclear. Cao et al. [29][94] studied the effect of SO42− anions on the adsorption of oleate anions on a dolomite (104) surface and compared the adsorption behavior of SO42− anions onto both perfect and CO3-defect dolomite surfaces using DFT. The results indicated that only the SO42− anions are adsorbed on the CO3-defect surface, where they bound to Ca atoms. The rest of the Mg and Ca atoms at the defect sites will further interact with oleate anions to produce new Mg−O and Ca−O ionic bonds. SO42− anions and oleate might coexist on a dolomite surface. This phenomenon helped to illustrate the flotation process of dolomite treated with H2SO4.
Feldspar-group minerals (KAlSi3O8, NaAlSi3O8, and CaAl2Si2O8) are both aluminosilicate ores and major sources of Na. Oxalic acid, as a pH modifier, is a commonly used organic substance in feldspar flotation, and it has three different configurations (C2O42−, HC2O4−, and H2C2O4) depending on the pH value of the aqueous solutions. Xue et al. [30][95] employed DFT calculations, classical molecular dynamic (CMD) simulations, and frequency calculations to understand the adsorption mechanism of oxalic acid at the water–feldspar interface. The results demonstrated that the adsorption of H2C2O4 on a feldspar surface was a physical outer-sphere adsorption with the formation of hydrogen bonds, while the adsorption of C2O42− and HC2O4− belonged to inner-sphere adsorption, which preferred to coordinate with Al active sites on a feldspar surface rather than Si active sites. The order of adsorbed substances on feldspar surface was C2O42− > HC2O4− > H2O > H2C2O4. At a lower pH of 0.00, water instead of H2C2O4 would attack the Al active sites to promote Al dissolution. At higher pH values of 2.76 and 6.00, HC2O4− and C2O42−, but not water, would attack the Al active sites to facilitate Al dissolution; however, at acidic conditions, H+ would attack Si active sites instead of Al active sites to promote Si dissolution.
1.2. Non-Ferrous Sulfide Minerals
For non-ferrous sulfide minerals, Liu et al. [31][96] used DFT calculations to investigate the structure–activity relations between four chelating collectors (including diisobutyl monothiophosphinate (DIBMTPI), diisobutyl monothiophosphate (DIBMTPA), diisobutyl dithiophosphinate (DIBDTPI), and diisobutyl dithiophosphate (DIBDTPA)) and the Cu-, Au-, Ag-, and Pb-bearing sulfide minerals and provided a potential method for the molecular design of new reagents for improving metal recovery. Moreover, Cui et al. [32][97] introduced the principle of coordination chemistry and the DFT method to study the interactions of methyl xanthate as a collector with galena and sphalerite under weak acidic and neutral conditions, which contributed to a systematic understanding of the interaction mechanism of xanthate collectors on the surfaces of non-ferrous sulfide minerals and provided a theoretical basis for future studies.
1.2.1. Copper Sulfide Minerals
Zhao et al. [33][98] synthesized two ether thionocarbamates, O-(2-butoxy-1-methylethoxy) isopropyl-N-ethoxycarbonyl thionocarbamate (BMIPECTC), and O-butoxy isopropyl-N-ethoxycarbonyl thionocarbamate (BIPECTC) and studied their collection powers using adsorption measurements, ultraviolet spectra (UV) and FTIR analysis, flotation tests, and DFT calculations. Quantum chemistry calculations revealed that both BIPECTC and BMIPECTC exhibited greater collection power towards copper minerals in terms of binding model simulation with Cu ions, molecular hydrophobicity, and frontier molecular orbital analysis compared to O-isobutyl-N-ethoxycarbonyl thionocarbamate (IBECTC) and O-isopropyl-N-ethyl thionocarbamate (IPETC). Li et al. [34][99] understood the mechanism of kerosene influence on chalcopyrite floatability in a Mg2+-bearing solution (which simulated seawater as flotation medium) by means of DFT calculations and extended the Derjaguin–Landau–Verwey–Overbeek (EDLVO) theory. The results indicated that kerosene was selectively adsorbed on the Mg(OH)2 surface and formed agglomerates, thus hindering the adsorption of Mg(OH)2 precipitates on the chalcopyrite surface. When additional kerosene was dosed, hydrophobic agglomerates were also formed due to the adsorption of kerosene on chalcopyrite, which further improved the floatability of chalcopyrite. Mkhonto et al. [35][100] employed DFT to investigate the adsorption energies, electronic properties, and bonding behavior related to the reactivity of five collectors, including O-butyl- O-butyl-N-butoxycarbonyl-thiocarbamate (BBCTC), N-ethoxycarbonyl-thiocarbamate (BECTC), O-isobutyl-N-butoxycarbonyl-thiocarbamate (IBBCTC), O-isobutyl-N-isobutoxycarbonyl-thiocarbamate (IBIBCTC), and O-isobutyl-N-ethoxycarbonyl-thiocarbamate (IBECTC), with a chalcopyrite (112) surface. The results showed that BECTC and BBCTC are better collectors for the selective flotation of chalcopyrite, indicating that collectors possessing a straight hydrocarbon chain might be preferable to those possessing a branched hydrocarbon chain. Moreover, He et al. [36][101] proposed a model combining quantum chemistry and machine learning to facilitate the screening of solidophilic reagents for chalcopyrite flotation. A 47-molecule flotation set was built at the level of B3LYP/def2-TZVP under solvation effects to acquire information about their affinity to potential active sites on a chalcopyrite surface (e.g., Fe(II), Cu(I), Cu(II) sites).
In addition to the research on flotation reagents for chalcopyrite itself, the reagents used to separate chalcopyrite from pyromorphite, pyrite, and galena have attracted a great deal of attention. Molybdenite and chalcopyrite have similar floatability under the action of collectors; hence, it is difficult to selectively separate them. Therefore, depressants are essential to achieve the necessary Cu-Mo separation. Conventional inorganic depressants, such as lime, cyanide, and sulfides, inevitably cause low selective depression for the flotation separation of various sulfide minerals. Therefore, organic depressants have become one of the research focuses in recent years because of their biodegradability, excellent selectivity, high flexibility, potential modifications, and rich sources. The adsorption mechanisms of multiple depressants such as L-cysteine [37][102], rhodanine-3-acetic acid [38][103], disodium carboxymethyl trithiocarbonate [39][104], thioglycolic acid [40][105], 3-amino-5-mercapto-1,2,4-triazole [41][106], 2-((5-mercapto-1,3,4-thiadiazol-2-yl)thio)acetic acid [42][107], and 5-amino-1,3,4-thiadiazole-2-thiol [43][108] on a chalcopyrite surface were studied using DFT supplemented with micro-flotation and bench-scale flotation experiments and various advanced characterization technologies, including ultraviolet-visible (UV-vis) spectroscopy, FTIR, contact angle and XPS analysis, time-of-flight secondary ion mass spectrometry (Tof-SIMS) measurements, adsorption capacity, and zeta potential measurements, which helped to offer a theoretical basis of the molecular design of organic depressants with respect to chalcopyrite.
Pyrite (FeS2) often associates with chalcopyrite, which is a common gangue mineral in copper flotation. Therefore, depressants are often required to restrict the flotation of pyrite to acquire qualified concentrates during chalcopyrite–pyrite flotation separation. In this case, Wu et al. [44][109] analyzed the sodium butyl xanthate (SBX) adsorption on Cu- and Fe-deficient chalcopyrite surfaces using DFT calculations and examined the galvanic effect on flotation behaviors using mixed mineral flotation tests. The DFT calculations revealed that the Cu-/Fe-deficient surface could not facilitate SBX adsorption. The galvanic interaction of the chalcopyrite–pyrite couple increased pyrite recovery by means of copper activation but decreased chalcopyrite recovery due to a weaker SBX adsorption, making their flotation separation more difficult. Mkhonto et al. [45][110] adopted DFT combined with micro-flotation tests, electronic property analysis, and XPS to study the interaction mechanisms of three thiocarbamate collectors, Sallyl-N-diethyl-dithiocarbamate (ADEDTC), O-isopropyl-N-diethyl-thionocarbamate (IPDETC), and IPETC, on pyrite (100) and reconstructed chalcopyrite (112) surfaces. The results demonstrated that the active sites on the chalcopyrite surface were Cu atoms rather than Fe atoms. Among the three collectors, the adsorption of ADEDTC was the strongest, and its adsorption on chalcopyrite was stronger than that on the pyrite surface. Zhang et al. [46][111] systematically investigated the chalcopyrite–pyrite separation mechanism at high alkaline conditions using adsorption studies, DFT calculations, flotation tests, FTIR analysis, and zeta potential measurements. The flotation tests and various measurements indicated that the flotation separation of chalcopyrite and pyrite could be realized at highly alkaline conditions. The DFT calculations further confirmed that the adsorption of SBX on chalcopyrite Cu sites was stronger than on pyrite Fe sites. Since the hydroxyl ion had a stronger affinity towards pyrite, it could adsorb efficiently on the pyrite surface rather than on the chalcopyrite surface.
Complex and refractory Cu-Pb polymetallic sulfide minerals such as galena and chalcopyrite have always been difficult to separate because of their similar surface wettability. Hence, in galena–chalcopyrite flotation separation, depressants should be added to enlarge the difference in their floatability, which is regarded as the most common method in their separation. Liu et al. [47][112] systematically investigated the selectivity of three mercapto acids (mercaptoacetic acid, 3-mercaptoisobutyric acid, and 3-mercaptopropionic acid) for the separation of galena and chalcopyrite using flotation experiments combined with first-principles calculations. Both the experimental and calculation results demonstrated that mercapto acids have a higher affinity towards chalcopyrite, and, among them, the selectivity of 3-mercaptopropionic and 3-mercaptoisobutyric acids are better than that of mercaptoacetic acid, which makes them the most selective depressants in the improved flotation separation of chalcopyrite and galena. Zhang et al. [48][113] synthesized a novel depressant, dithiocarbamated poly (acrylamide-allyamine) (DTC-PAA), and employed it as a depressant for galena in chalcopyrite–galena flotation separation. The DFT calculation results showed that when using O-isopropyl-N-ethyl thiocarbamate (IPETC) as the collector, DTC-PAA was chemisorbed onto galena surface Pb sites through the dithiocarbamate groups in DTC-PAA, consequently achieving the effective flotation separation of galena from chalcopyrite. These studies have enabled the development of reagent molecules with high selectivity by quantum chemical means, exemplifying the potential for the effective separation of Cu-Pb polymetallic sulfide minerals.
Quantum chemical studies on flotation reagents for covellite (CuS) have also been reported. Porento and Hirva [49][114] performed ab initio calculations to study the interaction of three different sulfhydryl surfactants, 1,1,1-butanetrithiol (BTT), diethyl dithiocarbamate, and ethyl xanthate, with a covellite (001) surface. The results suggested that the mineral–reagent interaction of BTT was the strongest, making it a potential collector for the covellite flotation. Ma et al. [50][115], using single-mineral and mixed-mineral flotation tests, in conjunction with adsorption measurements, an FTIR analysis, and DFT calculations, studied the collection mechanism of ethyl isobutyl xanthogenic acetate (EIBXAC) in the flotation of secondary copper sulfide minerals (for instance, covellite and digenite). The results confirmed that EIBXAC could be chemisorbed on the covellite (001) and digenite (001) surfaces, showing the possibility of using EIBXAC to collect secondary copper sulfide minerals. Botero et al. [51][116] studied the interaction mechanisms of potassium amyl xanthate (PAX) and O-isopropyl-N-ethyl thionocarbamate (IPETC) as collectors on a covellite surface. The DFT calculations predicted that PAX bonded to the surface Cu atoms via the C–S and C=S groups, whereas IPETC bonded to the surface Cu atoms only through the C=S group.
Nevertheless, quantum chemical studies of two other common copper sulfide minerals, chalcocite (Cu2S) and bornite (Cu3FeS3), have been reported only in terms of surface properties and electronic structures [52][53][117,118], and they do not report on the interaction mechanisms involving reagent adsorption. As a consequence, their study may become one of the main directions for future research.
1.2.2. Lead Sulfide Minerals
Galena, PbS, is the most abundant lead mineral in nature, often associated with sphalerite, chalcopyrite, and pyrite, and thus, the flotation separation reagents for it are the invariable subject of research for many scholars. Ma et al. [54][119], Jia et al. [55][120], and Jia et al. [56][121] synthesized novel collectors, including S-benzoyl-N,N-diethyldithiocarbamate (BEDTC), β-oxo thioamide surfactant 3-(ethylamino)-N-phenyl-3-thioxopropanamide (EAPhTXPA), and trimethylacetyl thiobenzamide (TTBA), respectively, and investigated their interaction mechanisms in the flotation separation of galena and sphalerite via flotation tests, adsorption measurements, FTIR and XPS analyses, and DFT calculations. The results showed that all of these collectors have stronger collection powers than traditional collectors and have greater selectivity towards galena against sphalerite. The BEDTC acted as a bidentate ligand, bonding with galena Pb atoms through the carbonyl O and thiol S atoms to form two different adsorption geometries, one with two distinct Pb atoms to form a bullet-shaped complex, and the other with the same surface Pb atom to form a six-membered ring complex [54][119]. Whereas EAPhTXPA interactes with PbS by forming Pb–O, Pb–N, and Pb–S bonds [55][120], TTBA interacts with PbS via the formation of Pb–O and Pb–S bonds [56][121]. In addition, Zhang et al. [57][122] developed a reagent scheme consisting of aerofloat collectors as well as Zn2+ and SO32− depressants for the flotation separation of galena from sphalerite-rich sulfide minerals. Ab initio molecular dynamics (AIMD) simulations and static calculations were used to investigate the mechanism of the employed reagent scheme on the flotation separation of galena and sphalerite at the atomic level. The results suggested that Zn2+ and SO32− have synergistic effects on depressing sphalerite, whereas among them, aerofloat collectors exhibit greater selectivity towards galena.
Based on the DFT calculation, Zhang et al. [58][123] designed and synthesized polymaleamide-propyl dithiocarbamate (PMA-PDTC) as a novel depressant and investigated its depression effect in separating galena from chalcopyrite. Wei et al. [59][124] employed three 2-mercaptobenzimidazole derivatives, including 1-benze-2-mercapto-benzimidazole (BMBI), 1-ethyl-2-mercapto-benzimidazole (EMBI), and 1-propyl-2-mercapto-benzimidazole (PMBI), as chelating collectors to understand their collection mechanisms in the flotation separation of galena from pyrite via lab-scale flotation tests and DFT simulations. The results indicated that the floatability of these collectors follows the increasing order of EMBI < PMBI < BMBI. Later on, Chen et al. [60][125] adopted computational simulations and the microcalorimetry method to study the adsorption of xanthate, dithiocarbamate, and dithiophosphate on pyrite and galena surfaces. The results revealed that pyrite Fe atoms are more active than galena Pb atoms, and the reagents coordinated mainly to the surfaces through interactions between their S atoms and surface Pb/Fe atoms. The adsorption of xanthate on the pyrite surface was stronger than that on the galena surface, while those of dithiocarbamate and dithiophosphate were the opposite, and they showed good selectivity in the separation of pyrite and galena. Furthermore, Dong et al. [61][126] used a novel collector S-benzyl-N-ethoxycarbonyl thiocarbamate (BET) to study its interaction mechanism for selectively separating galena from a polymetallic sulfide ore using flotation experiments, adsorption tests, and an FTIR spectra analysis in conjunction with DFT. The results manifested that BET is chemisorbed on the galena surface via the formation of normal covalent bonds between carbonyl S atoms and surface Pb atoms and back donation covalent bonds between carbonyl O atoms and surface Pb atoms. BET showed strong collection power and good selectivity for galena and was therefore considered to have a wide range of industrial application prospects.
Quantum chemical studies of flotation reagents for jamesonite (Pb4FeSb6S14) have also been reported. Cui et al. [62][127] adopted the mixed collectors of sodium diethyldithiocarbamate (DDTC) and sodium diisobutyl dithiophosphinate (3418A) to enhance the flotation of jamesonite, and the adsorption mechanism was studied by means of flotation experiments; FTIR, scanning electron microscopy, and energy-dispersive X-ray spectroscopy (SEM-EDS) analyses; and dispersion-corrected density functional theory (DFT-D) calculations. In this study, the dispersion correction was added to make the calculation results more consistent with the experimental values, as the dispersion interaction caused by the incorrect long-range behavior of the correlation potential was corrected [62][127]. The results showed that the DDTC/3418A mixture with a 2:1 molar ratio exhibits excellent selectivity and significantly enhances the flotation performance of natural refractory Pb-Sb-Zn minerals. Such mixture could perform synergistic chemisorption on a jamesonite surface, with 3418A rapidly overcoming the binding interactions of the surrounding molecules from the micelles and then forming effective adsorption on the jamesonite surface, while DDTC reinforces the hydrophobic layer through the formation of H…N hydrogen bonds. Additionally, Li et al. [63][128], using flotation tests supplemented with DFT calculations, compared the depression performance of ten kinds of organic depressants for the flotation of marmatite, jamesonite, and pyrite. The results showed that jamesonite could be well depressed via pyrogallic acid and 4-amino-hydroxybenzene, indicating that the presence of a benzene ring in the molecule could enhance the depression performance. This study also proposed that the calculations of frontier orbitals could well explain the interactions between sulfide minerals and organic depressants, and the hydrophilicity of organic depressants should be taken into account when applying frontier orbitals to investigate their depression effects on sulfide minerals.
1.2.3. Zinc Sulfide Minerals
Sphalerite and wurtzite are two polymorphs of ZnS and represent the most widespread zinc sulfide minerals in nature. Of these two, the quantum chemistry of sphalerite flotation reagents has been studied since the beginning of the 21st century, with research topics being mainly focused on non-activated collection, activated collection, and the development and mechanistic study of collectors and depressants. Regarding the non-activated collection of sphalerite, Liu et al. [64][129] tested three thiophenol collectors, including 2-fluoro thiophenol, 2-hydroxy thiophenol, and 2-amino thiophenol, for the flotation of marmatite (Fe-bearing sphalerite) without the addition of an activator, copper sulfate (CuSO4). Both the flotation tests and quantum chemical calculations confirmed that 2-amino thiophenol has the strongest collection power among the three reagents.
For the activated collection, Porento et al. [65][130] employed ab initio cluster model calculations, and Liu et al. [66][67][68][131,132,133] adopted DFT calculations to investigate the effect of Cu atoms on the adsorption of ethyl xanthate (EX) on sphalerite (111) and (110) surfaces, respectively. These studies revealed that Cu adsorbed on sphalerite S atoms [68][133] and Cu substituted with sphalerite Zn atoms [67][132] could lead to the activation of sphalerite. The S 3p orbitals of EX and the Cu 3d orbitals of Cu-activated sphalerite overlapped exactly to the maximum extent near the Fermi level (EF), implying stable chemisorption. Moreover, Sarvaramini et al. [69][134] combined flotation tests and DFT simulations to study the interactions of the collector DIBDTPI with un-activated and Pb-activated sphalerite. Unlike copper activation, it was impossible to substitute Zn cations in the lattice with Pb at the surface due to the larger van der Waals radius of Pb and the pronounced lattice deformations of the sphalerite structure. Dissolved collectors are attached to the Pb-activated sphalerite surface via adsorbed Pb cations or Pb(OH)2. The adsorbed Pb cations, in return, are able to attach DIBDTPI through forming two bidentate covalent bonds between the collector S atoms and Pb cations. The interactions of surface Pb(OH)2 with the collector is realized by the formation of covalent bonds between the S head of DIBDTPI and the Pb cations of Pb(OH)2. Aside from these, Long et al. [66][131] performed a DFT study to investigate the interaction mechanism of collector EX with non-activated/Cu-activated sphalerite (110) surfaces in the absence and presence of water molecules. The calculation showed that the adsorption of water molecules drastically changes the properties of the sphalerite surface, leading to a decrease in the reactivity of surface Zn atoms with xanthate, but the presence of water has a rare effect on the properties of the Cu-activated sphalerite surface.
The N,N-dimethyldi-thiocarbamate (DMDC), the lowest homologue of dialkyldithiocarbamate salts, has been found to be effective in chalcopyrite flotation and to significantly depress Cu-activated marmatite (the Fe-rich variety of sphalerite), with excellent selectivity in Cu-Zn sulfide minerals. However, the separation mechanisms of Cu-Zn sulfide minerals are unclear. Therefore, Qin et al. [70][135] adopted UV-vis spectroscopy, FTIR, and a first-principles study to investigate the effects of the sodium salt of DMDC with or without BX on the flotation of chalcopyrite, marmatite, and sphalerite. It was found that the presence of Fe could hinder the activation of sphalerite, and thus, marmatite was more likely to be depressed than sphalerite. DMDC could enhance the recovery of chalcopyrite at pH 7.5, but it is detrimental to the recovery of Cu-activated sphalerite/mamatite in the presence of BX. Meanwhile, the Cu-activated marmatite was depressed more obviously. The adsorption of DMDC on mineral surfaces occurred through the interaction of S 3p orbitals with 3p, the 3d orbitals of Cu atoms, and 3d orbitals of Zn atoms. The electrons transferred from the Cu and Zn atoms to S atoms, respectively.
Zhu et al. [71][136] exploited a green depressant 1-hydroxyethylidene-1,1-diphosphonic acid (HEDP) for sphalerite and investigated its interaction mechanism in the flotation separation of sphalerite and galena via zeta potential tests, FTIR, XPS, ToF-SIMS, and DFT calculations. The results demonstrated that when using sodium isobutyl xanthate (SIBX) as a collector, HEDP selectively depresses sphalerite rather than galena, which achieves effective flotation separation results. This observation was attributed to the selective adsorption of HEDP on the sphalerite surface, but the pre-adsorbed HEDP on the galena surface could be easily substituted via SIBX. HEDP was attached to the sphalerite surface by coordinating Zn atoms through two distinct phosphonic acid groups, generating a six-membered chelating ring.
Previous research works have not examined the depression of Ca via the Fe content in the Fe-bearing sphalerite, nor has convincing microscopic evidence of Ca adsorption been obtained to support the depression of Ca-containing ions on the flotation of Fe-bearing sphalerite. Thus, Zhang et al. [72][137] investigated the effects of the Fe content and the presence of Ca on the flotation depression of marmatite under high-alkalinity environments. The following conclusions could be obtained through the results of various experiments and calculations. The increased adsorption of hydroxides (especially iron hydroxide) and Ca significantly hinders the adsorption of Cu and xanthate and increases the hydrophilicity of a high-iron sphalerite surface, which ultimately leads to an increase in the depression degree of high-iron sphalerite with the increasing Fe content.
1.2.4. Other Non-Ferrous Sulfide Minerals
Cao et al. [73][138] investigated the adsorption mechanism of Pb2+ as an activator on a stibnite (Sb2S3) (010) surface using DFT calculations, inductively coupled plasma mass spectrometry (ICP-MS) experiments, and micro-flotation tests. The calculation results indicated that BX could be adsorbed on both the Sb sites on the non-activated surface and the Pb sites on the Pb-activated surface, and that BX adsorption on the Pb-activated surface is more stable.
Apart from stibnite, quantum chemical studies of flotation reagents acting on other non-ferrous sulfide minerals containing Ni, Co, Sb, Hg, Cd, Bi, Al, Mg, Na, Sr, Ba, etc., for instance, pentlandite, (Ni,Fe)9S8; violarite, Ni2FeS4; millerite, NiS; linnaeite, Co3S4; carrollite, CuCo2S3; cobaltite, CoAsS; cinnabar, HgS; corderoite, Hg3S2(Cl,Br)2; etc., are rarely reported.
2. Ferrous Metals
“Ferrous metals” is the industrial term for Fe, Cr, and Mn, including the alloys of these three metals. Iron is widely distributed in nature, and it is the earliest discovered and most abundant metal, of which the most important and most industrially utilized mineral sources are magnetite, hematite, and ilmenite. Previous quantum chemical studies of flotation reagents for Fe-bearing minerals concentrated mostly on these three minerals, followed by Mn-bearing minerals. However, at present, no quantum chemical studies on flotation reagents for Cr-bearing minerals have been reported.
2.1. Iron Oxide Minerals
For hematite, Fe2O3, its separation from quartz via reverse flotation has been the primary focus of research. Reverse flotation is the most commonly used industrial beneficiation method for hematite. In reverse cationic flotation, carboxymethyl cellulose (CMC), dextrin, and modified starch are among the most efficient hematite depressants in practice, while quartz is first activated by metal ions (for instance, Ca2+ ions) and then collected by sodium oleate (OL) and DDA, which has been widely applied commercially. By using AFM in conjunction with DFT, Li et al. [74][139] studied the depression of hematite in the oleate–starch–hematite reverse flotation system. The results showed that oleate and starch alone could be adsorbed onto the hematite (001) surface through the formation of covalent bonds. Nevertheless, in the oleate–starch–hematite system, the presence of starch hindered the adsorption of oleate on the hematite surface. In addition, the depression mechanism of causticized cassava starch (CCS) in the reverse flotation system of DDA-CCS-hematite was also studied by Zhang et al. [75][140]. The DFT calculations demonstrated that starch has a stronger depression effect on a hydrated hematite surface than quartz, whereas the collector RNH3+ (one of the main components of DDA) has a better selective adsorption to the hydrated surface of starch-modified quartz, leading to the selective separation of hematite and quartz. Moreover, by employing flotation tests, zeta potential measurements, XPS, and CMD simulations, Wang et al. [76][141] used carboxymethyl chitosan (CMCS) as a depressant to investigate its interaction mechanism in the reverse flotation of quartz from hematite using collector DDA. Similar to starch, the results suggested that CMCS can prevent the adsorption of DDA on a hematite surface, while it rarely affects the interaction of DDA with a quartz surface, and thus, CMCS has a better selective depression ability than starch. Therefore, CMCS can replace starch as one of the effective depressants in the hematite–quartz reverse flotation system in practice. Liu et al. [77][78][79][142,143,144] screened and firstly introduced N, N-bis (2-hydroxyethyl)-N-methyl dodecyl ammonium chloride (BHMDC), N,N-dimethyl-N′-(2-hydroxyethyl)-N′-dodecyl-1,3-propanediamine (DMPDA), and N, N-Dimethyl-N′-dodecyl-1,3-propanediamine (DPDA) as collectors in the reverse flotation of hematite based on DFT calculations, and employed flotation experiments combined with various surface characterization methods, including zeta potential measurements, XPS, and FTIR, to study the interaction mechanisms of these collectors on quartz and hematite surfaces. The results indicated that all three collectors have higher selectivity than the conventional collector DDA. BHMDC and DMPDA are mainly adsorbed on mineral surfaces through hydrogen bonding and electrostatic interaction. The introduction of hydroxyl groups can improve the surface activity of the collectors and selective adsorption on the target mineral surfaces. The excellent selectivity of DPDA, on the other hand, can be attributed to a greater number of active sites and a larger polar group size.
Studies on ilmenite (FeTiO3) collectors have also been widely reported. Zhang et al. [80][145] systematically assessed the adsorption mechanisms of the commonly used collectors with O-containing functional groups on the ilmenite (104) surface via DFT calculations. The adsorption energy results manifested that, for ilmenite beneficiation, the O-containing pnictogen compounds are the best among all of the O-containing collectors, and the collection performance decreases along the row of arsenic acid, phosphorous acid, and phosphoric acid ester. A partial density of states (PDOS) analysis further confirmed that the hybridization of ilmenite Ti 3d orbitals and collector O 2p orbitals contributes to the adsorption of collectors on an ilmenite surface, in which the O 2p electrons occupy the empty Ti 3d orbitals to complete the coordination shell of surface Ti atoms. Furthermore, the adsorption mechanisms of the collectors 2-ethyl-2-hexenoic hydroxamic acid (EHHA) [81][146], α-hydroxyoctyl phosphonic acid (HPA) [82][147], and benzohydroxamic acid (BHA) [83][148] on the ilmenite surface were evaluated via DFT simulation supplemented with flotation tests and multiple surface characterization tools. The experimental results confirmed the superior affinity of the three collectors for ilmenite, in which EHHA may be chemisorbed on the ilmenite surface in the form of 5-membered chelates, HPA may be chemisorbed on the ilmenite surface through the formation of HPA2− species, and the strong selectivity of BHA is attributed to the abundance of adsorption sites and the solid adsorption of five-membered rings. These works provide new ideas for the screening of solidophilic functional groups for the reagent design of ilmenite beneficiation and help to increase the understanding of the adsorption mechanisms of O-containing collectors on oxide mineral surfaces.
Ren et al. [84][149] studied the adsorption mechanism of salicylhydroxamic acid (SA) as a novel collector on a coulmbite surface using DFT calculations, flotation tests, and zeta potential determination. The results indicated that the dianion of SA exhibits a higher atomic charge value, HOMO energy, and greater dipole moment, and therefore, it has a stronger collecting power for coulmbite. In addition, Rath et al. [85][150] used DFT calculations to compare the interaction mechanism between oleate, as a collector, and magnetite (111), hematite (110), and goethite (010) surfaces. The results suggested that magnetite forms the most stable complexes with oleate, followed by hematite, and the least stable complexes are formed by goethite; meanwhile, this trend was verified by the contact angle measurements and flotation studies of hematite, magnetite, and goethite with OL at different pH and collector concentrations. However, quantum chemical studies of flotation reagents for other iron oxide minerals, including maghemite (γ-Fe2O3), limonite (a mixture of iron oxides and hydroxides, mostly goethite), and siderite (FeCO3), have not been reported.
2.2. Iron Sulfide Minerals
Pyrite, marcasite, and pyrrhotite are the three most common iron sulfide minerals, and they commonly coexist with other metal sulfides, such as copper, lead, and zinc sulfide minerals. The collection and depression of iron sulfides is essential for the recovery of other valuable metal sulfides. Hence, many DFT studies have been undertaken to investigate the collection and depression mechanisms in the flotation separation of pyrite, marcasite, and pyrrhotite from their associated minerals.
Xanthates are the most important and commonly used collectors in pyrite flotation. Therefore, it is necessary to understand the flotation chemistry of pyrite with xanthates during flotation. Han et al. [86][151] combined experimental and computational studies to evaluate the flotation chemistry between pyrite and isomeric xanthates (i.e., BX and iso-BX). The quantum chemical calculations elucidated that the iso-BX presents higher reactivity than that of the corresponding BX based on the frontier molecular orbital theory of chemical reactivity. Yang et al. [87][152], using the DFT method, studied the quantum chemical properties of xanthate derivatives, ROC=SS− (R = amyl, ethyl, or isobutyl), and their interactions with Fe(OH)2+, Fe(OH)2+, Fe3+, and pyrite cluster under solvation effects. Based on the results of the electronic chemical potential and frontier orbital energies, the flotation performance of the alkyl xanthates was predicted to be in a decreasing order from amyl xanthate (AMX), through iso-BX to EX. According to these studies, the experimental results of the xanthate collectors agree with the theoretical prediction, suggesting that the quantum chemical calculation is one of the effective tools for the rational design and selection of flotation reagents.
Kumar et al. [88][153] compared the adsorption of 2-mercaptobenzothiazole (MBT) on chalcopyrite and pyrite surfaces via DFT, explaining the selectivity of MBT towards pyrite in flotation. Mkhonto et al. [89][154] designed and synthesized a novel collector di-sodium 2,6-dithio-4-butyl-amino-1,3,5-triazine (SDTBAT) and investigated its adsorption mechanism on a pyrite surface using experimental methods combined with the computational DFT with dispersion correction and U-parameter (DFT-D3+U), demonstrating that SDTBAT has the potential to be an alternative collector to xanthates due to its high collection power in the separation of sulfide minerals.
NaOH, cyanide, and lime (CaO) are the three most common depressants in the flotation separation of sulfide minerals. The flotation of polymetallic sulfides is generally carried out at a pH of about 12, and at pH values below 12.5, the main components of CaO dissolved in water are the calcium hydroxyl ions, [Ca(OH)]+. Zhao et al. [90][91][155,156] employed DFT to investigate the depression mechanisms of cyanide and [Ca(OH)]+ with the pyrite (100), marcasite (010), and pyrrhotite (001) surfaces. The calculation results indicated that the depression effect of both cyanide and [Ca(OH)]+ is in the increasing order of pyrite < pyrrhotite < marcasite. As shown in Figure 23a–c, after CN− adsorption, the C atom interacts with one Fe atom on the pyrite surface; for marcasite, the C atom interacts with one S atom, while the N atom interacts with one Fe atom on the surface; and for pyrrhotite, only the N atom interacts with one Fe atom on the surface. The different adsorption configurations resulted in different charge transfers and adsorption energies, causing different flotation behaviors of these three iron sulfide minerals. After [Ca(OH)]+ adsorption, for marcasite and pyrite, the O atom interacts with one Fe atom, the Ca atom interacts with two surface S atoms, and there exists a Ca–Fe anti-bonding on the pyrite surface. For pyrrhotite, the Ca atom is attached to three S atoms on the pyrrhotite surface (see Figure 23d–f). Li et al. [92][157] also reported that the adsorption of [Ca(OH)]+ on the pyrite (100) surface is greater than the adsorption of (OH)−. After adsorption, partial surface S atoms are covered by the Ca atoms of [Ca(OH)]+, which is unfavorable for Cu activation, and thus, the Cu activation of pyrite after depression via lime is tougher than after depression via sodium hydroxide.
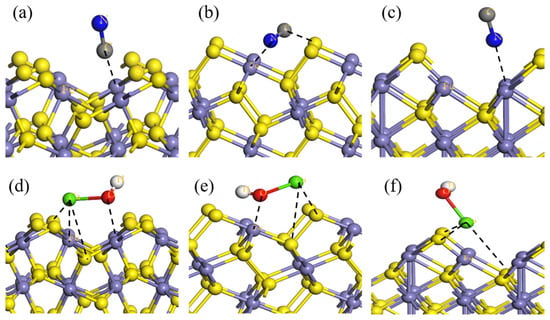
Figure 23. Schematic representation of CN− and [Ca(OH)]+ adsorbed on pyrite (100), marcasite (010), and pyrrhotite (001) surfaces: CN− adsorbed on (a) pyrite, (b) marcasite, (c) pyrrhotite (data from [90][155]) and [Ca(OH)]+ adsorbed on (d) pyrite, (e) marcasite, and (f) pyrrhotite (data from [91][156]).
Hydrogen peroxide (H2O2) is another efficient pyrite depressant. Cao et al. [93][158] studied the interaction between H2O2 and hydrated pyrite (100) surfaces using DFT calculations. The results showed that the H2O2 molecule is prone to react with the pyrite surface to generate an H2O molecule and one S=O bond.
2.3. Manganese Oxide Minerals
Minerals with a high Mn content are not common, and there are seven most typical types of industrially mined Mn-bearing minerals: pyrolusite, MnO2; “psilomelane” (a mixture of manganese oxides and hydroxides; mMnO·MnO2·nH2O); manganite, MnO (OH)2; hausmannite, Mn3O4; braunite, Mn2O3; rhodochrosite, MnCO3; and alabandite, MnS. The first six of them belong to the oxide minerals, while the last one is a sulfide. There are a few quantum chemical studies on flotation reagents for Mn oxide minerals, including rhodochrosite, but none were reported for Mn sulfide minerals. Zhao et al. [94][159] first introduced a novel collector, tert-butyl benzohydroxamic acid (TBHA), into the flotation of rhodochrosite, and compared the interaction mechanisms of TBHA and a commonly used collector BHA on the mineral surface using DFT calculations as well as micro-flotation tests, zeta potential measurements, and XPS analysis. Both the experimental and theoretical investigation results suggested that TBHA has a stronger collecting power to rhodochrosite than BHA, i.e., the substitution of tert-butyl groups on benzene rings greatly enhances the affinity of the hydroxamic acid to rhodochrosite. Consequently, TBHA is considered to be an ideal candidate collector for the flotation of rhodochrosite or other oxide minerals.
