Phenolic compounds (PheCs) are particularly relevant in many different frameworks due to their pro-oxidant and antioxidant activities. In fact, on the one hand, they are considered very dangerous pro-oxidant agents that can be present in the environment as pollutants in wastewater and soil from different industrial and agricultural industries. On the other hand, the antioxidant influence of PheCs available in natural products (including foods) is nowadays considered essential for preserving human health. Conventional techniques for detecting PheCs present some disadvantages, such as requiring expensive instrumentation and expert users and not allowing in situ measurements. This is the reason why there is a high interest in the development of simple, sensitive, specific, and accurate sensing methods for PheCs. Enzymes are often used for this purpose, and laccases with unique optical properties are adopted as bio-elements for sensing schemes.
- laccases
- phenolic compounds
- optical properties
- optical biosensors
1. Optical Properties of Laccase
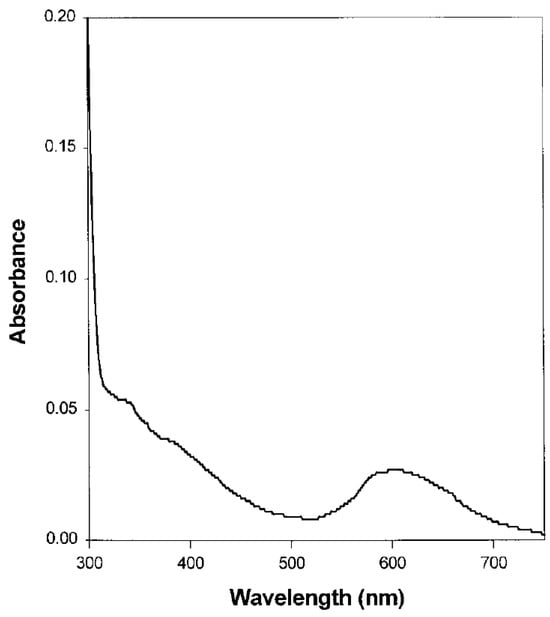
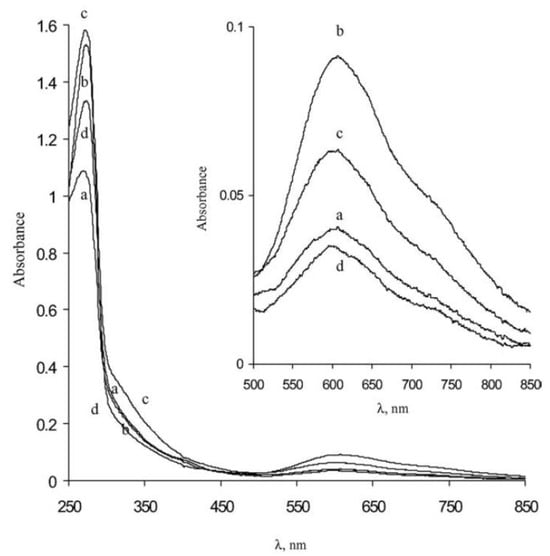
1.1. UV–Vis Absorption Spectroscopy
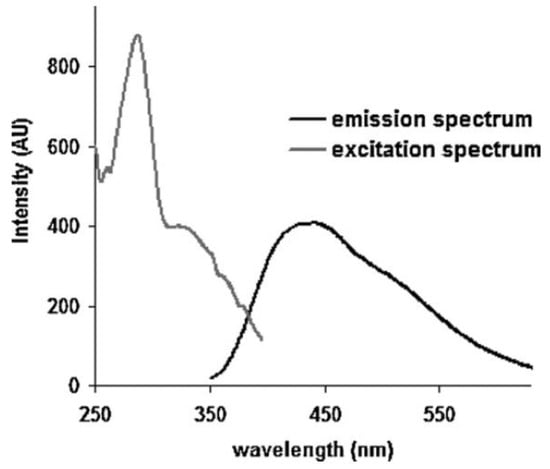
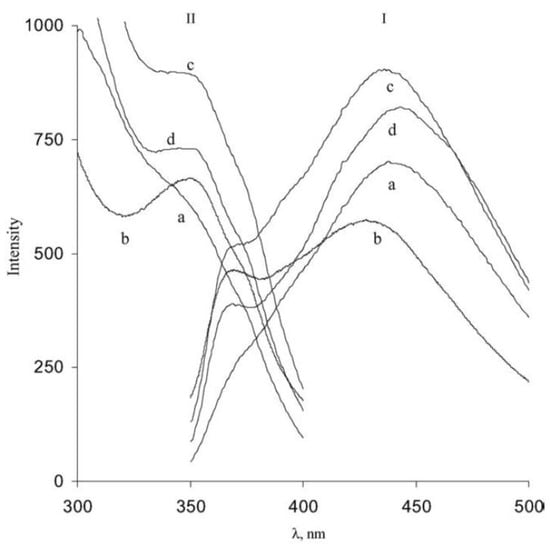
1.2. Fluorescence Spectroscopy
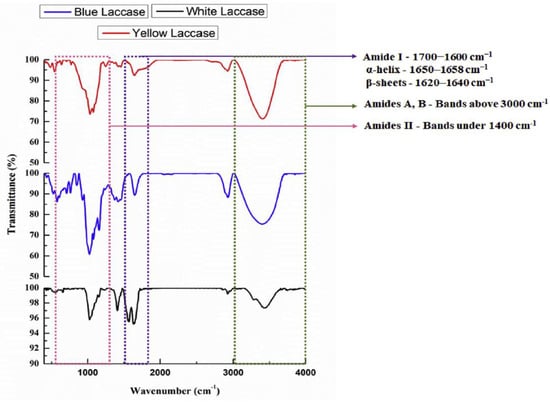
1.3. Fourier Transform InfraRed (FT-IR) Spectroscopy
2. Laccase-Based Sensor Development
- (a)
-
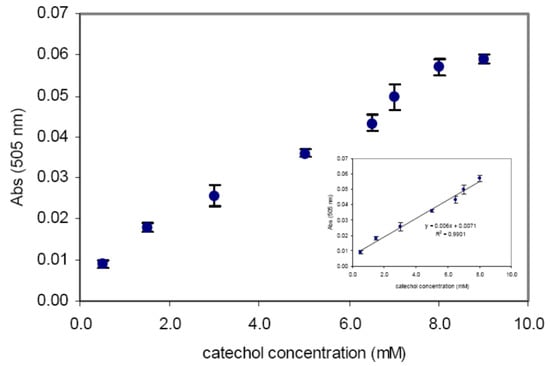
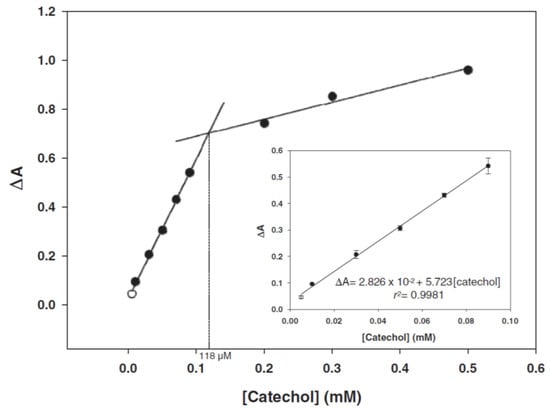
Sensor based on optical absorption spectroscopy.
- (b)
-
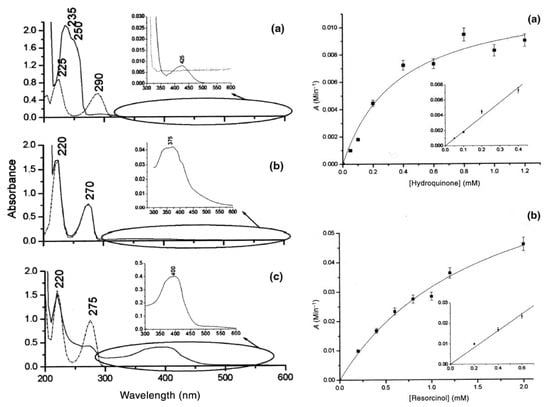
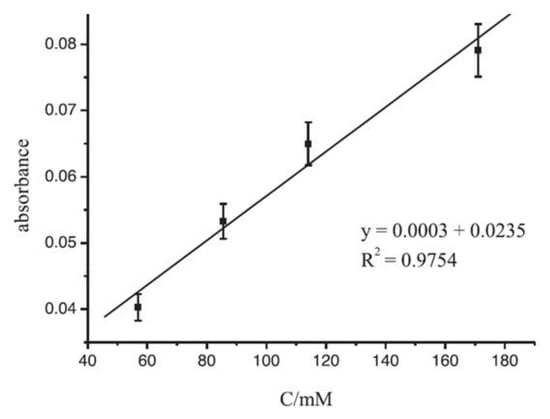
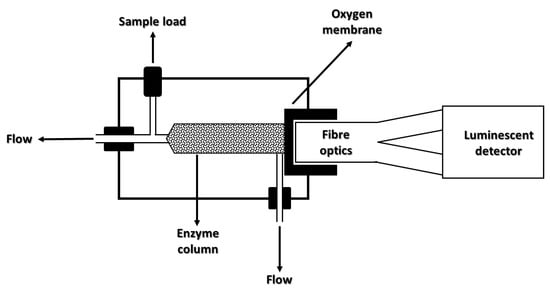
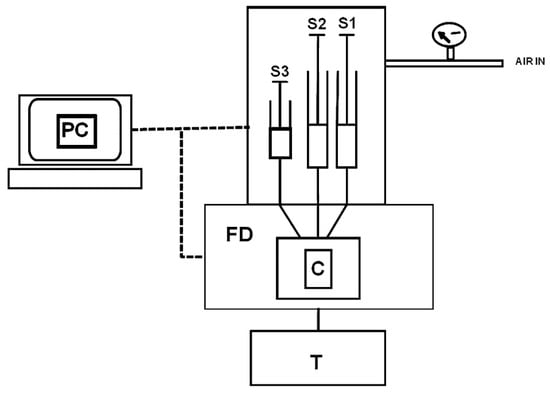
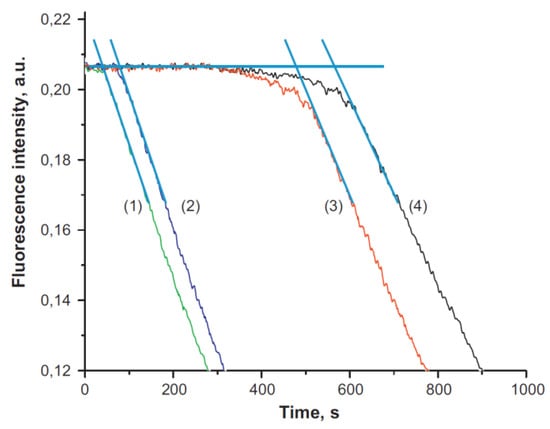
Sensors based on fluorescence spectroscopy
References
- Messerschmidt, A. Special structure of ascorbate oxidase, laccase and related proteins implication for catalytic mechanism. In Multicopper Oxidase; Messerschmidt, A., Ed.; World Scientific Publishing: Singapore, 1997; pp. 23–80.
- Wang, Y.; Wu, N.; Chen, Z.-H. Insight of the optical property of laccase during polymerics formation for application in real-time biosensing. J. Mater. Sci. 2021, 56, 14368–14380.
- Riva, S. Laccases: Blue Enzymes for Green Chemistry. Trends Biotechnol. 2006, 24, 219–226.
- Claus, H. Laccases: Structure, Reactions, Distribution. Micron 2004, 35, 93–96.
- Dennison, C. Investigating the Structure and Function of Cupredoxins. Coord. Chem. Rev. 2005, 249, 3025–3054.
- Lallawmsanga; Leo, V.V.; Passari, A.K.; Muniraj, I.K.; Uthandi, S.; Hashem, A.; Abd Allah, E.F.; Alqarawi, A.A.; Singh, B.P. Elevated levels of laccase synthesis by Pleurotus pulmonarius BPSM10 and its potential as a dye decolorizing agent. Saudi J. Biol. Sci. 2019, 26, 464–468.
- Palmieri, G.; Giardina, P.; Bianco, C.; Scaloni, A.; Capasso, A.; Sannia, G. A Novel White Laccase from Pleurotus Ostreatus. J. Biol. Chem. 1997, 272, 31301–31307.
- Jiao, X.; Li, G.; Wang, Y.; Nie, F.; Cheng, X.; Abdullah, M.; Lin, Y.; Cai, Y. Systematic Analysis of the Pleurotus ostreatus Laccase Gene (PoLac) Family and Functional Characterization of PoLac2 Involved in the Degradation of Cotton-Straw Lignin. Molecules 2018, 23, 880.
- Leontievsky, A.A.; Vares, T.; Lankinen, P.; Shergill, J.K.; Pozdnyakova, N.N.; Myasoedova, N.M.; Kalkkinen, N.; Golovleva, L.A.; Cammack, R.; Thurston, C.F.; et al. Blue and Yellow Laccases of Ligninolytic Fungi. FEMS Microbiol. Lett. 2006, 156, 9–14.
- Navas, L.E.; Martínez, F.D.; Taverna, M.E.; Fetherolf, M.M.; Eltis, L.D.; Nicolau, V.; Estenoz, D.; Campos, E.; Benintende, G.B.; Berretta, M.F. A Thermostable Laccase from Thermus sp. 2.9 and Its Potential for Delignification of Eucalyptus Biomass. AMB Express 2019, 9, 24.
- Agrawal, K.; Verma, P. Multicopper Oxidase Laccases with Distinguished Spectral Properties: A New Outlook. Heliyon 2020, 6, e03972.
- Mot, A.C.; Coman, C.; Hadade, N.; Damian, G.; Silaghi-Dumitrescu, R.; Heering, H. “Yellow” Laccase from Sclerotinia sclerotiorum Is a Blue Laccase That Enhances Its Substrate Affinity by Forming a Reversible Tyrosyl-Product Adduct. PLoS ONE 2020, 15, e0225530.
- Leontievsky, A.; Myasoedova, N.; Pozdnyakova, N.; Golovleva, L. ‘Yellow’ Laccase of Panus Tigrinus Oxidizes Non-phenolic Substrates without Electron-transfer Mediators. FEBS Lett. 1997, 413, 446–448.
- Haibo, Z.; Yinglong, Z.; Feng, H.; Peiji, G.; Jiachuan, C. Purification and Characterization of a Thermostable Laccase with Unique Oxidative Characteristics from Trametes Hirsuta. Biotechnol. Lett. 2009, 31, 837–843.
- Zhang, L.B.; Deng, Z.Q.; Qiu, T.T.; Yang, W.W.J.; Zhu, F.; Ye, X.Y. Characterisation of a laccase isolated from Trametes hirsuta and its application in the oligomerisation of phenolic compounds. Fungal Biol. 2023, 127, 872–880.
- Zhao, D.; Cui, D.Z.; Mu, J.S.; Zhang, X.; Wang, Y.; Zhao, M. Induction of a White Laccase from the Deuteromycete Myrothecium Verrucaria NF-05 and Its Potential in Decolorization of Dyes. Biocatal. Biotransformation 2014, 32, 214–221.
- Sondhi, S.; Sharma, P.; George, N.; Chauhan, P.S.; Puri, N.; Gupta, N. An Extracellular Thermo-Alkali-Stable Laccase from Bacillus Tequilensis SN4, with a Potential to Biobleach Softwood Pulp. 3 Biotech 2015, 5, 175–185.
- Wang, S.-N.; Chen, Q.-J.; Zhu, M.-J.; Xue, F.-Y.; Li, W.-C.; Zhao, T.-J.; Li, G.-D.; Zhang, G.-Q. An Extracellular Yellow Laccase from White Rot Fungus Trametes sp. F1635 and Its Mediator Systems for Dye Decolorization. Biochimie 2018, 148, 46–54.
- Pozdnyakova, N.N.; Turkovskaya, O.V.; Yudina, E.N.; Rodakiewicz-Nowak, Y. Yellow Laccase from the Fungus Pleurotus Ostreatus D1: Purification and Characterization. Appl. Biochem. Microbiol. 2006, 42, 56–61.
- Shin, K.-S.; Lee, Y.-J. Purification and Characterization of a New Member of the Laccase Family from the White-Rot Basidiomycete Coriolus Hirsutus. Arch. Biochem. Biophys. 2000, 384, 109–115.
- Revina, A.A.; Khaikin, G.I.; Protasova, E.L.; Mezentseva, N.B.; Stepanova, E.V.; Skorobogat’ko, O.V. Oxygen Effects in Aqueous Solutions of Laccases: Absorption Spectra and Reactivity. Russ. Chem. Bull. 2000, 49, 1464–1467.
- Shleev, S.V.; Morozova, O.V.; Nikitina, O.V.; Gorshina, E.S.; Rusinova, T.V.; Serezhenkov, V.A.; Burbaev, D.S.; Gazaryan, I.G.; Yaropolov, A.I. Comparison of Physico-Chemical Characteristics of Four Laccases from Different Basidiomycetes. Biochimie 2004, 86, 693–703.
- Delfino, I.; Viola, D.; Cerullo, G.; Lepore, M. Ultrafast Excited-State Charge-Transfer Dynamics in Laccase Type I Copper Site. Biophys. Chem. 2015, 200–201, 41–47.
- Goldberg, M.; Pecht, I. Fluorescence Enhancement of Laccase Induced by Reduction of Cu(II) Sites. Proc. Nat. Acad. Sci. USA 1974, 71, 4684–4687.
- Wynn, R.M.; Sarkar, H.K.; Holwerda, R.A.; Knaff, D.B. Fluorescence Associated with the Type 3 Copper Center of Laccase. FEBS Lett. 1983, 156, 23–28.
- Moţ, A.C.; Pârvu, M.; Damian, G.; Irimie, F.D.; Darula, Z.; Medzihradszky, K.F.; Brem, B.; Silaghi-Dumitrescu, R. A “Yellow” Laccase with “Blue” Spectroscopic Features, from Sclerotinia Sclerotiorum. Process Biochem. 2012, 47, 968–975.
- Saoudi, O.; Ghaouar, N.; Othman, T. Fluorescence study of laccase from Trametes versicolor under the effects of pH, chemical denaturants and ionic liquids. J. Mol. Liq. 2017, 225, 56–63.
- Susi, H.; Byler, D.M. Resolution-Enhanced Fourier Transform Infrared Spectroscopy of Enzymes. Methods Enzymol. 1986, 130, 290–311.
- Yang, H.; Yang, S.; Kong, J.; Dong, A.; Yu, S. Obtaining Information about Protein Secondary Structures in Aqueous Solution Using Fourier Transform IR Spectroscopy. Nat. Protoc. 2015, 10, 382–396.
- Baker, M.J.; Trevisan, J.; Bassan, P.; Bhargava, R.; Butler, H.J.; Dorling, K.M.; Fielden, P.R.; Fogarty, S.W.; Fullwood, N.J.; Heys, K.A.; et al. Using Fourier Transform IR Spectroscopy to Analyze Biological Materials. Nat. Protoc. 2014, 9, 1771–1791.
- Errico, S.; Moggio, M.; Diano, N.; Portaccio, M.; Lepore, M. Different experimental approaches for Fourier-transform infrared spectroscopy applications in biology and biotechnology: A selected choice of representative results. Biotechnol. Appl. Biochem. 2023, 70, 937–961.
- Ragusa, S.; Cambria, M.T.; Pierfederici, F.; Scirè, A.; Bertoli, E.; Tanfani, F.; Cambria, A. Structure–Activity Relationship on Fungal Laccase from Rigidoporus Lignosus: A Fourier-Transform Infrared Spectroscopic Study. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2002, 1601, 155–162.
- Portaccio, M.; Della Ventura, B.; Mita, D.G.; Manolova, N.; Stoilova, O.; Rashkov, I.; Lepore, M. FT-IR Microscopy Characterization of Sol–Gel Layers Prior and after Glucose Oxidase Immobilization for Biosensing Applications. J. Solgel Sci. Technol. 2011, 57, 204–211.
- Samui, A.; Sahu, S.K. One-Pot Synthesis of Microporous Nanoscale Metal Organic Frameworks Conjugated with Laccase as a Promising Biocatalyst. New J. Chem. 2018, 42, 4192–4200.
- Almeida, L.C.; Correia, R.D.; Marta, A.; Squillaci, G.; Morana, A.; La Cara, F.; Correia, J.P.; Viana, A.S. Electrosynthesis of poly-dopamine films-tailored matrices for laccase-based biosensors. Appl. Surf. Sci. 2019, 480, 979–989.
- Perna, V.; Baum, A.; Ernst, H.A.; Agger, J.W.; Meyer, A.S. Laccase activity measurement by FTIR spectral fingerprinting. Enzym. Microb. Technol. 2019, 122, 64–73.
- Cantarella, G.; d’Acunzo, F.; Galli, C. Determination of Laccase Activity in Mixed Solvents: Comparison between Two Chromogens in a Spectrophotometric Assay. Biotechnol. Bioeng. 2003, 82, 395–398.
- Zuyun, H.; Houping, H.; Ruxiu, C.; Yun’e, Z. Organic Solvent Enhanced Spectrofluorimetric Method for Determination of Laccase Activity. Anal. Chim. Acta 1998, 374, 99–103.
- Sun, X.; Bai, R.; Zhang, Y.; Wang, Q.; Fan, X.; Yuan, J.; Cui, L.; Wang, P. Laccase-Catalyzed Oxidative Polymerization of Phenolic Compounds. Appl. Biochem. Biotechnol. 2013, 171, 1673–1680.
- Huang, J.; Fang, H.; Liu, C.; Gu, E.; Jiang, D. A Novel Fiber Optic Biosensor for the Determination of Adrenaline Based on Immobilized Laccase Catalysis. Anal. Lett. 2008, 41, 1430–1442.
- Navarro, J.; Galban, J.; de Marcos, S. A label-free platform for dopamine biosensing. Bioanalysis 2018, 10, 11–21.
- Baluta, S.; Zając, D.; Szyszka, A.; Malecha, K.; Cabaj, J. Enzymatic Platforms for Sensitive Neurotransmitter Detection. Sensors 2020, 20, 423.
- Sangubotla, R.; Kim, J. Fiber-optic biosensor based on the laccase immobilization on silica-functionalized fluorescent carbon dots for the detection of dopamine and multi-color imaging applications in neuroblastoma cells. Mater. Sci. Eng. C 2021, 122, 111916.
- Pimpilova, M.; Kamarska, K.; Dimcheva, N. Biosensing Dopamine and L-Epinephrine with Laccase (Trametes pubescens) Immobilized on a Gold Modified Electrode. Biosensors 2022, 12, 719.
- Castañeda-Arriaga, R.; Pérez-González, A.; Reina, M.; Alvarez-Idaboy, J.R.; Galano, A. Comprehensive Investigation of the Antioxidant and Pro-Oxidant Effects of Phenolic Compounds: A Double-Edged Sword in the Context of Oxidative Stress? J. Phys. Chem. B 2018, 122, 6198–6214.
- Rodríguez-Delgado, M.M.; Alemán-Nava, G.S.; Rodríguez-Delgado, J.M.; Dieck-Assad, G.; Martínez-Chapa, S.O.; Barceló, D.; Parra, R. Laccase-Based Biosensors for Detection of Phenolic Compounds. TrAC Trends Anal. Chem. 2015, 74, 21–45.
- Simkus, R.A.; Laurinavicius, V.; Boguslavsky, L.; Skotheim, T.; Tanenbaum, S.W.; Nakas, J.P.; Slomczynski, D.J. Laccase Containing Sol-Gel Based Optical Biosensors. Anal. Lett. 1996, 29, 1907–1919.
- Gupta, R.; Chaudhury, N.K. Entrapment of Biomolecules in Sol–Gel Matrix for Applications in Biosensors: Problems and Future Prospects. Biosens. Bioelectron. 2007, 22, 2387–2399.
- Klein, L.; Aparicio, M.; Jitianu, A. (Eds.) Handbook of Sol-Gel Science and Technology; Springer International Publishing: Cham, Switzerland, 2018.
- Figueira, R.B.; de Sorrentino, J.M.; Ferreira, B.; Coelho, L.; Silva, C.J.R. Optical Fiber Sensors Based on Sol–Gel Materials: Design, Fabrication and Application in Concrete Structures. Mater. Adv. 2021, 2, 7237–7276.
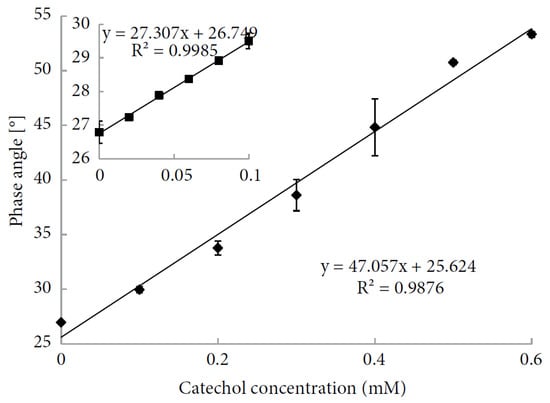
- Abdullah, J.; Ahmad, M.; Heng, L.; Karuppiah, N.; Sidek, H. An Optical Biosensor Based on Immobilization of Laccase and MBTH in Stacked Films for the Detection of Catechol. Sensors 2007, 7, 2238–2250.
- Karweik, D.H. Quantitative Analysis, 4th Edition (Day, R.A.; Underwood, A.L.). J. Chem. Educ. 1982, 59, A105.
- Navas Díaz, A.; Ramos Peinado, M.C.; Torijas Minguez, M.C. Sol–Gel Horseradish Peroxidase Biosensor for Hydrogen Peroxide Detection by Chemiluminescence. Anal. Chim. Acta 1998, 363, 221–227.
- Xu, F. Oxidation of Phenols, Anilines, and Benzenethiols by Fungal Laccases: Correlation between Activity and Redox Potentials as Well as Halide Inhibition. Biochemistry 1996, 35, 7608–7614.
- Delfino, I.; Portaccio, M.; Della Ventura, B.; Manzo, G.; Mita, D.G.; Lepore, M. Optical Properties of Sol-Gel Immobilized Laccase: A First Step for Its Use in Optical Biosensing; Berghmans, F., Mignani, A.G., De Moor, P., Eds.; SPIE: Bellingham, WA, USA, 2012; p. 84391X.
- Lepore, M.; Portaccio, M. Optical Detection of Different Phenolic Compounds by Means of a Novel Biosensor Based on Sol–Gel Immobilized Laccase. Biotechnol. Appl. Biochem. 2017, 64, 782–792.
- Sanz, J.; de Marcos, S.; Galbán, J. Autoindicating Optical Properties of Laccase as the Base of an Optical Biosensor Film for Phenol Determination. Anal. Bioanal. Chem. 2012, 404, 351–359.
- Jędrychowska, A.; Malecha, K.; Cabaj, J.; Sołoducho, J. Laccase Biosensor Based on Low Temperature Co-Fired Ceramics for the Permanent Monitoring of Water Solutions. Electrochim. Acta 2015, 165, 372–382.
- Cano-Raya, C.; Dencheva, N.V.; Braz, J.F.; Malfois, M.; Denchev, Z.Z. Optical Biosensor for Catechol Determination Based on Laccase-immobilized Anionic Polyamide 6 Microparticles. J. Appl. Polym. Sci. 2020, 137, 49131.
- Sorrentino, I.; Giardina, P.; Piscitelli, A. Development of a Biosensing Platform Based on a Laccase-Hydrophobin Chimera. Appl. Microbiol. Biotechnol. 2019, 103, 3061–3071.
- Papkovsky, D.B.; Ghindilis, A.L.; Kurochkin, I.N. Flow-Cell Fibre-Optic Enzyme Sensor for Phenols. Anal. Lett. 1993, 26, 1505–1518.
- Andreu-Navarro, A.; Fernández-Romero, J.M.; Gómez-Hens, A. Determination of Polyphenolic Content in Beverages Using Laccase, Gold Nanoparticles and Long Wavelength Fluorimetry. Anal. Chim. Acta 2012, 713, 1–6.
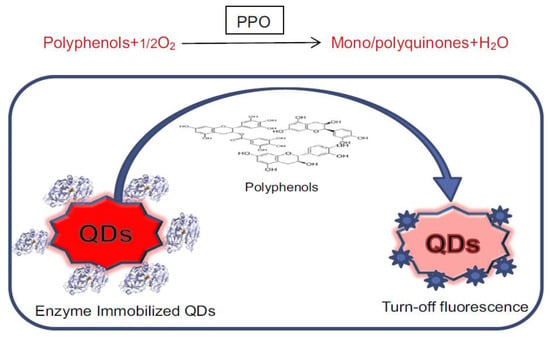
- Akshath, U.S.; Shubha, L.R.; Bhatt, P.; Thakur, M.S. Quantum Dots as Optical Labels for Ultrasensitive Detection of Polyphenols. Biosens. Bioelectron. 2014, 57, 317–323.
- Mediavilla, M.; Revenga-Parra, M.; Gutierrez-Sanchez, C.; Hernandez-Apaolaza, L.; Pariente, F.; Lorenzo, E. Fluorescent enzymatic assay for direct total polyphenol determination in food-related samples. Talanta 2022, 247, 123576.
- Bilir, K.; Weil, M.-T.; Lochead, J.; Kök, F.N.; Werner, T. Construction of an Oxygen Detection-Based Optic Laccase Biosensor for Polyphenolic Compound Detection. Turk. J. Biol. 2016, 40, 1303–1310.
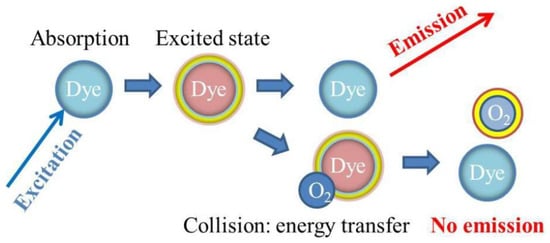
- Lakowicz, J.R.; Weber, G. Quenching of Fluorescence by Oxygen. Probe for Structural Fluctuations in Macromolecules. Biochemistry 1973, 12, 4161–4170.
