Aneurysmal subarachnoid hemorrhage (aSAH) provokes a cascade reaction that is responsible for early and delayed brain injuries mediated by intracranial hypertension, hydrocephalus, cerebral vasospasm (CV), and delayed cerebral ischemia (DCI), which result in increased morbidity and mortality. During open microsurgical repair, cisternal access is achieved essentially to gain proximal vascular control and aneurysm exposition. Cisternostomy also allows brain relaxation, removal of cisternal clots, and restoration of the CSF dynamics through the communication between the anterior and posterior circulation cisterns and the ventricular system, with the opening of the Membrane of Liliequist and lamina terminalis, respectively. Continuous postoperative CSF drainage through a cisternal drain (CD) is a valuable option for treating acute hydrocephalus and intracranial hypertension. Moreover, it efficiently removes the blood and toxic degradation products, with a potential benefit on CV, DCI, and shunt-dependent hydrocephalus. Finally, the CD is an effective pathway to administer vasoactive, fibrinolytic, and anti-oxidant agents and shows promising results in decreasing CV and DCI rates while minimizing systemic effects.
1. Pathophysiology of Delayed Cerebral Ischemia
DCI occurs in up to 30% of aSAH patients and results in poor functional outcomes in half of them despite adequate treatment
[1][2][3]. CV is defined as a narrowing of the angiographically visible cerebral arteries that may occur in 30–70% of patients following aSAH
[4]. It has been considered for decades the principal cause of DCI. Recent evidence demonstrated that CV in the major cerebral vessels is a contributing factor and not the determinant one
[2]. Blood degradation products in the subarachnoid space trigger a molecular cascade that may lead to CV, microcirculatory dysfunction, excitotoxicity, oxidative stress, and inflammatory cascade
[2][5][6]. The glymphatic system is responsible for clearing the CSF toxic products
[7]. However, lymphatic vessel disruption and glymphatic system dysfunction following aSAH may exacerbate neuroinflammatory response and contribute to DCI
[8][9][10]. Continuous CSF drainage seems a valuable option to increase clots evacuation, reduce DCI, and improve functional outcomes, but contrasting results are reported with different output pathways
[11][12][13][14][15][16]. The studies reporting the impact of external CSF drains in CV and DCI are summarized in
Table 1.
Table 1. The studies investigating the role of the lumbar drain, external ventricular drain, and cisternal drain for cerebral vasospasm and delayed cerebral ischemia management are summarized here. CD: cisternal drain; DCI: delayed cerebral ischemia; EVD: external ventricular drain; LD: lumbar drain; RCT: randomized controlled trial; STD: standard.
| Author and Year |
Design |
Intervention |
Outcome |
| Wolf et al., 2023 [12] |
RCT |
LD (144) vs. STD care * (143) |
LD reduced DCI (p = 0.04) and unfavorable outcomes at 6 months (p = 0.04). |
| Al-Tamimi et al., 2012 [13] |
RCT |
LD (105) vs. STD care * (105) |
LD showed DCI reduction (p = 0.021) but no outcome improvement at 6 months. |
| Maeda et al., 2013 [11] |
Retrospective |
LD (34) vs. EVD (17) |
LD showed more rapid clot washout and a trend toward DCI reduction. |
| Klimo et al., 2004 [14] |
Retrospective |
LD (81) vs. STD care * (86) |
LD reduced CV (p < 0.001) and DCI (p = 0.05). |
| Ogura et al., 1988 [15] |
Retrospective |
CD (101) vs. no drain (31) |
The CD was associated with no significant reduction of CV, although positive trends were seen in patients with high Fisher grades. |
| Sakaki et al., 1987 [16] |
Retrospective |
CD (75) vs. STD care * (74) |
Early CD significantly reduced DCI (p < 0.05) and improved outcomes (p < 0.01). |
Moreover, increased intracranial pressure combined with micro and macrovascular changes may further reduce cerebral perfusion pressure and have been related to the risk of developing hypoperfusion and ischemia
[17][18][19]. DCI is a complex process resulting from multiple pathological pathways secondary to aSAH and has been correlated with the extent of subarachnoid hemorrhage, as expressed by the modified Fisher grade
[20][21][22]. The role of vasospasm has probably been overemphasized, and a therapeutic approach to all these factors should be preferred.
2. Cisternal Drainage, Cerebral Vasospasm and Delayed Cerebral Ischemia
Extensive and early evacuation of cisternal clots shortly after aSAH and before the process of blood degradation takes place could be a valid option to stop the pathological cascade and prevent DCI. Evacuation of a subarachnoid hemorrhage within 48h showed a reduction in CV and DCI in preclinical studies
[23][24]. Moreover, the amount of postoperative clots and clot-clearance rate on serial brain CT seems to predict the risk of CV, consequent infarction, and unfavorable outcomes
[25][26].
Yasargil pioneered the interest in understanding the anatomy of the basal cisterns. He was the first to report routine basal cistern opening, including LT (
Figure 1) and MoL (
Figure 2) fenestration to obtain brain relaxation, proximal exposure, extensive evacuation of the subarachnoid hemorrhage, and CSF flow restoration
[27]. In the following years, the advent of microneurosurgery allowed widespread diffusion of cisternal procedures
[27][28][29].
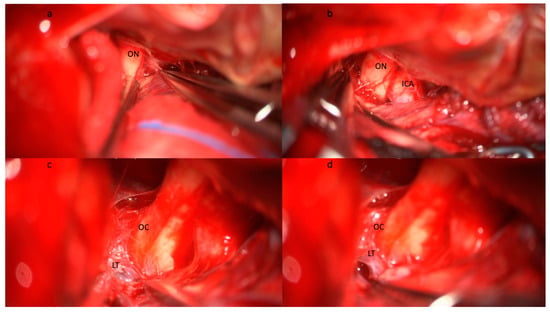
Figure 1. Step-by-step illustration of a cisternostomy performed during microsurgical clipping of an aneurysm of the right middle cerebral artery bifurcation. (a) Subfrontal approach and identification of the optic nerve (ON) with (b) progressive opening of the optico-carotid cistern and exposure of the internal carotid artery (ICA). (c) The dissection continues medially and posteriorly following the ON until the exposure of the optic chiasma (OC) and the lamina terminalis (LT). (d) The opening of the lamina terminalis gives access to the third ventricle and allows CSF drainage from the ventricular system. Brain relaxation is generally obtained at this stage after blood clot evacuation from the basal cisterns, the opening of the optico-carotid cisterns, and the LT. Abbreviations: CSF: cerebrospinal fluid; ICA: internal carotid artery; LT: lamina terminalis; OC: optic chiasma; ON: optic nerve.
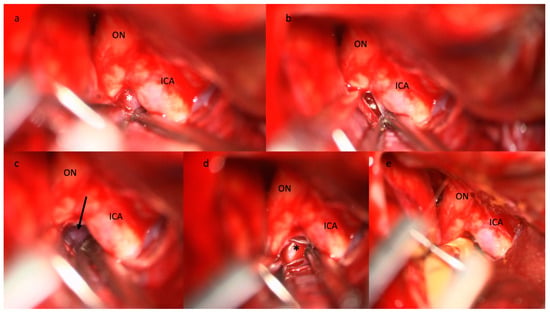
Figure 2. Step-by-step illustration of a cisternostomy performed during microsurgical clipping of an aneurysm of the right middle cerebral artery bifurcation. (a) The diencephalic leaf of the Membrane of Liliequist (white asterisk) is seen through the optico-carotid triangle and (b) progressively opened, (c) enabling the exposure of the mesencephalic leaf (black arrow) that is finally opened. (d) The basilar artery (black asterisk) is visible in the pre-pontine cistern. (e) The tip of the cisternal drain is positioned between the ICA and the ON in the pre-pontine cistern. Abbreviations: ICA: internal carotid artery; ON: optic nerve.
Extensive subarachnoid clot removal was first described by Suzuki and Yoshimoto in 1976
[30]. Early surgery and clot evacuation within 24 h from the bleeding greatly lessen the risk of CV and improve functional outcomes, especially in patients with high Fisher grades aSAH
[30][31]. A few reports published in Japan in the seventies and eighties confirmed the positive impact of early operation and clot evacuation on CV and delayed ischemia
[25][32][33][34][35]. Moreover, the Japanese register showed an association between surgical clipping and a lower risk of cerebral infarction
[36].
A few studies have been published dealing with the impact of cisternostomy on CV, DCI, and functional outcome, and the results are summarized in
Table 2 [15][16][37][38][39][40][41][42].
Table 2. The studies investigating the role of the cisternal drain in CV and DCI management are summarized here. CD: cisternal drain; DCI: delayed cerebral ischemia; STD: standard.
| Author and Year |
Design |
Intervention |
Outcomes |
| Inagawa T et al., 1991 [40] |
Retrospective |
CD (140) |
The total amount of CSF cisternal drainage was correlated with decreased CV and DCI. |
| Ogura et al., 1988 [15] |
Retrospective |
CD (101) vs. no drain (31) |
The CD was associated with no significant reduction of CV, although positive trends were seen in patients with high Fisher grades. |
| Sakaki et al., 1987 [16] |
Retrospective |
CD (75) vs. STD care * (74) |
Early CD significantly reduced DCI (p < 0.05) and improved outcomes (p < 0.01). |
| Kawakami et al., 1987 [39] |
Retrospective |
CD (22) |
Symptomatic CV occurred in 22% of cases and good functional outcome in 95% of cases. |
| Ito el al., 1986 [38] |
Retrospective |
CD (38) |
Effective cisternal drainage was correlated with reduced CV and improved outcomes. |
To researchers' knowledge, Ito et al. were the first to combine the insertion of cisternal drainage with cisternostomy to enhance their positive effects
[38]. Cisternal drainage improves toxic substance clearance compared to EVD and may remove more than 1g of hemoglobin per day
[16]. Its position in the basal cisterns creates a gravitational gradient to CSF circulation and clearance similar to lumbar drains but achieving it closer to the bleeding source
[11][12][14][43]. Moreover, despite the emerging efficacy of LD in reducing DCI and unfavorable outcomes, obstructive hydrocephalus and compressed basal cisterns remain the main limitations in the use of LD, while both situations are efficiently treated with CD
[12][43]. Indeed, LT and MoL fenestration combined with CD increase daily CSF drainage, resolve obstructive hydrocephalus, and were significantly associated with a reduction of symptomatic CV
[38]. Kawakami et al. performed a continuous cisternal drainage for a minimum of fourteen days in 22 patients. Moderate CV occurred in 22% of cases, and good neurological outcome was observed in 95% of cases
[39]. Inagawa et al. evaluated the efficacy of continuous cisternal drainage on CV for 140 consecutive patients. They classified patients according to the total amount of drainage regardless of the duration. Drainage of cisternal CSF of more than 500 mL was shown to be associated with decreased DCI, angiographic and symptomatic CV, and improved functional outcome
[40]. Sakaki et al. reported that early clot evacuation combined with CD was associated with better neurological outcomes (
p = 0.01). Moreover, they observed a reduction of 50% for symptomatic CV and a less severe angiographic spasm
[16]. Ogura et al. assessed the efficacy of continuous postoperative cisternal drainage in a retrospective study and showed that it reduced CV and mortality incidence and improved outcomes in patients with severe clinical and radiological presentation
[15]. Moreover, continuous postoperative CSF drainage decreases ICP, is directly related to cerebral perfusion, and contributes to DCI
[15][42][44]. Cisternostomy may, thus, represent a valuable adjunct in ruptured aneurysms surgery
[31][45][46][47][48], as it may allow clots evacuation and postoperative cisternal drainage
[38], thus blocking the cascade responsible for DCI
[15][16][38][39][40]. Therefore, cisternal drainage, as part of the surgical treatment, has several advantages that allow ICP control and the reduction of several processes that contribute to the development of DCI.
3. CD and Drugs Administration
Intrathecal drug administration presents many anatomic and pharmacodynamic advantages compared to systemic administration. A higher drug concentration can be reached with minimal systemic effects
[49]. Cisternal injections, compared to ventricular and lumbar administration, allow drug delivery directly in the basal cisterns. It may enhance the pharmacologic effects on the large proximal arteries and improve the intraparenchymal diffusion through the perivascular Virchow spaces. Animal models confirmed a more effective and durable vasoactive response compared to intra-arterial administration
[50][51]. Prophylactic injections of intrathecal nicardipine and milrinone have shown a reduction in angiographic CV and DCI and an increase in mean cerebral blood flow but without a significant improvement in functional outcomes
[52][53][54][55][56][57]. Intraventricular nicardipine showed a significant reduction in DCI and improvement of functional outcomes in patients treated for significant CV
[58]. Shibuya et al. administered 2 mg of cisternal nicardipine three times a day for 10 days in 50 patients treated for an aSAH. Prophylactic nicardipine reduced the incidence of radiographic and symptomatic CV by 26% and 20%, respectively, and increased early good clinical outcomes by 15%. However, no statistical significance was reached
[55]. Similar results were obtained by Suzuki et al.
[52].
Magnesium sulfate showed several potential beneficial effects in aSAH patients, such as vasodilatation and attenuation of neuronal death. However, intravenous administration failed to prevent DCI and improve functional outcomes
[59], while continuous intracisternal administration significantly improved DCI and functional outcomes
[60].
In researchers' institution, they introduced intrathecal nicardipine as a treatment for moderate (>50% arterial narrowing at angioCT) to severe (>75% arterial narrowing at angioCT) CV in patients with aSAH in 2019. In reseaourchers' experience, intrathecal nicardipine showed a significant reduction in DCI rate and improved functional outcome (unpublished data). Moreover, cisternal administration showed a positive trend toward a further reduction of DCI when compared to ventricular administration.