Telomere dysfunction is implicated in vascular aging and shorter leucocyte telomeres are associated with an increased risk of atherosclerosis, myocardial infarction, and heart failure. Another pathophysiological mechanism that explains the causal relationship between telomere shortening and atherosclerosis development focuses on the clonal hematopoiesis of indeterminate potential (CHIP), which represents a new and independent risk factor in atherosclerotic cardiovascular diseases. Since telomere attrition has a central role in driving vascular senescence, understanding telomere biology is essential to modulate the deleterious consequences of vascular aging and its cardiovascular disease-related manifestations. Emerging evidence indicates that a class of long noncoding RNAs transcribed at telomeres, known as TERRA for “TElomeric Repeat-containing RNA”, actively participates in the mechanisms regulating telomere maintenance and chromosome end protection. However, the multiple biological functions of TERRA remain to be largely elucidated.
- TERRA
- telomere
- clonal hematopoiesis of indeterminate potential vascular aging
1. Long Telomeric Repeat-Containing RNA (TERRA) Transcription
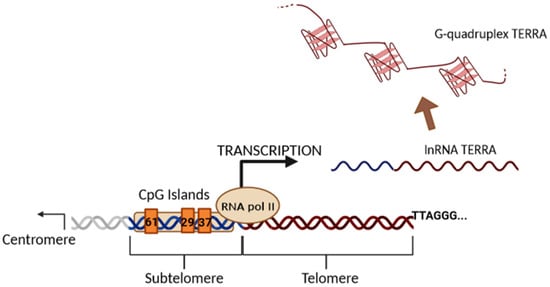
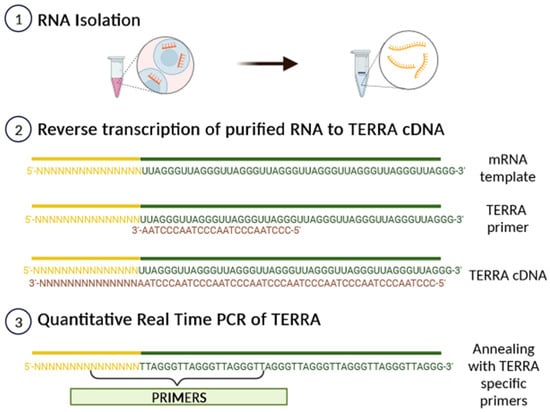
2. Biological Functions of TERRA
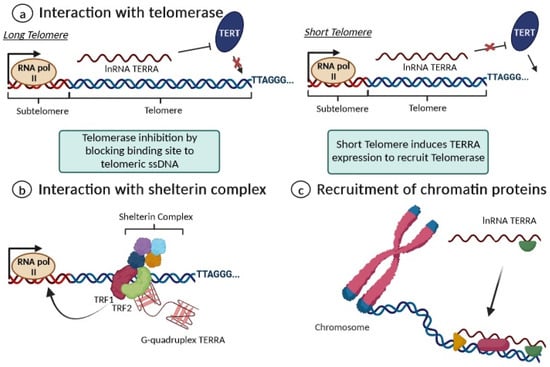
3. Dysregulation of TERRA in Human Studies
| Reference | Human Model | Study Design | Biological Sample | Method | Main Findings |
|---|---|---|---|---|---|
| Sampl S et al., 2012 [42][17] | Astrocytoma | Cancer tissue | Tissue | Real-Time PCR | Down-regulation |
| Vitelli V et al., 2018 [43][18] | Head and neck squamous cell carcinoma | Healthy tissue/Cancer tissue | Tissue | Real-Time PCR | Down-regulation |
| Adishesh M et al. 2020 [44][19] | Endometrial cancer | Healthy tissue/Cancer tissue | Endometrial biopsy | Real-Time PCR | Down-regulation |
| Bae SU et al., 2019 [45][20] | Colorectal cancer | Cancer tissue | Tissue | Real-Time PCR | Up-regulation |
| Storti CB et al., 2020 [46][21] | Non-small cell lung cancer | Healthy tissue/Cancer tissue | Tissue | Real-Time PCR | Up-regulation |
| Cao H et al., 2020 [47][22] | Hepatocellular carcinoma | Healthy tissue/Cancer tissue | Tissue | FISH | Down-regulation |
| Manganelli M et al., 2022 [48][23] | Hepatocellular carcinoma | Healthy tissue/Cancer tissue Case/Control | Tissue Plasma | Real-Time PCR | Down-regulation in tissue Up-regulation in plasma |
| Wang Z et al., 2015 [49][24] | Cancer | Case/Control | Plasma | RNA Seq | Up-regulation |
| Gao Y et al., 2017 [50][25] | Idiopathic pulmonary fibrosis | Case/Control | Blood | Real-Time PCR | Up-regulation |
| Diman A et al., 2016 [51][26] | Healthy runners | Pre/post exercise tissue | Muscle biopsy | FISH | Up-regulation |
| Chang KV et al., 2020 [52][27] | Sarcopenia | Case/Control | Leukocytes | Real-Time PCR | Down-regulation |
4. TERRA Expression and Vascular Biology
References
- Azzalin, C.M.; Reichenbach, P.; Khoriauli, L.; Giulotto, E.; Lingner, J. Telomeric repeat containing RNA and RNA surveillance factors at mammalian chromosome ends. Science 2007, 318, 798–801.
- Schoeftner, S.; Blasco, M.A. Developmentally regulated transcription of mammalian telomeres by DNAdependent RNA polymerase II. Nat. Cell. Biol. 2008, 10, 228–236.
- Porro, A.; Feuerhahn, S.; Delafontaine, J.; Riethman, H.; Rougemont, J.; Lingner, J. Functional characterization of the TERRA transcriptome at damaged telomeres. Nat. Commun. 2014, 5, 5379.
- Rivosecchi, J.; Cusanelli, E. TERRA beyond cancer: The biology of telomeric repeat-containing RNAs in somatic and germ cells. Front. Aging 2023, 4, 1224225.
- Fernandes, R.V.; Feretzaki, M.; Lingner, J. The makings of TERRA R-loops at chromosome ends. Cell Cycle 2021, 20, 1745–1759.
- Nergadze, S.G.; Farnung, B.O.; Wischnewski, H.; Khoriauli, L.; Vitelli, V.; Chawla, R.; Giulotto, E.; Azzalin, C.M. CpG-island promoters drive transcription of human telomeres. RNA 2009, 15, 2186–2194.
- Feretzaki, M.; Lingner, J. A practical qPCR approach to detect TERRA, the elusive telomeric repeat-containing RNA. Methods 2017, 114, 39–45.
- Feretzaki, M.; Renck Nunes, P.; Lingner, J. Expression and differential regulation of human TERRA at several chromosome ends. RNA 2019, 25, 1470–1480.
- Rippe, K.; Luke, B. TERRA and the state of the telomere. Nat. Struct. Mol. Biol. 2015, 22, 853–858.
- Azzalin, C.M.; Lingner, J. Telomere functions grounding on TERRA firma. Trends. Cell. Biol. 2015, 25, 29–36.
- Lalonde, M.; Chartrand, P. TERRA, a multifaceted regulator of telomerase activity at telomeres. J. Mol. Biol. 2020, 432, 4232–4243.
- Aguado, J.; d’Adda di Fagagna, F.; Wolvetang, E. Telomere transcription in ageing. Ageing Res. Rev. 2020, 62, 101115.
- Deng, Z.; Norseen, J.; Wiedmer, A.; Riethman, H.; Lieberman, P.M. TERRA RNA binding to TRF2 facilitates heterochromatin formation and ORC recruitment at telomeres. Mol. Cell. 2009, 35, 403–413.
- Schoeftner, S.; Blasco, M.A. Chromatin regulation and non-coding RNAs at mammalian telomeres. Semin. Cell. Dev. Biol. 2010, 21, 186–193.
- Abreu, P.L.; Lee, Y.W.; Azzalin, C.M. In Vitro Characterization of the Physical Interactions between the Long Noncoding RNA TERRA and the Telomeric Proteins TRF1 and TRF2. Int. J. Mol. Sci. 2022, 23, 10463.
- Yehezkel, S.; Segev, Y.; Viegas-Péquignot, E.; Skorecki, K.; Selig, S. Hypomethylation of subtelomeric regions in ICF syndrome is associated with abnormally short telomeres and enhanced transcription from telomeric regions. Hum. Mol. Genet. 2008, 17, 2776–2789.
- Sampl, S.; Pramhas, S.; Stern, C.; Preusser, M.; Marosi, C.; Holzmann, K. Expression of telomeres in astrocytoma WHO grade 2 to 4: TERRA level correlates with telomere length, telomerase activity, and advanced clinical grade. Trans. Oncol. 2012, 5, 56-IN4.
- Vitelli, V.; Falvo, P.; GNergadze, S.; Santagostino, M.; Khoriauli, L.; Pellanda, P.; Bertino, G.; Occhini, A.; Benazzo, M.; Morbini, P.; et al. Telomeric repeat-containing RNAs (TERRA) decrease in squamous cell carcinoma of the head and neck is associated with worsened clinical outcome. Int. J. Mol. Sci. 2018, 19, 274.
- Adishesh, M.; Alnafakh, R.; Baird, D.M.; Jones, R.E.; Simon, S.; Button, L.; Kamal, A.M.; Kirwan, J.; DeCruze, S.B.; Drury, J.; et al. Human endometrial carcinogenesis is associated with significant reduction in long non-coding RNA, TERRA. Int. J. Mol. Sci. 2020, 21, 8686.
- Bae, S.U.; Park, W.; Jeong, W.K.; Baek, S.K.; Lee, H.W.; Lee, J.H. Prognostic impact of telomeric repeat-containing RNA expression on long-term oncologic outcomes in colorectal cancer. Medicine 2019, 98, 14932.
- Storti, C.B.; de Oliveira, R.A.; de Carvalho, M.; Hasimoto, E.N.; Cataneo, D.C.; Cataneo, A.J.M.; De Faveri, J.; Vasconcelos, E.J.R.; Dos Reis, P.P.; Cano, M.I.N. Telomere-associated genes and telomeric lncRNAs are biomarker candidates in lung squamous cell carcinoma (LUSC). Exp. Mol. Pathol. 2020, 112, 104354.
- Cao, H.; Zhai, Y.; Ji, X.; Wang, Y.; Zhao, J.; Xing, J.; An, J.; Ren, T. Noncoding telomeric repeat-containing RNA inhibits the progression of hepatocellular carcinoma by regulating telomerase-mediated telomere length. Cancer Sci. 2020, 111, 2789–2802.
- Manganelli, M.; Grossi, I.; Corsi, J.; D’Agostino, V.G.; Jurikova, K.; Cusanelli, E.; Molfino, S.; Portolani, N.; Salvi, A.; De Petro, G. Expression of cellular and extracellular TERRA, TERC and TERT in hepatocellular carcinoma. Int. J. Mol. Sci. 2022, 23, 6183.
- Wang, Z.; Deng, Z.; Dahmane, N.; Tsai, K.; Wang, P.; Williams, D.R.; Kossenkov, A.V.; Showe, L.C.; Zhang, R.; Huang, Q.; et al. Telomeric repeat-containing RNA (TERRA) constitutes a nucleoprotein component of extracellular inflammatory exosomes. Proc. Natl. Acad. Sci. USA 2015, 112, 6293–6300.
- Gao, Y.; Zhang, J.; Liu, Y.; Zhang, S.; Wang, Y.; Liu, B.; Liu, H.; Li, R.; Lv, C.; Song, X. Regulation of TERRA on telomeric and mitochondrial functions in IPF pathogenesis. BMC Pulm. Med. 2017, 17, 16.
- Diman, A.; Boros, J.; Poulain, F.; Rodriguez, J.; Purnelle, M.; Episkopou, H.; Bertrand, L.; Francaux, M.; Deldicque, L.; Decottignies, A. Nuclear respiratory factor 1 and endurance exercise promote human telomere transcription. Sci. Adv. 2016, 2, 1600031.
- Chang, K.V.; Chen, Y.C.; Wu, T.; Shen, H.J.; Huang, K.C.; Chu, H.P.; Han, D.S. Expression of telomeric repeat-containing RNA decreases in sarcopenia and increases after exercise and nutrition intervention. Nutrients 2020, 12, 376.
- Hamczyk, M.R.; Nevado, R.M.; Barettino, A.; Fuster, V.; Andrés, V. Biological versus chronological aging: JACC focus seminar. J. Am. Coll. Cardiol. 2020, 75, 919–930.
- Ungvari, Z.; Tarantini, S.; Sorond, F.; Merkely, B.; Csiszar, A. Mechanisms of vascular aging a geroscience perspective: JACC focus seminar. J. Am. Coll. Cardiol. 2020, 75, 931–941.
- Suda, M.; Paul, K.H.; Minamino, T.; Miller, J.D.; Lerman, A.; Ellison-Hughes, G.M.; Tchkonia, T.; Kirkland, J.L. Senescent Cells: A Therapeutic Target in Cardiovascular Diseases. Cells 2023, 12, 1296.
- Boniewska-Bernacka, E.; Pańczyszyn, A.; Klinger, M. Telomeres and telomerase in risk assessment of cardiovascular diseases. Exp. Cell Res. 2020, 397, 112361.
- Haycock, P.C.; Heydon, E.E.; Kaptoge, S.; Butterworth, A.S.; Thompson, A.; Willeit, P. Leucocyte telomere length and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ 2014, 349, 4227.
- D’Mello, M.J.; Ross, S.A.; Briel, M.; Anand, S.S.; Gerstein, H.; Paré, G. Association between shortened leukocyte telomere length and cardiometabolic outcomes: Systematic review and meta-analysis. Circ. Cardiovasc. Genet. 2015, 8, 82–90.
- Emami, M.; Agbaedeng, T.A.; Thomas, G.; Middeldorp, M.E.; Thiyagarajah, A.; Wong, C.X.; Elliott, A.D.; Gallagher, C.; Hendriks, J.M.L.; Lau, D.H.; et al. Accelerated Biological Aging Secondary to Cardiometabolic Risk Factors Is a Predictor of Cardiovascular Mortality: A Systematic Review and Meta-analysis. Can. J. Cardiol. 2022, 38, 365–375.
- Li, C.; Stoma, S.; Lotta, L.A.; Warner, S.; Albrecht, E.; Allione, A.; Arp, P.P.; Broer, L.; Buxton, J.L.; Alves, A.D.S.C.; et al. Genome-wide Association Analysis in Humans Links Nucleotide Metabolism to Leukocyte Telomere Length. Am. J. Hum. Genet. 2020, 106, 389–404.
- Deng, Y.; Li, Q.; Zhou, F.; Li, G.; Liu, J.; Lv, J.; Li, L.; Chang, D. Telomere length and the risk of cardiovascular diseases: A Mendelian randomization study. Front. Cardiovasc. Med. 2022, 9, 101261.
- Vecoli, C.; Borghini, A.; Pulignani, S.; Mercuri, A.; Turchi, S.; Carpeggiani, C.; Picano, E.; Andreassi, M.G. Prognostic value of mitochondrial DNA4977 deletion and mitochondrial DNA copy number in patients with stable coronary artery disease. Atherosclerosis 2018, 276, 91–97.
- Vecoli, C.; Borghini, A.; Pulignani, S.; Mercuri, A.; Turchi, S.; Picano, E.; Andreassi, M.G. Independent and Combined Effects of Telomere Shortening and mtDNA4977 Deletion on Long-term Outcomes of Patients with Coronary Artery Disease. Int. J. Mol. Sci. 2019, 20, 5508.
- Yeh, J.K.; Lin, M.H.; Wang, C.Y. Telomeres as therapeutic targets in heart disease. JACC Basic Transl. Sci. 2019, 4, 855–865.
- Vecoli, C.; Borghini, A.; Andreassi, M.G. The molecular biomarkers of vascular aging and atherosclerosis: Telomere length and mitochondrial DNA4977 common deletion. Mutat. Res. Rev. Mutat. Res. 2020, 784, 108309.
- Hoffmann, J.; Richardson, G.; Haendeler, J.; Altschmied, J.; Andrés, V.; Spyridopoulos, I. Telomerase as a therapeutic target in cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1047–1106.
- Bär, C.; Bernardes de Jesus, B.; Serrano, R.; Tejera, A.; Ayuso, E.; Jimenez, V.; Formentini, I.; Bobadilla, M.; Mizrahi, J.; de Martino, A.; et al. Telomerase expression confers cardioprotection in the adult mouse heart after acute myocardial infarction. Nat. Commun. 2014, 5, 5863.
- Bernardes de Jesus, B.; Schneeberger, K.; Vera, E.; Tejera, A.; Harley, C.B.; Blasco, M.A. The telomerase activator TA-65 elongates short telomeres and increases health span of adult/old mice without increasing cancer incidence. Aging Cell. 2011, 10, 604–621.
- Fragkiadaki, P.; Renieri, E.; Kalliantasi, K.; Kouvidi, E.; Apalaki, E.; Vakonaki, E.; Mamoulakis, C.; Spandidos, D.A.; Tsatsakis, A. Τelomerase inhibitors and activators in aging and cancer: A systematic review. Mol. Med. Rep. 2022, 25, 158.
- Rossi, M.; Gorospe, M. Noncoding RNAs controlling telomere homeostasis in senescence and aging. Trends Mol. Med. 2020, 26, 422–433.
- Sinha, S.; Shukla, S.; Khan, S.; Farhan, M.; Kamal, M.A.; Meeran, S.M. Telomeric Repeat Containing RNA (TERRA): Aging and Cancer. CNS Neurol. Disord. Drug Targets 2015, 14, 936–946.
- Marzano, S.; Pagano, B.; Iaccarino, N.; Di Porzio, A.; De Tito, S.; Vertecchi, E.; Salvati, E.; Randazzo, A.; Amato, J. Targeting of Telomeric Repeat-Containing RNA G-Quadruplexes: From Screening to Biophysical and Biological Characterization of a New Hit Compound. Int. J. Mol. Sci. 2021, 22, 10315.
- Scionti, F.; Juli, G.; Rocca, R.; Polerà, N.; Nadai, M.; Grillone, K.; Caracciolo, D.; Riillo, C.; Altomare, E.; Ascrizzi, S.; et al. TERRA G-quadruplex stabilization as a new therapeutic strategy for multiple myeloma. J. Exp. Clin. Cancer Res. 2023, 42, 71.
- Rossiello, F.; Aguado, J.; Sepe, S.; Iannelli, F.; Nguyen, Q.; Pitchiaya, S.; Carninci, P.; d’Adda di Fagagna, F. DNA damage response inhibition at dysfunctional telomeres by modulation of telomeric DNA damage response RNAs. Nat. Commun. 2017, 8, 15344.
- Aguado, J.; Sola-Carvajal, A.; Cancila, V.; Revêchon, G.; Ong, P.F.; Jones-Weinert, C.W.; Wallén Arzt, E.; Lattanzi, G.; Dreesen, O.; Tripodo, C.; et al. Inhibition of DNA damage response at telomeres improves the detrimental phenotypes of Hutchinson-Gilford Progeria Syndrome. Nat. Commun. 2019, 10, 4990.
