1. Biodegradable Systems
A novel approach is the use of hydrogels as a depot for the various formulations in the intravesical delivery
[1][107]. Hydrogels are three-dimensional hydrophilic or amphiphilic polymer networks prepared via the formation of intermolecular bonds which can be chemical (covalently cross-linked networks) or physical (hydrophobic interactions, ionic or hydrogen bonds) in nature. Hydrogels can swell in water without disrupting their original structure and form an insoluble three-dimensional network with tunable degradability. Biodegradable polymer hydrogels provide a high concentration and a sustained release of the drugs at a tumor site, which eliminates the need for frequent drug administration
[1][2][3][4][5][6][9,107,108,109,110,111]. Injectable physically crosslinked hydrogels have some advantages over chemically crosslinked formulations because they do not require photoirradiation, organic solvents, or crosslinking catalysts. In addition, physical crosslinking methods do not result in the production of heat during polymerization, which can affect the incorporated therapeutics, cells, and surrounding tissues. Physically cross-linked hydrogels are more readily eliminated after drug release and uptake into the urothelial tissues than hydrogels prepared using covalently bonded polymers. However, a balance is required between the prolonged duration of action and biodegradability so that covalently linked hydrogels do not cause any harm to the body. Among the wide variety of thermosensitive gels under investigation, only a few have been tested for intravesical delivery.
1.1. Ion-Sensitive Formulations
The best-known examples of physically crosslinked hydrogels that can be gelled due to ionic interactions are polysaccharides, e.g., alginate, carrageenan, and chitosan. A significant advantage of polysaccharides lies in the possibility of their cross-linking at room temperature and physiological pH values. The mucoadhesive properties of polysaccharide carriers enable the desired attachment to the inner wall of the bladder and provide optimum release rate of the encapsulated agent. As regards in vivo-tested systems, calcium alginate rods containing mitomycin C (2 mm in diameter and 15 mm length in the dry form containing 0.2 mg of the drug) were implanted into the rabbit bladder
[7][112]. Ultrasonographic observations showed that the alginate implant is retained at the injection site for 1 week, during which the drug is released fairly evenly, and 85% of the cargo drug was released at the end of the week. The authors observed calcification, congestion, and mixed-type inflammatory reaction, but those effects were minor and detected just around the implantation site of the alginate carriers. However, the essential fact is that alginate gels are destabilized during the extraction of calcium ions with chelating agents, which leads to their gradual dissolution.
Some classes of injectable hydrogel demonstrate a sol–gel phase transition upon injection in response to external stimuli such as temperature, pH, and light
[3][108]. Among the different types that have been developed, thermosensitive hydrogels have gained increasing attention
[3][4][8][108,109,113].
1.2. Thermosensitive Formulations
Thermosensitive hydrogels are formed by chemical cross-linking using a covalent bond between polymer chains or hydrophobic reactions. Chitosan is a very attractive cationic polymer because of its biocompatibility with negligible immunostimulatory activities. Moreover, positively charged polymers can alter the penetration of drugs into the bladder wall because of the electrostatic interaction with the negatively charged glycosaminoglycan layer of the bladder. To control the chitosan solubility through hydrophobic interactions and hydrogen bonds, β-glycerophosphate (approved by the FDA for intravenous administration) was proposed. Chitosan/β-glycerophosphate mixtures are transparent below physiological temperature due to electrostatic attraction between phosphate groups of β-glycerophosphate and ammonium groups of chitosan; moreover, the additional hydration due to the hydrogen bonding of glycerophosphate with water molecules prevent gel formation. The sol–gel transformation occurs upon heating to 37 °C, and the gelation can be attributed to a thermally induced shift in interchain forces of attraction compared to repulsion, which may arise through several mechanisms
[9][114]. Generally, glycerol provides a protective and hydroresistant layer around the chitosan chains, and increasing the temperature eliminates this polyol layer and allows the chitosan to be in equilibrium through stronger hydrophobic bonds, thereby generating gels. The rheological data, mucoadhesion and retention on the porcine bladder mucosa, syringeability through the urethral catheter, and the mitomycin C release profiles (up to 39% of the mitomycin C were released in 6 h from a gel) reveal that chitosan with the molecular weight of 370 kDa, combined with β-glycerophosphate, exhibited excellent resistance to urine wash-out
[10][115].
The main drawback of the chitosan/β-glycerophosphate gels is that the β-glycerophosphate impairs the mucoadhesive properties of chitosan (which is related to the reduction in positive values of zeta potential for these formulations compared to chitosan alone). So, in terms of the bladder retention of the formulation, additional improvements may include the introduction of magnetic nanoparticles into the chitosan/β-glycerophosphate gel. It was shown that chitosan/glycerophosphate/Fe
3O
4 with encapsulated Bacillus Calmettee–Guérin (BCG) or mitomycin C adhered to the bladder walls and resisted being washed away during urine voiding, and the gel system can withstand the hostile environment of rat urinary bladder for a limited period of 48
[11][116] or 72 h
[12][117], correspondingly. The antitumor effect and increased survival rates after intravesical mitomycin C delivery by chitosan/glycerophosphate/Fe
3O
4 hydrogel were registered in
[12][117]. The uptake of mitomycin C through the bladder mucosa increases from 7.80 ± 0.46 to 21.25 ± 1.55 μg in the case of delivery using chitosan/glycerophosphate/Fe
3O
4 gel compared with the free substance
[12][117].
Alongside magnetically driven localization, an additional option for preventing urinary tract obstruction is related to the floating hydrogel
[4][109]. It was shown that an in situ gelling system based on thermosensitive Poloxamer 407 and NaHCO
3, loaded with adriamycin or gemcitabine, can float owing to the decomposition of NaHCO
3 in the presence of H
+ [13][14][118,119]. The production of carbon dioxide that is attached to the surface of hydrogel allows the material to float in a model environment (
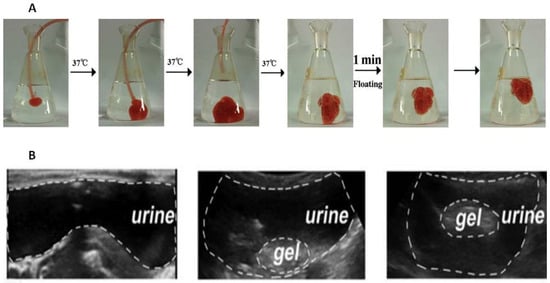
Figure 1A).
Figure 1A comprises a sequence of photographs demonstrating the introduction of a liquid substance into a model medium, simulating the introduction of a gel into the bladder in vivo. Photos 1–3 demonstrate the immediate formation of a hydrogel from the liquid mixture containing 35% Poloxamer 407 and 8% NaHCO
3. In the third photograph, the catheter responsible for introducing the mixture was removed, and its supply was terminated. Meanwhile, NaHCO
3 undergoes decomposition in acidic environments, such as citric acid buffer and acidified urine, leading to the production of numerous CO
2 bubbles (Photo 4). The adriamycin-loaded hydrogel is further supported by microbubbles which are generated on its surface and within it. This contributes to its fast ascent (Photos 5–6) and acts as evidence that it would not cause bladder obstruction.
It has been mentioned that to achieve the excellent floating and bladder retention effect, the urine needed to be acidified, which makes the approach less acceptable due to the potential irritation of the bladder caused by low pH. To address this issue, an updated version of the intravesical floating gel contained only pure P407 (without NaHCO
3) is administered to the animals in an oxygenated state
[15][120]. Oxygenated-by-shaking concentrated poloxamer solution is saturated with self-generating microbubbles, which can be viewed as gas-filled micelles formed by surfactant molecules, whose hydrophobic tail groups face the hydrophobic gas, and whose hydrophilic head groups face the aqueous phase. Due to the viscosity of P407 solution, microbubbles were suspended for a certain amount of time and did not escape easily. In vivo release experiments showed that the drug was released continually from hydrogel for 10 h during the lengthened dissolution process comparatively to P407/NaHCO
3 composition
[15][120]. In a bladder simulation model that involves emptying the bladder every 2 h, the remaining fraction of the P407 gel decreased by approximately 15% at every pouring point, with a reduction of approximately 60% at 8 h, and erosion followed zero order kinetics
[16][121].
Another approach to making the Poloxamer 407-based floating gel is to add perfluoropentane to its composition
[17][122]. The ultrasonically emulsified mixture of Poloxamer 407-perfluoropentane was administered intravesically, and since the boiling point of perfluoropentane is 29 °C, it evaporated in the rabbit bladder to form microbubbles in the hydrogel. Furthermore, perfluoropentane is biologically inert and clinically approved as an ultrasound contrast agent, which allows the gel to be monitored in vivo (
Figure 1B).
Figure 1. Adriamycin-loaded Poloxamer 407-NaHCO
3 floating hydrogel with the peripheral microbubbles (
A)
[13][118]. Poloxamer 407-perfluoropentane hydrogel floating in the rabbit bladder after 2 min after injection (
B)
[17][122].
It has been established that the gel formulation containing F127 and deguelin-loaded nanoparticles was syringeable at room temperature, and the residence time of the drug in the bladder was maintained for 6 h
[18][123]. When comparing gels based on chitosan and Poloxamer 188/407, samples based on chitosan showed the best performance in terms of their stability in the Tyrode solution, which mimics the conditions in the bladder (Poloxamer gels lost their in situ gelling properties at body temperature), bioadhesion to the bladder mucosa, and the percentage of the cargo drug gemcitabine permeating the bladder mucosa (the amount of gemcitabine was almost twice as high in case of chitosan hydrogel)
[19][124].
Biodegradable poly(N-isopropylacrylamide) (PNIPAM) is another well-known thermosensitive polymer with a gelation temperature of 32 °C, which serves as a base for intravesical degradable formulations
[5][110]. A comparison of the amount of cisplatin accumulated in bladder tissues after administration in the form of its solution and in the composition of PNIPAM or PNIPAM grafted sequentially with hyaluronic acid and gelatin (PNIPAM-HA-G) gels was made
[20][125]. In vivo results showed a seven-fold and two-fold benefit after 6 h exposure when using the PNIPAM or PNIPAM-HA-G depot systems correspondingly, presumably because of the mucoadhesiveness of the hydrogels (owing to cationic nature of PNIPAM). Although histological examination showed no adverse change in the urothelium, PNIPAM caused partial desquamation of umbrella cells
[20][125]. Unfortunately, there is information that monomeric acrylamide, which is a main metabolite of PNIPAM in the body, exhibits carcinogenic or teratogenic toxicity
[21][126], which sufficiently limits the clinical application of PNIPAM.
Modified thermosensitive triblock co-polymer polyethylene glycol-poly[lactic acid-co-glycolic acid]-polyethylene glycol (PEG-PLGA-PEG) is the another type of in situ-formed gel
[8][22][113,127]. Gelation at body temperature extended the residence time of the cargo molecules in the bladder from 8 to 24 h, while additionally, the emollient properties of the gel were helpful
[8][113]. Moreover, thiol-bearing 2-(acetylthio)ethylacrylate (ATEA)-based gels showed good intravesical retention and helped to resist the washout of encapsulated doxorubicin molecules due to covalent disulphide bridges with the cysteine-rich regions of urothelial mucins
[23][128]. In general, mucoadhesiveness and modification with cationic groups (-amine, -thiol, etc.) are often used to increase the bioavailability and duration of action of intravesical drugs
[4][109].
Mini-tablets, extrudates, and mini-molds with a lipid matrix can be profitable dosage forms for long-term intravesical treatment. As the hydrolysis of lipids would not occur in the urinary bladder, the lipid-based formulations would maintain the integrity for a long time and provide the long-term control-released properties in the bladder. In addition, the density of the lipids was lower than urine. For this reason, different small-sized glyceryl tristearate-based dosage forms were prepared to permit application through the urethra
[24][25][129,130]. The release kinetics depend on the shape of the formulation: spherical tablets with a diameter of 2 mm provide a five-day release; while 4 mm-diameter tablets allow for almost five-times-slower release
[24][129]. In vivo evaluation showed that the prepared long-term floating preparations could maintain an effective 5-fluorouracil concentration in the bladder for about one month; furthermore, in this period, the 5-fluorouracil concentration in blood was always far less than that in urine
[25][130].
1.3. Combined Particle–Hydrogel Systems for Intravesical Delivery
To enhance stability of nanoparticles and to minimize their susceptibility to washout, different combined systems where nanoparticles are incorporated in a hydrogel have been developed. Men et al.
[18][123] designed a delivery system for deguelin, which is poorly water soluble. For this, deguelin was encapsulated in cationic DOTAP and monomethoxy poly(ethylene glycol)–poly(3-caprolactone) hybrid nanoparticles, with subsequent dispersion into a thermosensitive Pluronic F127 hydrogel. The hydrogel enhances the tissue absorption and cellular uptake of the nanoparticles and prevents their elimination during urination. GuhaSahar et al.
[26][131] developed in situ a gelling liposome-in-gel system composed of fluidizing liposomes incorporated into a urine-triggered hydrogel. The liposomes enhance cellular penetration through the urothelial barrier, while the hydrogel co-delivers the suspended liposomes and enhances adhesion on the mucin layer of the urothelium. The paclitaxel-loaded liposome-in-gel system showed drug retention for at least 7 days, which is higher than the free drug approach (a few hours). Another liposome-in-gel system was proposed in
[27][132], wherein the rapamycin-loaded folate-modified liposomes were dispersed in the same Pluronic F127 hydrogel. The gel with folate-modified liposomes loaded with rapamycin showed higher inhibition of tumor growth compared to unmodified liposomes. Karavana et al.
[28][133] prepared bioadhesive gemtabicine-loaded microspheres from Carbopol 2020 NF and Eudragit E100 and dispersed them in two gel formulations: mucoadhesive chitosan gel and in situ Poloxamer gel. Intravesical treatment with a once-weekly Poloxamer-based delivery system was found to be more effective than the chitosan-based system and the delivery of gemtabicine-loaded microspheres without a gel. Unfortunately, this result is blurred by the fact that upon the dilution with an artificial urine, the Poloxamer-based gel lost its in situ gelling properties at body temperature.
As for the gels that have reached clinical trials, several potential thermosensitive gel systems should be noted. TCGel
® is a hydrogel with reverse thermal gelation properties, produced by TheraCoat Ltd. (Raanana, Israel), containing Pluronic F-127, PEG-400, and a small amount of hydroxypropyl methylcellulose. Following instillation, the gel solidifies and acts as a sustained drug release depot in situ. It exhibited improved safety and residence within the bladder cavity between 6 and 8 h with the release of mitomycin C during this time
[2][29][9,134]. TCGel
® is slowly excreted from the bladder during urination. It is 100% biocompatible and harmless to the body. TheraCoat Ltd. has commenced several efficacy clinical trials
[30][31][135,136].
UGN-102 is another investigational agent designed for primary non-surgical treatment of NMIBC and to potentially obviate the need for repetitive TURBT. UGN-102 consists of mitomycin and a proprietary reverse thermal hydrogel (UroGen Pharma, Raanana, Israel). The ablative effect of UGN-102 was evaluated after six intravesical once-weekly instillations of UGN-102
[29][134]. The gel slowly disintegrates over a 6 h period and is eliminated through normal urine flow, allowing for the sustained release of mitomycin over a period of 4 to 6 h, enabling the ten-fold-higher drug content in the bladder after 2 and 6 h after instillation as compared to the free drug
[32][137]. At the same time, levels of mitomycin C in the plasma were low, confirming the safety of the treatment. The achieved disease-free level was 95, 73, and 25% after 6, 9, and 12 months after treatment initiation, respectively
[29][134]. Assuming positive findings from the ENVISION
[33][138] and ATLAS
[34][139] Phase-III studies in the summer of 2023, UroGen anticipates submitting a new drug application for UGN-102 in 2024. If approved, UGN-102 would be the first non-surgical primary therapeutic to treat a subset of bladder cancer characterized by high recurrence rates and multiple surgeries.
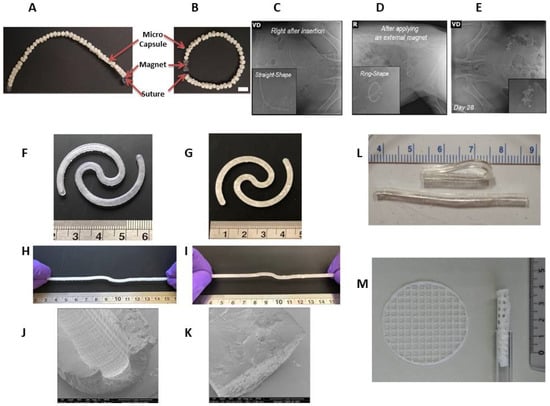
In addition, there is an intermediate product worth mentioning in this section—an intravesical delivery system composed of drug-encapsulating biodegradable polycaprolactone (PCL) microcapsules and connected by a bioabsorbable Polydioxanone (PDS) suture with NdFeB magnets in the end (Figure 2A,B). The implant can be easily inserted into the bladder and forms a “ring” shape under the magnet exposure (Figure 2C,D).
2. Non-Biodegradable Indwelling Devices
2.1. Elastomer-Based Devices
The mechanical properties of elastomers can be advantageously used for the insertion and retention of products based on them in hollow organs. But in contrast to biodegradable products, their significant drawback is the need for a transurethral removal procedure, which inevitably reduces the patient’s compliance. More recently, over the past few years, bladder devices based on elastic polymers (elastic resin) have been fabricated via stereolithographical technology to achieve the desired complex geometrical structures, shape, and mechanical properties of the implant to enable its insertion via catheter
[35][141]. A modification of the 3D printing process allows for the formation of two variants: hollow and solid implants with a diameter of 3 mm and a length of about 130 mm in an extended shape (
Figure 2F–K). This option allows for the adjustment of the release duration of the cargo molecules from the bladder-retentive devices in the range of 4–14 days
[35][141]. Additionally, this technique can be used to print drug-containing devices regardless of their solubility in water as they can either be dissolved or dispersed in the liquid resin.
A resorbable elastomer with poly(glycerol-co-sebacic acid) composition
[36][142] may be of interest for intravesical delivery owing to better biocompatibility than poly(DL-lactide-co-glycolide) when tested in vivo
[37][143]. Unlike poly(DL-lactide-co-glycolide), poly(glycerol-co-sebacic acid) primarily degrades via surface erosion, which gives a linear degradation profile of mass, preservation of geometry and intact surface, and retention of mechanical strength. A poly(glycerol-co-sebacic acid) tube with a laser-drilled orifice (
Figure 2L) allowed for the drug payload release via osmotically-driven water permeation over a time period of a few weeks
[36][142], and modulation of the release rate can be achieved by varying the orifice size. In vitro experiments have shown that the elastomer is susceptible to hydrolytic degradation, indicating the possibility of creating a completely resorbable device.
A number of semi-solid printing implants were fabricated via pressure-assisted micro syringe printing based on polycaprolactone and ethylene vinyl acetate copolymer
[38][144]. They were flexible enough to be inserted using a common urinary catheter (
Figure 2M) and remain inside the urinary bladder for up to several weeks.
Figure 2. Optical image of a partially biodegradable ring-shaped implantable device based on polycaprolactone, polydioxanone, and NdFeB magnets before (
A) and after (
B) exposure to a magnet. Scale bar = 1 cm. Radiograph images of the implant immediately after insertion (
C), after applying an external magnet (
D), and 28 days after implantation in a swine model (
E)
[39][140]. Photographs of hollow (
F) and solid (
G) bladder devices (elastic resin) in their intact form and under stretching (
H,
I). Scale is in cm. SEM images of sections of the empty hollow (
J) and empty solid (
K) devices
[35][141]. Poly(glycerol-co-sebacic acid) tubes demonstrating flexibility of material (
L)
[36][142]. Printed net-shaped polycaprolactone/ethylene vinyl acetate copolymer implants unfolded (left) and coiled up (right) for insertion via catheter
[38][144] (
M).
2.2. Osmotic Pumps
Among non-degradable implantable systems that provide long-term drug delivery with a minimal risk of infection and minimal risk to patient’s life, a number of state-of-the-art osmotic devices should be noted, e.g., LiRIS, UROS, GemRIS, and Duros.
A continuous lidocaine-releasing intravesical system LiRIS (TARIS Biomedical, Lexington, USA) is the only indwelling bladder device that has advanced to clinical trials
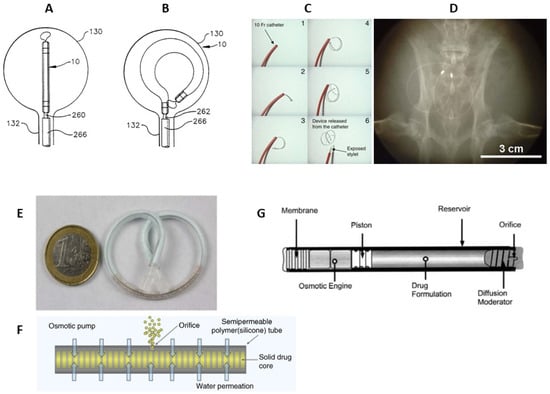
[40][41][145,146]. Prior to LiRIS, an intravesical horseshoe-shaped device, UROS (Situs Corporation, Houston, TX, USA), was developed (
Figure 3A,B)
[42][43][147,148]. This indwelling pump was 10–15 cm in the largest diameter and released cargo for up to 28 days
[44][149]. Phase I/II-trials were relatively unsuccessful due to the poor tolerability associated with the large size of the device; thus, UROS has not entered clinical practice
[44][149]. Thereafter, LiRIS was developed in order to minimalize the size to avoid discomfort
[40][145]. LiRIS is a dual-lumen silicone tube that contains the drug in the form of mini-tablets in one lumen (enabling a higher dose of 2 mg) and a super elastic shape memory nitinol wire in the other (
Figure 3C). To enable intravesical administration, the wire is mechanically forced into an elongated shape. The device can be inserted into the bladder with a foley catheter and adopts a “pretzel” conformation once inside the bladder, preventing it from being accidentally voided. A silicone container absorbs urine to dissolve the lidocaine contents, whereas the created osmotic pressure forces the solution out of the container through a small orifice in a controlled release over 14 days
[40][45][145,150].
Promising results were achieved when the device was tried in a rabbit model (
Figure 3D); lidocaine was detected in the bladder tissue during the 3-day period, while a single instillation yielded immeasurable amounts within 24 h
[45][150]. The small proof of concept study in women with ulcerative interstitial cystitis and Hunner′s lesions demonstrated a favorable safety profile as well as long-lasting improvements in lesions, pain, and voiding frequency after 2 weeks of LiRIS therapy
[46][151]. Expanded Phase-II/III trials conducted in 2021 to evaluate the efficacy of a LiRIS device in female patients did not demonstrate a therapeutic effect of LiRIS compared to placebo
[41][146].
The upgraded version of the silicone “pretzel” pump with a shape memory wire is adapted to gemcitabine bladder delivery (GemRIS or TAR-200, TARIS Biomedical)
[47][152]. The device consists of a 5 cm semipermeable silicone tube that slowly releases dissolving gemcitabine tablets (
Figure 3E,F), and as a result, 60–70% of the cargo drug is delivered over 2 weeks, compared to the 2 h conventional dwell time for intravesical drugs
[47][152]. GemRIS proved its safety and tolerability during the 7-day indwelling time and demonstrated encouraging preliminary efficacy
[48][153]. It showed very promising results during Phase-I trials completed in 2019–2020 for single-agent delivery in patients with cisplatin-ineligible muscle-invasive bladder cancer
[49][50][51][154,155,156]. Along with safety and tolerability, the aim of a Phase-I study was to unleash the potential of TAR-200 for intravesical drug delivery in muscle-invasive bladder cancer in the neoadjuvant setting in combination with nivolumab
[52][157]. A currently ongoing Phase-III trial of combination treatment with intravesical TAR-200 and systemic cetrelimab, with the last update in August 2023, aims to evaluate the efficacy in participants with muscle-invasive urothelial carcinoma of the bladder
[53][158]. In a recruiting Phase-III study started at the beginning of 2023, metronomic dosing of intravesical gemcitabine, delivered via TAR-200 (alone or in combination with cetrelimab), will be evaluated and compared against intravesical Bacillus Calmette–Guérin in participants with high-risk NMIBC
[54][159]. A few years earlier, the FDA granted fast track designation to TARIS Biomedical for GemRIS (TAR-200) for the treatment of patients with muscle-invasive bladder cancer
[55][160].
A completely different type of implantable device consists of a cylindrical titanium alloy drug reservoir (4 mm in diameter by 45 mm in length and holds approximately 150 µL of formulation), capped at one end by a polyurethane rate-controlling semipermeable membrane (
Figure 3F). The osmotic engine consists mainly of NaCI adjacent to the membrane, and a sliding elastomeric piston isolates the engine from the drug formulation. At the far end of the cylinder is the diffusion moderator that contains the orifice through which the drug is released. The DUROS (ALZA Corporation, Vacaville, USA) osmotic pump has been commercialized for the subcutaneous implantation and palliative treatment of prostate cancer (Viadur system)
[56][161]. This implant provides zero-order release kinetics
[56][57][161,162] and can be adapted to biomolecules delivery that requires long-term controlled administration, including those that have a narrow therapeutic window or a short half-life. DUROS delivery technology is capable of delivering a wide range of therapeutic peptides and proteins (1.2 kDa–25 kDa) for durations ranging from 3 to 12 months
[56][58][161,163] and may be applicable to the chronic delivery.
Figure 3. Scheme of the UROS device during insertion into the bladder through a catheter (
A) and after taking on a horseshoe shape (
B)
[42][147]. The representation of deployment of the LiRIS by the catheter–stylet system (
C). X-ray image of an implanted device within the bladder of a rabbit in a ventral–dorsal view (
D)
[45][150]. GemRIS (TAR-200) device after self-coiling into a pretzel shape (
E) and its scheme (
F)
[47][152]. Sketch of DUROS implant (
G)
[56][161].