Volatile compounds not only contribute to the distinct flavors and aromas found in foods and beverages, but can also serve as indicators for spoilage, contamination, or the presence of potentially harmful substances. As the odor of food raw materials and products carries valuable information about their state, gas sensors play a pivotal role in ensuring food safety and quality at various stages of its production and distribution. Among gas detection devices that are widely used in the food industry, metal oxide semiconductor (MOS) gas sensors are of the greatest importance. Ongoing research and development efforts have led to significant improvements in their performance, rendering them immensely useful tools for monitoring and ensuring food product quality.
- metal oxide semiconductor gas sensors
- E-nose
- electronic nose
- thermally modulated MOS gas sensor
- heterostructures
- nanostructures
- nanomaterials
- doping with noble metals
- conducting polymers
- food safety
1. Introduction
2. MOS Gas Sensors—Design and Operation
2.1. Design
MOS gas sensors are based on changes in the physical and chemical properties of the device-sensing material under the influence of gas components undergoing redox reactions on its surface [1]. The key components of a typical MOS gas sensor include the substrate, the metal oxide sensing layer, electrodes, a microheater, and a protective layer [1,8,9][1][8][9]. Figure 1 shows the schematic design of an MOS gas sensor.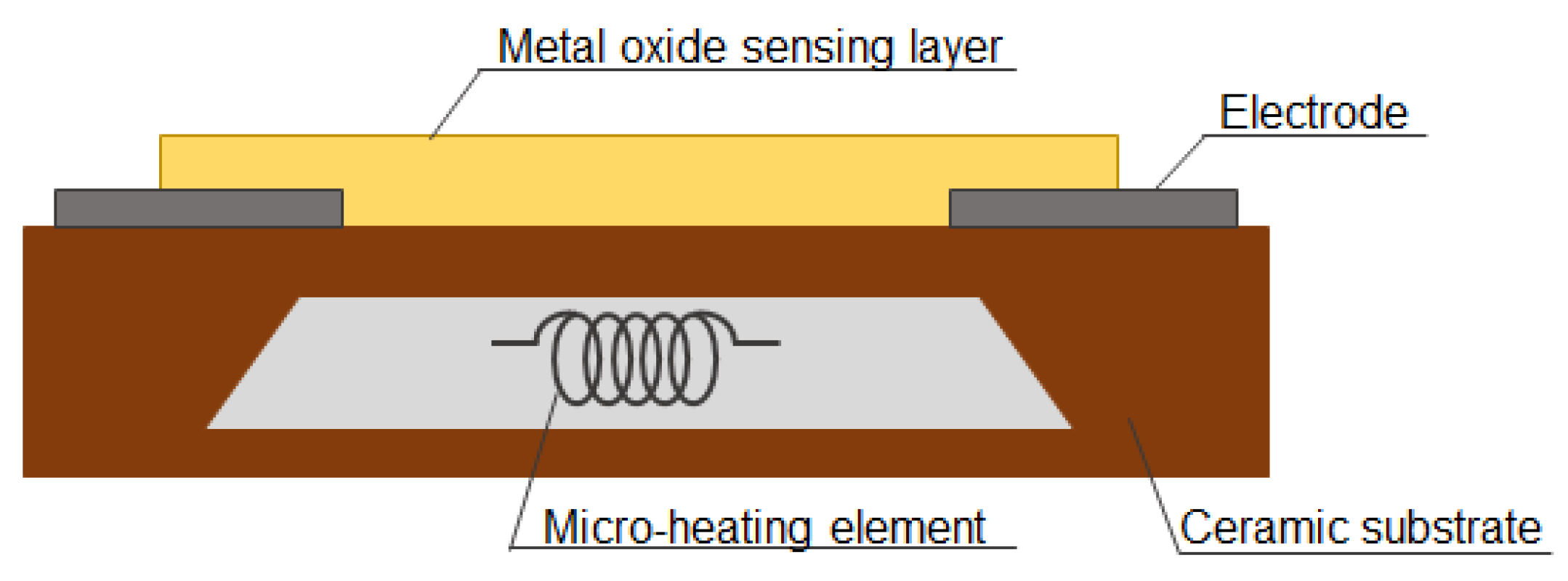
2.2. Principles of Operation
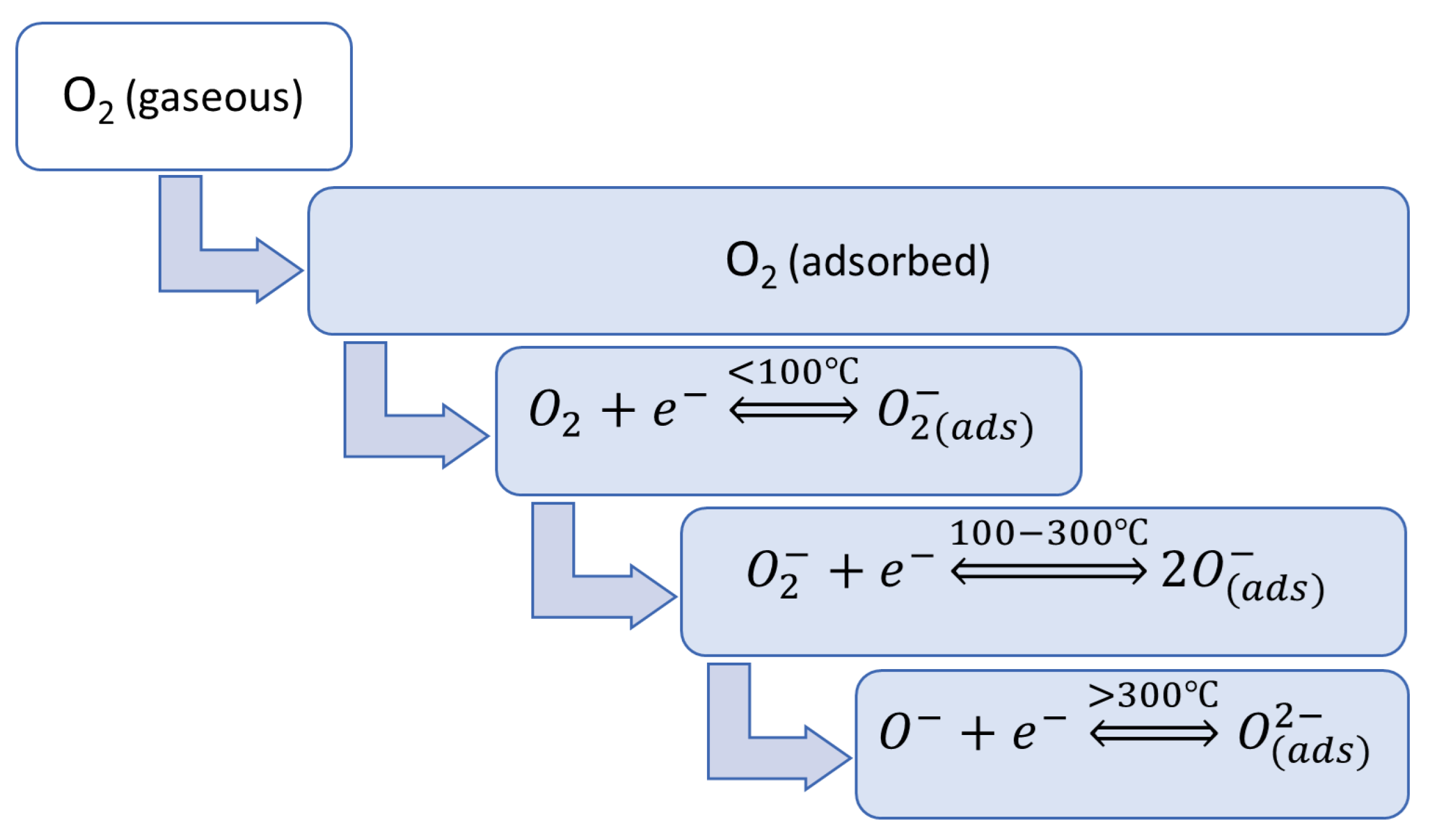
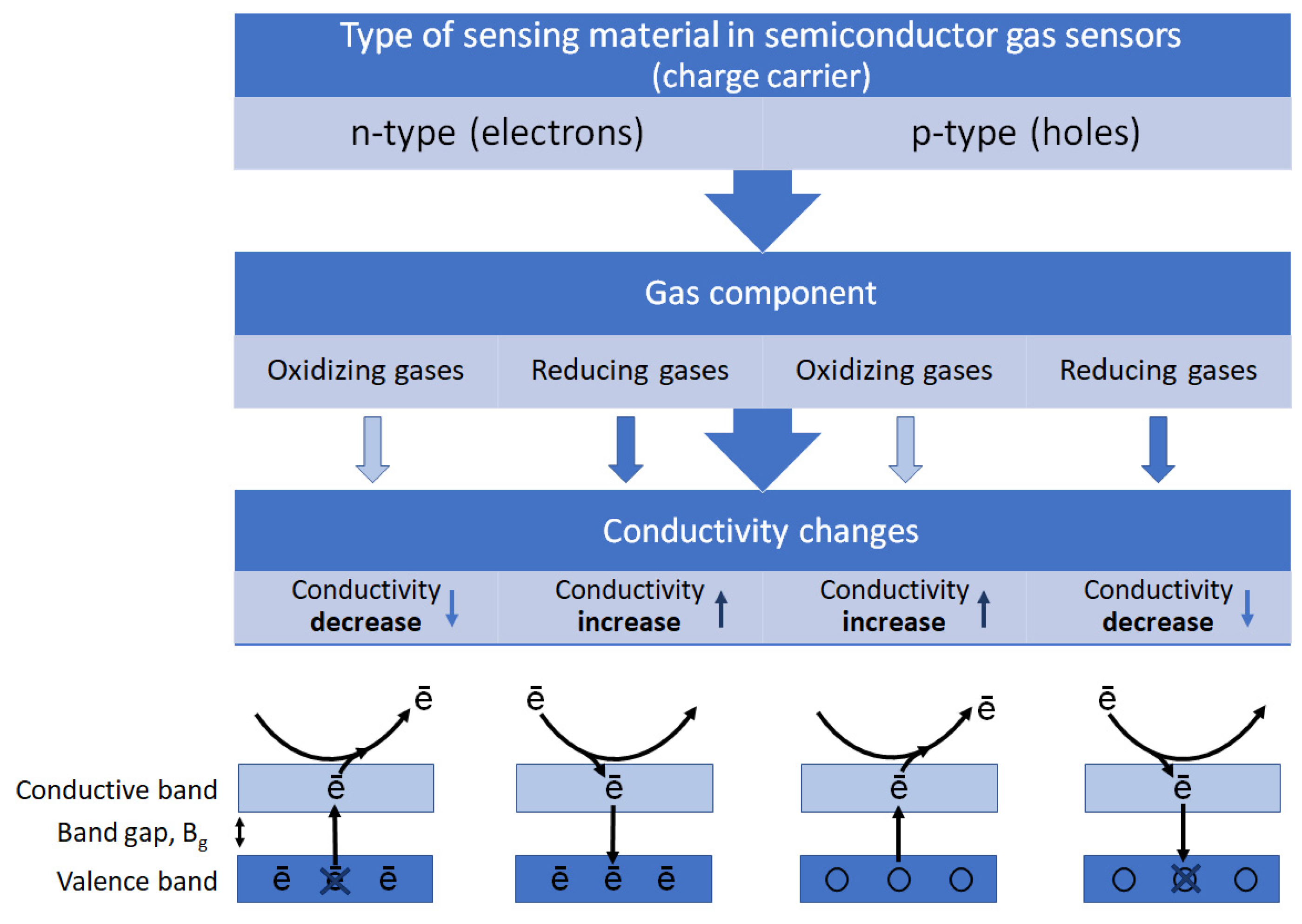
The operation of the MOS gas sensor relies on changes in electrical properties of the semiconductor material when exposed to reducing or oxidizing gases in the surrounding environment [36,37][18][19]. In this way, the non-electric chemical information acquired from selective interactions of the target analytes with the sensing material is transformed into an analytically useful and readily measured electrical signal. A basic diagram of stages in the operation of MOS gas sensors is shown in Figure 2.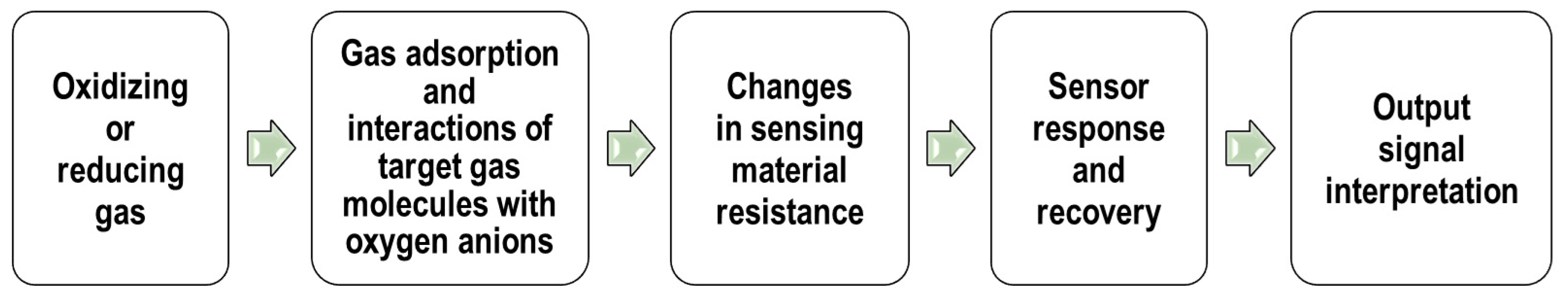
3. Advancements in Metal Oxide Gas Sensors
Sensitivity, selectivity, and stability defined as specific interactions between the sensing material and target gas molecules are widely recognized as key parameters of gas sensors [12,50][12][36]. Recent research on improving these parameters of MOS gas sensors mainly involves designing new or modified sensing materials [7[7][36],50], whereas selectivity enhancement may be additionally achieved thanks to the development of new measurement methodologies [51][37] and elaboration of new techniques to interpret sensor responses by extracting specific features of the measured signal [5].3.1. Progress in Sensing Materials
Enhancing adsorption of gas molecules on the sensing surface is crucial for improving gas sensor sensitivity, selectivity, and stability [52][38]. This can be achieved through several strategies (Figure 5), including increasing the number of available adsorption sites, promoting the formation of oxygen vacancies, and enhancing surface catalytic activity. The following sections of the entreviewy present some methods that have recently been used to reach this goal.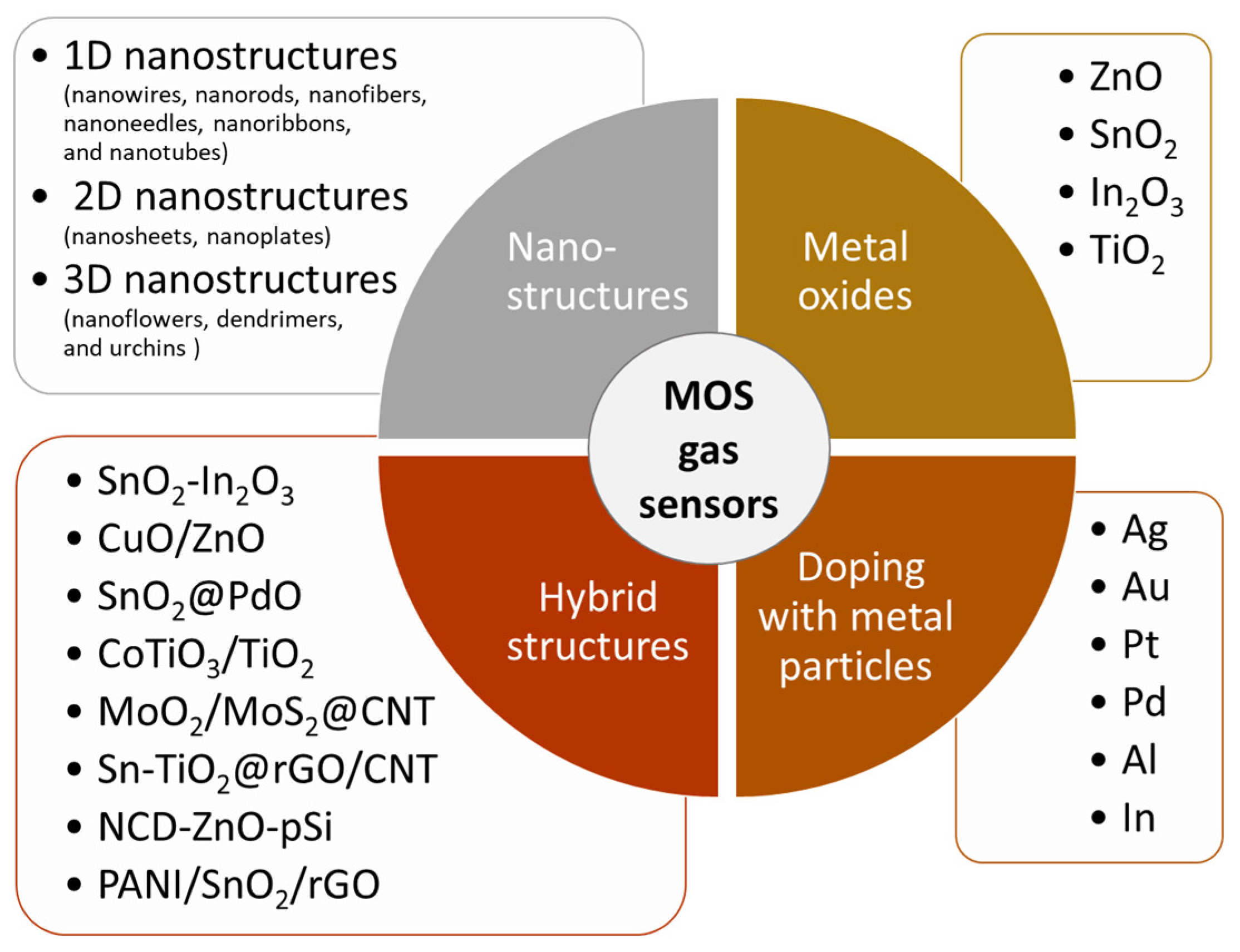
3.2. Sensor Thermal Modulation


3.3. MOS Gas Sensor Arrays
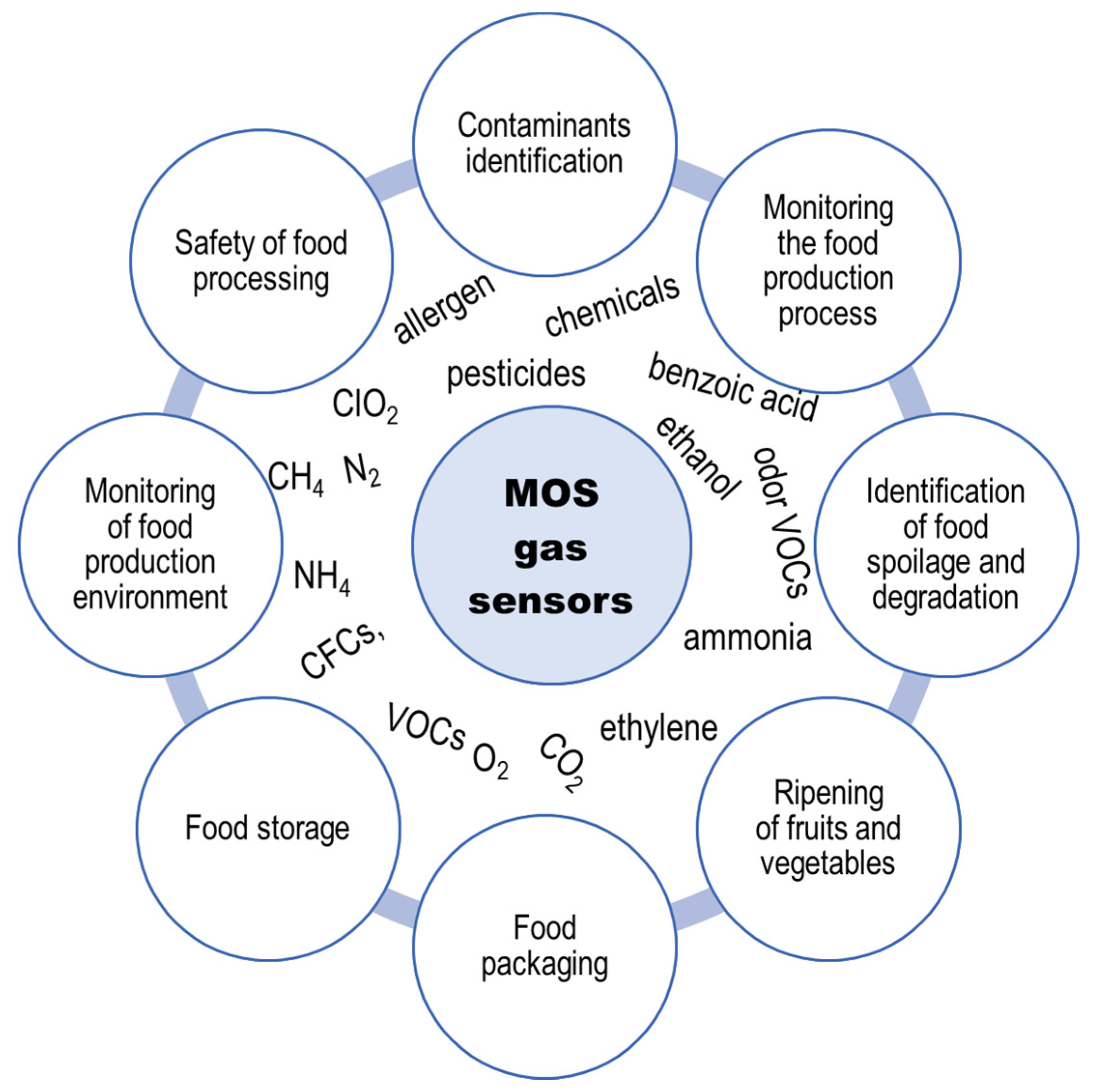
4. Application of MOS Gas Sensors in the Food Industry
References
- Nazemi, H.; Joseph, A.; Park, J.; Emadi, A. Advanced micro-and nano-gas sensor technology: A review. Sensors 2019, 19, 1285.
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal oxide gas sensors: Sensitivity and influencing factors. Sensors 2010, 10, 2088–2106.
- Krishna, K.G.; Parne, S.; Pothukanuri, N.; Kathirvelu, V.; Gandi, S.; Joshi, D. Nanostructured metal oxide semiconductor-based gas sensors: A comprehensive review. Sens. Actuators A Phys. 2022, 341, 113578.
- Nikolic, M.V.; Milovanovic, V.; Vasiljevic, Z.Z.; Stamenkovic, Z. Semiconductor gas sensors: Materials, technology, design, and application. Sensors 2020, 20, 6694.
- Wawrzyniak, J. Methodology for Quantifying Volatile Compounds in a Liquid Mixture Using an Algorithm Combining B-Splines and Artificial Neural Networks to Process Responses of a Thermally Modulated Metal-Oxide Semiconductor Gas Sensor. Sensors 2022, 22, 8959.
- Ji, H.; Yuan, Z.; Zhu, H.; Qin, W.; Wang, H.; Meng, F. Dynamic Temperature Modulation Measurement of VOC Gases Based on SnO2 Gas Sensor. IEEE Sens. J. 2022, 1, 14708–14716.
- Zappa, D.; Galstyan, V.; Kaur, N.; Munasinghe Arachchige, H.M.M.; Sisman, O.; Comini, E. Metal oxide -based heterostructures for gas sensors—A review. Anal. Chim. Acta 2018, 1039, 1–23.
- Kakoty, P.; Bhuyan, M. Fabrication of Micromachined SnO2 Based MOS Gas Sensor with Inbuilt Microheater for Detection of Methanol. Sens. Transducers J. 2016, 204, 58–67.
- Yun, J.; Cho, M.; Lee, K.; Kang, M.; Park, I. A review of nanostructure-based gas sensors in a power consumption perspective. Sens. Actuators B Chem. 2022, 372, 132612.
- Gonzalez, O.; Roso, S.; Calavia, R.; Vilanova, X.; Llobet, E. NO2 sensing properties of thermally or UV activated In2O3 nano-octahedra. Procedia Eng. 2015, 120, 773–776.
- Meng, Z.; Stolz, R.M.; Mendecki, L.; Mirica, K.A. Electrically-transduced chemical sensors based on two-dimensional nanomaterials. Chem. Rev. 2019, 119, 478–598.
- Korotcenkov, G. Metal oxides for solid-state gas sensors: What determines our choice? Mater. Sci. Eng. B 2007, 139, 1–23.
- Drmosh, Q.A.; Yamani, Z.H.; Mohamedkhair, A.K.; Hendi, A.H.Y.; Hossain, M.K.; Ibrahim, A. Gold nanoparticles incorporated SnO2 thin film: Highly responsive and selective detection of NO2 at room temperature. Mater. Lett. 2018, 214, 283–286.
- Barquinha, P.; Fortunato, E.; Gonçalves, A.; Pimentel, A.; Marques, A.; Pereira, L.; Martins, R. A study on the electrical properties of ZnO based transparent TFTs. Mater. Sci. Forum 2006, 514–516, 68–72.
- Phan, D.T.; Chung, G.S. Effects of different morphologies of ZnO films on hydrogen sensing properties. J. Electroceramics 2014, 32, 353–360.
- Fine, G.F.; Cavanagh, L.M.; Afonja, A.; Binions, R. Metal oxide semi-conductor gas sensors in environmental monitoring. Sensors 2010, 10, 5469–5502.
- Garzella, C.; Comini, E.; Tempesti, E.; Frigeri, C.; Sberveglieri, G. TiO2 thin films by a novel sol-gel processing for gas sensor applications. Sens. Actuators B Chem. 2000, 68, 189–196.
- Hulanicki, A.; Glab, S.; Ingman, F. Chemical sensors: Definitions and classification. Pure Appl. Chem. 1991, 63, 1247–1250.
- Chai, H.; Zheng, Z.; Liu, K.; Xu, J.; Wu, K.; Luo, Y.; Liao, H.; Debliquy, M.; Zhang, C. Stability of Metal Oxide Semiconductor Gas Sensors: A Review. IEEE Sens. J. 2022, 22, 5470–5481.
- Pimentel, A.; Ferreira, S.H.; Nunes, D.; Calmeiro, T.; Martins, R.; Fortunato, E. Microwave synthesized ZnO nanorod arrays for UV sensors: A seed layer annealing temperature study. Materials 2016, 9, 299.
- Sun, Y.F.; Liu, S.B.; Meng, F.L.; Liu, J.Y.; Jin, Z.; Kong, L.T.; Liu, J.H. Metal oxide nanostructures and their gas sensing properties: A review. Sensors 2012, 12, 2610–2631.
- Gautam, Y.K.; Sharma, K.; Tyagi, S.; Ambedkar, A.K.; Chaudhary, M.; Pal Singh, B. Nanostructured metal oxide semiconductor-based sensors for greenhouse gas detection: Progress and challenges. R. Soc. Open Sci. 2021, 8, 201324.
- Choopun, S.; Hongsith, N.; Wongrat, E. Metal-Oxide Nanowires for Gas Sensors. In Nanowires; Peng, X., Ed.; IntechOpen: Rijeka, Croatia, 2012.
- Belysheva, T.V.; Bogovtseva, L.P.; Kazachkov, E.A.; Serebryakova, N. V Gas-Sensing Properties of Doped In2O3 Films as Sensors for NO2 in Air. J. Anal. Chem. 2003, 58, 583–587.
- Tyagi, S.; Chaudhary, M.; Ambedkar, A.K.; Sharma, K.; Gautam, Y.K.; Singh, B.P. Metal oxide nanomaterial-based sensors for monitoring environmental NO2 and its impact on the plant ecosystem: A review. Sens. Diagn. 2022, 1, 106–129.
- Nunes, D.; Pimentel, A.; Barquinha, P.; Carvalho, P.A.; Fortunato, E.; Martins, R. Cu2O polyhedral nanowires produced by microwave irradiation. J. Mater. Chem. C 2014, 2, 6097–6103.
- Pimentel, A.; Samouco, A.; Nunes, D.; Araújo, A.; Martins, R.; Fortunato, E. Ultra-fast microwave synthesis of ZnO nanorods on cellulose substrates for UV sensor applications. Materials 2017, 10, 1308.
- Devan, R.S.; Patil, R.A.; Lin, J.H.; Ma, Y.R. One-dimensional metal-oxide nanostructures: Recent developments in synthesis, characterization, and applications. Adv. Funct. Mater. 2012, 22, 3326–3370.
- Kolmakov, A.; Moskovits, M. Chemical sensing and catalysis by one-dimensional metal-oxide nanostructures. Annu. Rev. Mater. Res. 2004, 34, 151–180.
- Chaloeipote, G.; Prathumwan, R.; Subannajui, K.; Wisitsoraat, A.; Wongchoosuk, C. 3D printed CuO semiconducting gas sensor for ammonia detection at room temperature. Mater. Sci. Semicond. Process. 2021, 123, 105546.
- Nakate, U.T.; Yu, Y.T.; Park, S. Hierarchical CuO nanostructured materials for acetaldehyde sensor application. Microelectron. Eng. 2022, 251, 111662.
- Kim, H.J.; Lee, J.H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B Chem. 2014, 192, 607–627.
- Miller, D.R.; Akbar, S.A.; Morris, P.A. Nanoscale metal oxide-based heterojunctions for gas sensing: A review. Sens. Actuators B Chem. 2014, 204, 250–272.
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217.
- Ji, H.; Zeng, W.; Li, Y. Gas sensing mechanisms of metal oxide semiconductors: A focus review. Nanoscale 2019, 11, 22664–22684.
- Liu, X.; Cheng, S.; Liu, H.; Hu, S.; Zhang, D.; Ning, H. A survey on gas sensing technology. Sensors 2012, 12, 9635–9665.
- Zheng, Z.; Zhang, C. Electronic noses based on metal oxide semiconductor sensors for detecting crop diseases and insect pests. Comput. Electron. Agric. 2022, 197, 106988.
- Korotcenkov, G.; Cho, B.K. Metal oxide composites in conductometric gas sensors: Achievements and challenges. Sens. Actuators B Chem. 2017, 244, 182–210.
- Seekaew, Y.; Pon-On, W.; Wongchoosuk, C. Ultrahigh Selective Room-Temperature Ammonia Gas Sensor Based on Tin-Titanium Dioxide/reduced Graphene/Carbon Nanotube Nanocomposites by the Solvothermal Method. ACS Omega 2019, 4, 16916–16924.
- Krivetskiy, V.V.; Andreev, M.D.; Efitorov, A.O.; Gaskov, A.M. Statistical shape analysis pre-processing of temperature modulated metal oxide gas sensor response for machine learning improved selectivity of gases detection in real atmospheric conditions. Sens. Actuators B Chem. 2021, 329, 129187.
- Karmakar, M.; Mondal, B.; Pal, M.; Mukherjee, K. Acetone and ethanol sensing of barium hexaferrite particles: A case study considering the possibilities of non-conventional hexaferrite sensor. Sens. Actuators B Chem. 2014, 190, 627–633.
- Migas, D.B.; Turchenko, V.A.; Rutkauskas, A.V.; Trukhanov, S.V.; Zubar, T.I.; Tishkevich, D.I.; Trukhanov, A.V.; Skorodumova, N.V. Temperature induced structural and polarization features in BaFe12O19. J. Mater. Chem. C 2023, 11, 12406–12414.
- Hossein-Babaei, F.; Amini, A. A breakthrough in gas diagnosis with a temperature-modulated generic metal oxide gas sensor. Sens. Actuators B Chem. 2012, 166–167, 419–425.
- Chutia, R.; Bhuyan, M. Best frequency for temperature modulation of tin oxide gas sensor for chemical vapor identification. Int. J. Eng. Technol. 2014, 6, 1158–1166.
- Morati, N.; Contaret, T.; Seguin, J.; Bendahan, M.; Morati, N.; Contaret, T.; Seguin, J.; Bendahan, M.; Djedidi, O.; Morati, N.; et al. Data Analysis-Based Gas Identification with a Single Metal Oxide Sensor Operating in Dynamic Temperature Regime. In Proceedings of the ALLSENSORS 2020, The Fifth International Conference on Advances in Sensors, Actuators, Metering and Sensing, Valencia, Spain, 21–25 November 2020; pp. 20–23.
- Shi, X.; Zhang, H.; Ji, H.; Meng, F. Dynamic Measurement of VOCs with Multiple Characteristic Peaks Based on Temperature Modulation of ZnO Gas Sensor. Chemosensors 2022, 10, 226.
- Bora, A.; Chandra, S.K. A Temperature Modulation Circuit for Metal Oxide Semiconductor Gas Sensor. Indian J. Sci. Technol. 2015, 8, 1–7.
- Durán, C.; Benjumea, J.; Carrillo, J. Response optimization of a chemical gas sensor array using temperature modulation. Electronics 2018, 7, 54.
- Szczurek, A.; Maciejewska, M.; Bąk, B.; Wilk, J.; Wilde, J.; Siuda, M. Detecting varroosis using a gas sensor system as a way to face the environmental threat. Sci. Total Environ. 2020, 722, 137866.
- Mu, F.; Gu, Y.; Zhang, J.; Zhang, L. Milk source identification and milk quality estimation using an electronic nose and machine learning techniques. Sensors 2020, 20, 4238.
- Szczurek, A.; Maciejewska, M.; Bąk, B.; Wilk, J.; Wilde, J.; Siuda, M. Gas sensor array and classifiers as a means of varroosis detection. Sensors 2020, 20, 117.
- Liu, H.; Li, Q.; Yan, B.; Zhang, L.; Gu, Y. Bionic Electronic Nose Based on MOS Sensors Array and Machine Learning Algorithms Used for Wine Properties Detection. Sensors 2018, 19, 45.
- Han, L.; Yu, C.; Xiao, K.; Zhao, X. A new method of mixed gas identification based on a convolutional neural network for time series classification. Sensors 2019, 19, 1960.
- Voss, H.G.J.; Mendes Júnior, J.J.A.; Farinelli, M.E.; Stevan, S.L. A Prototype to Detect the Alcohol Content of Beers Based on an Electronic Nose. Sensors 2019, 19, 2646.
- Feng, S.; Farha, F.; Li, Q.; Wan, Y.; Xu, Y.; Zhang, T.; Ning, H. Review on smart gas sensing technology. Sensors 2019, 19, 3760.
- Seesaard, T.; Wongchoosuk, C. Recent Progress in Electronic Noses for Fermented Foods and Beverages Applications. Fermentation 2022, 8, 302.
- Teixeira, G.G.; Peres, A.M.; Estevinho, L.; Geraldes, P.; Garcia-Cabezon, C.; Martin-Pedrosa, F.; Rodriguez-Mendez, M.L.; Dias, L.G. Enose Lab Made with Vacuum Sampling: Quantitative Applications. Chemosensors 2022, 10, 261.
- Liu, Q.; Zhao, N.; Zhou, D.; Sun, Y.; Sun, K.; Pan, L.; Tu, K. Discrimination and growth tracking of fungi contamination in peaches using electronic nose. Food Chem. 2018, 262, 226–234.
- Radi; Barokah; Zamzami, L.F.; Setiawan, A. Performance Analysis of MOS Sensors on Electronic Nose for Synthetic Flavor Classification. Proc. Int. Conf. Sustain. Environ. Agric. Tour. (ICOSEAT 2022) 2023, 26, 552–558.
- Sudarmaji, A.; Margiwiyatno, A.; Sulistyo, S.B. Characteristics of array MOS gas sensors in detection of adulteration on patchouli oil with candlenut oil. AIP Conf. Proc. 2023, 2586, 70016.
- Teixeira, G.G.; Dias, L.G.; Rodrigues, N.; Marx, Í.M.G.; Veloso, A.C.A.; Pereira, J.A.; Peres, A.M. Application of a lab-made electronic nose for extra virgin olive oils commercial classification according to the perceived fruitiness intensity. Talanta 2021, 226, 122122.
- Machungo, C.W.; Berna, A.Z.; McNevin, D.; Wang, R.; Harvey, J.; Trowell, S. Evaluation of performance of metal oxide electronic nose for detection of aflatoxin in artificially and naturally contaminated maize. Sens. Actuators B Chem. 2023, 381, 133446.
- Jiang, X.; Jia, P.; Luo, R.; Deng, B.; Duan, S.; Yan, J. A novel electronic nose learning technique based on active learning: EQBC-RBFNN. Sens. Actuators B Chem. 2017, 249, 533–541.
- Machungo, C.; Berna, A.Z.; McNevin, D.; Wang, R.; Trowell, S. Comparison of the performance of metal oxide and conducting polymer electronic noses for detection of aflatoxin using artificially contaminated maize. Sens. Actuators B Chem. 2022, 360, 131681.
- Bax, C.; Robbiani, S.; Zannin, E.; Capelli, L.; Ratti, C.; Bonetti, S.; Novelli, L.; Raimondi, F.; Di Marco, F.; Dellacà, R.L. An Experimental Apparatus for E-Nose Breath Analysis in Respiratory Failure Patients. Diagnostics 2022, 12, 776.
- Tyagi, P.; Semwal, R.; Sharma, A.; Tiwary, U.S.; Varadwaj, P. E-nose: A low-cost fruit ripeness monitoring system. J. Agric. Eng. 2023, 54, 1389.
- Viejo, C.G.; Fuentes, S.; Hernandez-Brenes, C. Smart detection of faults in beers using near-infrared spectroscopy, a low-cost electronic nose and artificial intelligence. Fermentation 2021, 7, 117.
- Liu, K.; Zhang, C. Volatile organic compounds gas sensor based on quartz crystal microbalance for fruit freshness detection: A review. Food Chem. 2021, 334, 127615.
- Ali, A.; Mansol, A.S.; Khan, A.A.; Muthoosamy, K.; Siddiqui, Y. Electronic nose as a tool for early detection of diseases and quality monitoring in fresh postharvest produce: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2023, 22, 2408–2432.
- Kim, C.; Raja, I.S.; Lee, J.M.; Lee, J.H.; Kang, M.S.; Lee, S.H.; Oh, J.W.; Han, D.W. Recent trends in exhaled breath diagnosis using an artificial olfactory system. Biosensors 2021, 11, 337.
- Gancarz, M.; Wawrzyniak, J.; Gawrysiak-Witulska, M.; Wiącek, D.; Nawrocka, A.; Tadla, M.; Rusinek, R. Application of electronic nose with MOS sensors to prediction of rapeseed quality. Meas. J. Int. Meas. Confed. 2017, 103, 227–234.
- Gancarz, M.; Wawrzyniak, J.; Gawrysiak-Witulska, M.; Wiącek, D.; Nawrocka, A.; Rusinek, R. Electronic nose with polymer-composite sensors for monitoring fungal deterioration of stored rapeseed. Int. Agrophysics 2017, 31, 317–325.
- Konduru, T.; Rains, G.; Li, C. A Customized Metal Oxide Semiconductor-Based Gas Sensor Array for Onion Quality Evaluation: System Development and Characterization. Sensors 2015, 15, 1252–1273.
- Xu, Y.; Zhao, X.; Chen, Y.; Zhao, W. Research on a mixed gas recognition and concentration detection algorithm based on a metal oxide semiconductor olfactory system sensor array. Sensors 2018, 18, 3264.
- Tang, Y.; Xu, K.; Zhao, B.; Zhang, M.; Gong, C.; Wan, H.; Wang, Y.; Yang, Z. A novel electronic nose for the detection and classification of pesticide residue on apples. RSC Adv. 2021, 11, 20874–20883.
- Lee, C.H.; Chen, I.T.; Yang, H.C.; Chen, Y.J. An AI-powered Electronic Nose System with Fingerprint Extraction for Aroma Recognition of Coffee Beans. Micromachines 2022, 13, 1313.
- Štefániková, J.; Nagyová, V.; Hynšt, M.; Vietoris, V.; Martišová, P.; Nagyová, Ľ. Application of electronic nose for determination of Slovak cheese authentication based on aroma profile. Potravin. Slovak J. Food Sci. 2019, 13, 262–267.
- Wang, A.; Zhu, Y.; Zou, L.; Zhu, H.; Cao, R.; Zhao, G. Combination of machine learning and intelligent sensors in real-time quality control of alcoholic beverages. Food Sci. Technol. 2022, 42, e54622.
- Romani, S.; Rodriguez-Estrada, M.T. Chapter 5—Bakery Products and Electronic Nose. In Electronic Noses and Tongues in Food Science; Rodríguez Méndez, M.L., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 39–47. ISBN 978-0-12-800243-8.
- Yang, Y.; Xu, W.; Wu, M.; Mao, J.; Sha, R. Application of E-nose combined with ANN modelling for qualitative and quantitative analysis of benzoic acid in cola-type beverages. J. Food Meas. Charact. 2021, 15, 5131–5138.
- Matindoust, S.; Baghaei-Nejad, M.; Abadi, M.H.S.; Zou, Z.; Zheng, L.R. Food quality and safety monitoring using gas sensor array in intelligent packaging. Sens. Rev. 2016, 36, 169–183.
- Shaalan, N.M.; Ahmed, F.; Saber, O.; Kumar, S. Gases in Food Production and Monitoring: Recent Advances in Target Chemiresistive Gas Sensors. Chemosensors 2022, 10, 338.
- Intergovernmental Panel on Climate Change (IPCC). 2022: Summary for Policymakers. In Climate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Shukla, P.R., Skea, J., Slade, R., Al Khourdajie, A., van Diemen, R., McCollum, D., Pathak, M., Some, S., Vyas, P., Fradera, R., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2023; ISBN 9781107415416.
- Janudin, N.; Kasim, N.A.M.; Knight, V.F.; Halim, N.A.; Noor, S.A.M.; Ong, K.K.; Yunus, W.M.Z.W.; Norrrahim, M.N.F.; Misenan, M.S.M.; Razak, M.A.I.A.; et al. Sensing Techniques on Determination of Chlorine Gas and Free Chlorine in Water. J. Sensors 2022, 2022, 1898417.
- Xiao, Y.; Li, H.; Wang, C.; Pan, S.; He, J.; Liu, A.; Wang, J.; Sun, P.; Liu, F.; Lu, G. Room Temperature Wearable Gas Sensors for Fabrication and Applications. Adv. Sens. Res. 2023, 2300035.
