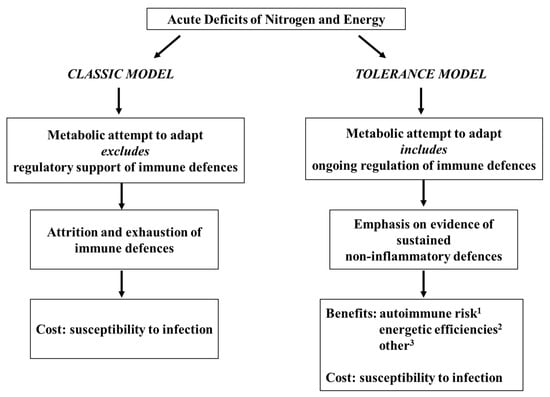
Figure 1. Classic model versus tolerance model of immune competence in acute pediatric malnutrition.
2. First Steps (1950s–1990s): Origins of a Tentative Tolerance-Centered Proposition
The core theme of the tolerance model was first proposed thirty years ago
[28][29][32,33] and remains unchanged
[1][3][30][7,9,34], viz. that the immune depression characteristic of acute pediatric malnutrition is one component, only, of a larger systemic attempt to adapt. According to the model, as first conceived, the immunological component of the adaptive attempt manifests as a remodeling of immune defences toward a non-inflammatory form of competence, and a presumptive benefit, secured at the cost of susceptibility to infection, is a reduction in the risk of inflammatory autoimmune sequelae to a catabolic challenge. Additional possible benefits have been indicated in more recent years, with some clinical and experimental support, center on the conservation of limited substrate and energy reserves in the face of the costs of inflammation. In any case, the model, notably, proposes nothing more than an adaptive attempt which, without external support, is unsustainable.
2.1. The Endocrine–Immune Nexus
Endocrine hormonal characteristics were among the early points of interest in relation to the physiology of acute pediatric malnutrition. By the mid-1970s, the proposition was widely acknowledged that at least some malnutrition-associated endocrine characteristics represent adaptive attempts (e.g.,
[31][32][33][41,42,43]), and this level of understanding quickly developed to take the form of a coordinated and systemic attempt (e.g.,
[32][34][35][36][42,44,45,46]). Importantly, the concept of neuroendocrine-mediated adaptation continues to develop in breadth and sophistication (e.g.,
[37][38][39][47,48,49]), recently extending to unanticipated and seemingly anomalous endocrine hormonal actions in support of the proffered adaptive attempt
[38][48]. In its earliest form, however, this adaptation-centered point of view took its shape from studies that invoked (without suggesting adaptive benefit) the high blood concentration of glucocorticoids, characteristic of acute malnutrition, as a contributor to the lymphoid atrophy and cell-mediated immune depression observed in this pathology. The first of these investigations was conducted using young adult rats
[40][50], but subsequent investigations of children
[41][51], weanling monkeys
[42][52] and weanling rodents
[42][43][52,53] placed attention on the prepubescent stage of life.
The cited early reports could not establish causation specific to the glucocorticoids, but they facilitated the formation of a connection, conceptually, between the endocrine and immunological components of the pathophysiology of prepubescent malnutrition in its acute forms. This point of understanding is now frequently invoked within the discipline (e.g.,
[44][54]) and is widely respected, also within the broader field relating diet and immune defence competence
[37][45][47,55]. Notably, the concept has grown to take the form of a response to nutrient quantity and balance that includes cytokines and neural mediators in addition to endocrine hormones
[45][55].
By the latter half of the decade of the 1970s, the concept connecting hormonal mediators and malnutrition-associated immune defences had achieved sufficient momentum to appear in secondary sources
[46][47][56,57], wherein endocrine candidates additional to the corticosteroids (notably epinephrine, insulin, growth hormone and the thyroid hormones) were named as possible players, albeit without experimental or other direct investigative evidence. This conceptual scaffolding, at the time more intriguing than compelling, prompted a series of investigations in which supplements of triiodothyronine were given to weanling mice subjected to either of two metabolically dissimilar forms of severe acute nitrogen and energy deficit. Regardless of the form of malnutrition, and despite advancing nutritional deficits, the hormonal intervention prevented depression in primary thymus-dependent and -independent antibody responses
[48][49][50][51][58,59,60,61] and in at least one primary cell-mediated response
[51][61], although it was ineffective against another
[49][59]. Importantly, these outcomes pertaining to function manifested in the face of unabated and profound lymphoid atrophy; in fact, in two models of extreme nitrogen deficits
[50][51][60,61], the intervention accelerated malnutrition-associated lymphoid involution. Through the lens of endocrine hormonal support, therefore, functional immune plasticity—incompatible with a model of unregulated debilitative attrition—was evident on the part of at least some adaptive defences despite advanced and ongoing catabolic malnutrition. Further investigation, therefore, could be encouraged from the standpoint of clinical prospects.
2.2. The Earliest Intervention Studies
The earliest intervention studies relevant to the tolerance model comprise a conglomerate of investigations that preceded the series outlined with respect to triiodothyronine and adaptive defences. These investigations are considered more fully elsewhere
[28][52][32,62], but seven are noted here to illustrate the diversity within the evidence base. In the first of the seven investigations, adrenalectomy prevented lymphopenia in adult rats subjected to an acute dietary nitrogen deficit
[40][50] and, twenty years later, the same surgical intervention prevented the involution of lymphoid organs when imposed on acutely protein-deficient weanling mice
[43][53]. Five studies followed shortly thereafter and, in each case, the strategy of administering bioactive compounds was adopted in favour of a surgical intervention. In the first of these investigations, acutely malnourished rabbits generated a fever response, despite ongoing severe weight loss, when administered chemically undefined pyrogens derived from animals fed a complete diet
[53][63]. Additional reports soon emerged of investigations in which rodents, despite various forms of severe acute malnutrition, exhibited immune defence capabilities when administered exogenous agents including the glucan lentinan
[54][64], a purified preparation of interleukin (IL)-1
[55][65] and a chemically ill-defined extract containing one or more thymic hormones
[56][57][66,67]. The response capabilities assessed included innate
[53][54][55][63,64,65] and adaptive
[56][57][66,67] defences as well as resistance to infectious disease challenges
[54][55][64,65].
Only two of the cited investigations
[43][54][53,64] addressed the weanling stage of life, although two other studies
[53][55][63,65] were initiated with somewhat older prepubescent animals. Nevertheless, the two studies employing surgical intervention (adrenalectomy)
[40][43][50,53] persuasively connected the endocrine and immunological consequences of acute malnutrition, although the findings were not rigorously specific to any particular hormonal candidate. Moreover, the five investigations that centered on a biochemical intervention were designed in a manner permitting discernment of immune restoration because, in each case, an intervention was initiated subsequently to the development of depression in the chosen index of inflammatory defence. Importantly, too, the immunological and disease resistance outcomes were assessed independently of any rehabilitative attempt and, in three studies of prepubescent animals
[53][54][55][63,64,65], the response manifested within twenty-four hours or less. Taken together, therefore, the findings constitute a body of evidence that immunological plasticity is sustained into the advanced stages of acute malnutrition, perhaps even during prepubescence. This was a substantive contribution toward the earliest explicit expression of the tolerance model
[28][29][52][32,33,62]. Moreover, the findings provided a segue to interventional studies using endocrine mediators, the series centered on triiodothyronine and adaptive defences being the first to follow.
2.3. An Adaptive Attempt: Tolerance of Self-Antigens Enters the Discussion
The body of early intervention studies, including evidence connecting endocrine and immunological characteristics of acute malnutrition, provided a modest basis for inferring that malnutrition-associated inflammatory immune depression is best understood as part of a systemic attempt to adapt to acute deficits of nitrogen and energy. This inference was first introduced explicitly in a presentation to the XIV International Congress of Nutrition (Seoul, Republic of Korea, 20–25 August 1989)
[52][62], then in journal format a few months thereafter
[28][32], and in a second conference setting two years later
[29][33]. In the same venues
[28][29][52][32,33,62], it was noted that a regulated adaptive attempt includes, ipso facto, an expectation of benefit. This point of reasoning led to a suggestion, prompted by considerations put forward by others regarding major physical trauma
[58][59][68,69], as to a possible benefit from the inflammatory immune depression of catabolic malnutrition
[28][29][32,33]. Physical trauma characteristically features hypercortisolemia, and the associated depression of inflammatory competence was proposed to reduce the risk of autoimmune pathologies pursuant to cortisol-mediated catabolic release of self-antigens
[58][59][68,69]. The hormonal and immunological similarities between physical trauma and acute forms of prepubescent malnutrition, therefore, formed the basis for extending the tolerance-centered conjecture regarding trauma to the setting of acute pediatric deficits of nitrogen and energy
[28][29][32,33]. The suggestion was entirely speculative. Moreover, it was acknowledged that any accruing benefits would come at the cost of susceptibility to opportunistic infections
[29][33].
3. The Tolerance Model Takes Shape (1990s–2006)
From its earliest days, the research effort regarding malnutrition-associated immune depression centered on the T cell system
[1][28][29][46][47][60][61][62][4,6,7,32,33,56,57,71]. This focus originated with, and was sustained by, reports documenting the blood lymphocyte profile, the exquisite sensitivity of the thymus (“a barometer of malnutrition”
[18][22]), the histopathology of secondary lymphoid organs and the particular vulnerability of the cell-mediated type of adaptive immune response. Therefore, the first research question to be addressed in relation to the nascent tolerance proposition
[28][29][32,33] addressed a prediction that the T cell compartment would restructure in a manner consistent with reduced inflammatory propensity.
3.1. T Cell Subset Balance Suggests Quiescence in Acute Pediatric Malnutrition
Balance in numbers between T cells and B cells and between CD4
+ and CD8
+ T cell sub-populations attracted early attention because of the type of information available from clinical studies
[46][47][56,57]. These important possibilities, however, came under challenge through studies of weanling mice that permitted invasive investigations of the total recirculating pool of lymphocytes
[63][72], the lymphocyte profile of secondary lymphoid compartments
[63][64][65][72,73,74] and a revealing comparison of the latter with the blood T cell profile
[65][74]. Consequently, beginning in the middle of the decade of the 1990s, investigations progressed to examination of subsets within the CD4
+ and CD8
+ sub-populations of T cells. Attention centered on a surface marker designated CD45RA because this protein identifies a quiescent, naïve-type subset with consequent stringent activation requirements. Thus, a series of studies using weanling mouse models revealed a particular abundance of CD45RA
+ elements within CD4
+ and CD8
+ sub-populations both in secondary lymphoid organs wherein immune responses arise
[64][66][67][68][73,75,76,77] and in the blood
[66][67][68][75,76,77]. In concordance with the model, the super-abundance of quiescent-phenotype T cells appeared to be independent of high-level antigenic challenge and, hence, to be a fundamental characteristic of the T cell system in acute malnutrition
[64][73]. Moreover, the phenomenon did not reflect a delay in immunologic ontogeny
[64][66][67][68][73,75,76,77], and a regulated adaptive purpose was further implicated by evidence that involution of the CD4
+ sub-population was confined to the effector/memory (CD45RA
−) subset
[67][76]. This series of investigations, although limited to experimental animals, provided the first intentional test of the nascent tolerance model. Importantly, a surface marker analysis of blood T cells from acutely malnourished children yielded corroborating findings soon thereafter, although interpretation was complicated somewhat by the factor of concurrent infection
[69][78].
3.2. Intervention Studies
Intervention studies have provided the most persuasive evidence that immune defence plasticity is sustained into the advanced stages of acute malnutrition, even its severe prepubescent forms. Investigations of this type were foundational to the first primitive formulation of the tolerance model hypothesis
[28][29][32,33] and, subsequently, necessary momentum leading to the formal expression of the paradigm in 2006
[2][8] was provided by eight additional investigations of interventional design.
Five investigations extended the database, revealing plasticity among innate defences. Triiodothyronine intervention prevented depression in natural killer cytotoxic activity in two mouse models of acute weanling malnutrition
[70][79] and, in concurrent work, three additional interventions centered on the macrophage
[71][72][73][80,81,82]. Thus, in an adult mouse model of severe nitrogen deficit, granulocyte-macrophage colony-stimulating factor restored the ability of macrophages to produce IL-6, superoxide and nitric oxide and, importantly, also restored resistance to a yeast infection
[71][80]. In other work, injections of macrophage colony-stimulating factor restored the development and numbers of Kupffer cells, albeit with no reported index of function, when administered to weanling mice subjected to a catabolic nitrogen deficit
[73][82]. Likewise, a glucocorticoid hormone receptor antagonist (mifepristone) eliminated depression in the capacity of peritoneal macrophages to produce superoxide and IL-6 following stimulation in vitro when administered throughout the progression of a catabolic nitrogen deficit in an adult mouse model
[72][81]. In this investigation, adrenalectomy effected the same outcome, but the interpretation of the report hinges primarily on the pharmacological specificity of the chemical intervention. This report provides probably the most forceful single piece of evidence implicating high concentrations of glucocorticoids in relation to the immunological characteristics of acute forms of malnutrition. Finally, in a fifth investigation, a probiotic intervention concurrent with severe dietary restriction invigorated the intraperitoneal influx of neutrophils on the part of young adult mice in response to a sterile irritant
[74][83]. The response to the intervention also manifested in terms of the production of several cytokines, viz. IL-6, IL-10 and macrophage inflammatory protein-2, by cells recovered from peritoneal exudates. Interpretation of this interesting study is hampered by the absence of a positive control; nevertheless, immune plasticity was evident in an advanced stage of ongoing catabolic malnutrition.
During this interval of time, a research front centered on leptin further extended the list of effective intervention mediators. Injections of leptin during a two-day starvation period enabled adult mice to generate a vigorous cell-mediated response, which was almost undetectable in the absence of the hormonal support
[16][20]. Soon thereafter, leptin interventions, using the same experimental system, were reported to attenuate splenic and thymic lymphoid involution
[17][21] and to sustain a balanced response to bacterial endotoxin on the part of the inflammatory cytokines, tumour necrosis factor (TNF)-α and interferon (INF)-γ
[19][23]. The relevance of this type of short-term, adult starvation model to prepubescent forms of acute malnutrition is unclear. Broadly, however, the findings contributed to the growing evidence base pointing to persistent endocrine hormonal control over immune defence capacities despite severe, catabolic malnutrition.
Collectively, the eight cited reports afforded a new breadth of support to the proposition that immune defences, both innate and adaptive, retain responsiveness to physiological mediators in advanced stages of acute malnutrition—perhaps even during prepubescence
[70][73][79,82]. Particularly forceful evidence of sustained immunological adaptability derives from the two studies
[71][73][80,82] in which intervention was initiated at an advanced stage of malnutrition and the response was achieved in the face of unabated weight loss. Further, one of the latter two studies addressed the weanling stage of life
[73][82] and, yet, the malnourished animals were able to respond to intervention within a matter of hours.
3.3. Cytokine Production and Blood Concentration Profiles: The Tolerance Model Formalized
By the beginning of the new century, an evidence base was established pointing to an emphasis on T cell quiescence in association with acute pediatric deficits of nitrogen and energy. In turn, this small body of information directed attention to the network of cytokines that determine T cell characteristics and the global disposition of the T cell system.
A small body of experimental and clinical evidence produced in the decade of the 1990s
[71][75][76][77][78][80,84,85,86,87] prompted a tentative suggestion to the 45th Nestle Nutrition Workshop (Bangkok, Thailand, 29 March–1 April 1999), that research should be directed toward the possibility that acute forms of malnutrition alter “the balance between Th1- and Th2-type cytokines”
[61][6]. Shortly thereafter, information relevant to this proposition was provided by a piece of work in which T cell mitogen-stimulated blood mononuclear cells of moderately wasted infants (weight-for-height z score, −1.3) produced more IL-4 and less INF-γ when first admitted for clinical care than following stabilization
[79][88]. Although T cell counts were not included in the report, this minor omission was tempered by the assessment of complementary cytokines. Within three years, a second study of a cytokine panel conferred a modicum of additional momentum to the proposition regarding T cell cytokine balance. In this investigation, an elevated expression of type 2 cytokines (IL-4 and IL-10) was reported together with diminished expression of inflammatory type 1 mediators (IL-2 and INF-γ) by T cells from blood samples of acutely malnourished children
[80][89]. The index applied in this instance was the percentage of T cells exhibiting intracellular cytokine expression. Notably, this report addressed constitutive cytokine expression, arguably revealing a baseline characteristic of the T cell compartment. By contrast, preceding investigations
[71][75][78][79][80,84,87,88] centered on T cell cytokine production elicited by either antigens or mitogens. Studies of humans are of obvious importance but are limited to the blood compartment, which may be unrepresentative of the sites wherein immune responses arise
[81][90]. It is worthy of note, therefore, that the findings pertaining to the cytokine profile of human blood T cells
[79][80][88,89] are consistent with preceding animal-based reports
[71][75][78][80,84,87] pertaining to T cells from secondary lymphoid organs.
Although easily overinterpreted, blood cytokine concentrations represent spillovers from extravascular sites and can be viewed as reflecting, while not representing, concentrations at extravascular sites of action
[82][91]. Arguably, therefore, assessment of blood cytokine concentrations provides a more broadly based view of systemic immunological character than can be gleaned from studies confined to cytokine-producing elements. Two reports pertaining to blood cytokine concentrations
[76][83][85,92] were particularly influential, leading to the suggestion
[61][6] that acute malnutrition tilts the balance of cytokines in a non-inflammatory direction. In the first place, elevated concentrations of IL-4 were found in blood samples taken from acutely malnourished children in a study that appeared to eliminate the confounder of nematode worm infestation
[76][85]. Subsequently, high blood levels of transforming growth factor (TGF)-β, a potent anti-inflammatory and tolerizing cytokine, were reported in acutely malnourished prepubescent guinea pigs following exposure to live tuberculosis organisms and challenge with tuberculin
[83][92].
As the body of information grew, the proposition was expanded to include cytokine-producing elements other than T cells and was refined to place emphasis on the potently anti-inflammatory and tolerogenic cytokine, IL-10
[84][93]. The evidence leading to this conceptual development included reports regarding macrophage cytokine production in vitro
[71][74][80,83], blood concentrations of IL-10 (at that time limited to conference presentations) and blood concentrations of macrophage-derived inflammatory cytokines
[19][23]. Reports pertaining to blood levels of IgE, characteristically high in acutely malnourished children
[47][57], also were influential. In particular, although IgE is a quintessential Th2 class of antibody
[85][94], its high blood levels emerged as independent of nematode worm infestation both in a cohort of acutely malnourished children
[76][85] and in two forms of acute weanling rodent malnutrition
[84][93]. Hence, a fundamental Th2-type disposition may be inferred. Notably, high blood levels of IgE did not reflect a mature, specific immune defence capability
[76][85]—a most important point emphasized again more recently
[86][95]. The proposition of type 2 and anti-inflammatory cytokine partiality in the context of acute pediatric malnutrition
[61][84][6,93] neither includes nor requires any suggestion of corresponding anti-infectious defence competence.
Blood cytokine levels are particularly informative when either complementary or opposing cytokines are considered together. This reasoning gave rise to an investigation of the blood concentrations of IL-10 and TGF-β in weanling mice subjected to acute forms of nitrogen and energy deficit
[2][8]. These cytokines were selected as the dominant cytokine mediators of peripheral tolerance, and their blood bioactivities were high in two metabolically distinct forms of acute malnutrition
[2][8]. Importantly, this outcome was not attributable simply to a delay in ontogeny that might attend a wasting disease at the weanling stage of life. Notably, also, the β1 isoform—the prepotent, perhaps exclusive, immunoregulatory member of the TGF-β family
[87][96]—accounted substantially for the 20-fold elevation in blood TGF-β bioactivity which was found in both forms of acute weanling malnutrition
[2][8].
Prompted by the perception that the blood cytokine profile reflects a systemic immunological character, the “tolerance model” was articulated in 2006
[2][8] to accommodate the growing body of reports collectively suggesting that immunologic adaptability and a predominantly non-inflammatory form of immune competence are sustained into advanced stages of acute prepubescent malnutrition. This synthesis was strengthened by the recognition that glucocorticoid hormones, well known to be found at high levels in the blood of acutely malnourished children and prepubescent animals
[32][33][34][35][36][41][42][43][88][42,43,44,45,46,51,52,53,97], would combine with IL-10 and TGF-β to comprise a potent triad of tolerogenic mediators
[2][8]. Of some note, high blood concentrations of the three mediators manifested together in advanced stages of acute malnutrition in each of two metabolically dissimilar weanling mouse models
[2][88][8,97], and this finding played a significant role in pointing to a probable functional unit of blood tolerogens.
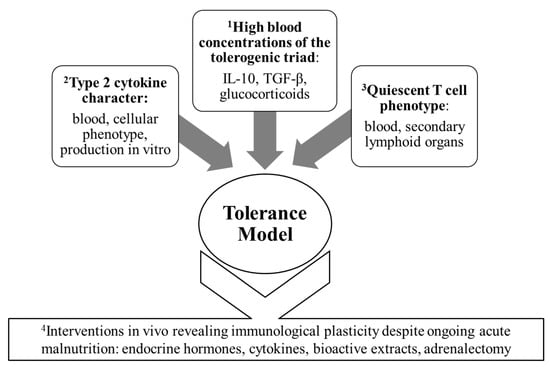
Figure 2 illustrates the distinct importance of the studies of interventional design while highlighting the four main threads of evidence that, collectively, prompted the formal expression of the tolerance model.
Figure 2. The four main clusters of immunological evidence leading to the formal articulation of the tolerance model.
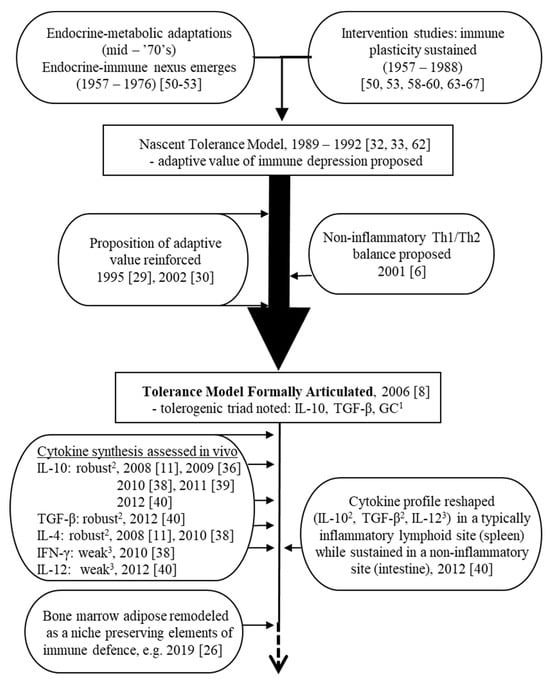
4. Ongoing Development of an Evidence Base Consistent with the Tolerance Model (2007 to the Present)
Several lines of evidence can be cited in the development of the platform for the tolerance model following its articulation in 2006. Broadly, these threads can be categorized as either primarily immunological in nature (i.e., centered on the cytokine profile or on the responsiveness of immune defences to intervention attempts), or primarily metabolic in nature. These ongoing research fronts serve both to uphold the model and to highlight potential growth points. A timeline is shown in Figure 3 highlighting milestones in the development of the tolerance model before and after its formal expression in 2006.
Figure 3. Timeline showing critical milestones in the continuing development of the tolerance model.
1 GC: glucocorticoid hormones;
2 production by malnourished either not different from, or greater than, well-nourished;
3 production by malnourished less than well-nourished.
[7][22][25][26][28][29][40][41][42][43][48][49][50][52][53][54][55][56][57][61][89][90][91][92][6,11,26,29,30,32,33,36,38,39,40,50,51,52,53,58,59,60,62,63,64,65,66,67].
4.1. Blood Cytokine Concentrations
The reports regarding constitutive blood cytokine concentrations that prompted the articulation of the tolerance model proposition focused on type 2 and anti-inflammatory mediators to the exclusion of type 1 cytokines. An early direct test of the formalized proposal, therefore, centered on INF-γ
[93][35]. In this piece of work, low constitutive blood bioactivities of INF-γ were found in both forms of acute weanling malnutrition that had been reported, in the previous year
[2][8], to elicit high bioactivities of IL-10 and TGF-β. Moreover, as reported in relation to these non-inflammatory, tolerizing cytokines
[2][8], the outcome pertaining to INF-γ was not attributable simply to a delay in ontogeny. Importantly, this animal-based investigation was soon corroborated and extended by reports pertaining to constitutive cytokine concentrations in the blood of acutely malnourished children, viz. low concentrations of INF-γ
[94][98], IL-2
[94][95][98,99] and IL-12
[8][94][12,98] coupled either with high concentrations of IL-10
[95][99] or, at least, with levels of this cytokine that were apparently unaffected despite catabolic malnutrition
[8][12].
In the meantime, young adult mice subjected to a wasting dietary nitrogen deficit were reported to exhibit low blood type 1 cytokine immunoactivities
[27][31] but high blood IL-10 immunoactivity
[7][11] in response to an inflammatory stimulus. A model of adult malnutrition may be of uncertain relevance to the prepubescent stage of life, but the consonant responses in terms of types 1 and 2 cytokines are noteworthy. Moreover, these findings could be interpreted as evidence pertaining to systemic production and/or turnover. In addition, one of the reports
[7][11] included the important interpretation that the finding in relation to IL-10 might reflect “an initial adaptation” and could represent “a mechanism to control the inflammatory response in…malnourishment.” Coming only two years after the formalization of the tolerance model
[2][8], this provided important independent support for a core aspect of the model, viz. an adaptive attempt leading to a pre-determined benefit.
During the same interval of years, additional studies
[89][96][36,37] confirmed high blood bioactivities of IL-10 and TGF-β in the advanced stages of acute weanling malnutrition and also provided evidence that the blood levels of these tolerogenic cytokines rose early in the progression of weight loss. These findings dovetailed with a similar outcome pertaining to the blood concentration of corticosterone in the same weanling animal systems
[88][97]. In view of the possible biphasic influence of glucocorticoids on inflammation, stimulatory at low concentrations and inhibitory at high levels
[97][100], the reported critical illness level of elevation in corticosterone concentration
[88][97] assumes particular significance. Of some note, the magnitude of the response, which remains worthy of research attention, came to light because of the availability and application of an exsanguination technique
[98][101] that minimizes the impact of pre-anesthesia stress. In sum, IL-10, TGF-β and the glucocorticoid hormones—previously submitted as a triadic unit upholding adaptive attempts during advanced stages of acute malnutrition (
[2][8])—emerged in position, also, to initiate the tolerogenic and non-inflammatory immunological character central to the tolerance model
[30][91][34,39].
The foregoing notwithstanding, blood concentrations of two inflammatory cytokines in particular, TNF-α and IL-6, have been reported over a span of many years to be either elevated or unaffected in cohorts of acutely malnourished children (e.g.,
[8][95][99][100][101][12,99,102,103,104]). Corroborating reports with respect to TNF-α
[83][92] and both TNF-α and IL-6
[102][105] in the blood of acutely nitrogen-deficient weanling rodents also must be noted. Endotoxemia, a common feature of acute pediatric malnutrition
[47][57], could be a factor in relation to these reports, having been found to trigger an exaggerated TNF-α response when imposed on an adult rodent starvation model
[19][23]. More than this, reports of robust blood concentrations of TGF-β
[83][92] and IL-10
[8][95][101][12,99,104] found together with high levels of IL-6 or TNF-α highlight a need to assess functional balance among cytokines, and to do so in a manner cognizant of the diversity of anti-inflammatory influences exerted by the tolerogenic cytokines (illustrated, for example, by the constellation of direct and indirect mechanisms coming to light with respect to IL-10
[103][106]).
It is also important to note that knowledge of the biological roles of IL-6 and TNF-α continues to grow in sophistication. Although generally considered invariantly inflammatory, these cytokines may be best understood as pleiotropic. For example, the IL-6–hepcidin axis promotes hypoferremia, a non-inflammatory defence against extracellular pathogens
[104][107], and this response is reportedly intact in adult rodents subjected to weight loss through caloric deficit
[105][108]. In addition, the cellular source of IL-6 is reported to determine whether the ensuing response (at least in adipose tissue) is inflammatory or non-inflammatory
[106][109]. Similarly, in rodent models, TNF-α appears to constrain type 1-based inflammatory over-reaction to facultative intracellular parasites
[107][108][110,111], perhaps in part by supporting the formation and maintenance of stable granulomas
[108][111]. TNF-α also is reported to promote the expansion and stability of regulatory T cell populations
[109][112], although this information is limited to tumour models, so its relevance to the pathogenesis of infectious disease remains to be determined. Clearly, TNF-α and IL-6 merit attention in the context of the tolerance model.
A In Section 6.4, herein, a larger related point is addressed regarding the retention of inflammatory capabilities in the face of acute prepubescent malnutrition.
4.2. The Cytokine Signature of Mononuclear Cells from the Blood and Lymphoid Organs
An important research front, centered on blood mononuclear cells of acutely malnourished children, continued to grow through the efforts of a group based in Mexico City
[94][110][111][98,113,114]. First, using the index of intracellular type 1 (IL-2, IL-18, IL-21 and INF-γ) and type 2 (IL-4 and IL-10) cytokine expression, the group showed that both CD4
+ and CD8
+ blood T cell populations from malnourished children exhibited type 2 cytokine polarization
[94][110][98,113]. In addition, the group reported corroborating results regarding the expression of mRNA for type 1 and type 2 cytokines on the part of the full blood mononuclear cell compartment
[94][111][98,114], and also found a reduced percentage of CD14
+ blood mononuclear cells expressing intracellular IL-12
[94][98]. Other research teams also reported a low percentage of T cells expressing IL-2
[95][99] and INF-γ
[8][95][12,99] in blood samples from acutely malnourished children. Further, one group extended the database to include the blood dendritic cell compartment which exhibited (on admission vs. following recovery) diminished constitutive production of IL-12 in vitro together with high-level constitutive production of IL-10
[8][12].
During the same period of years, two studies of young adult rodents subjected to an acute dietary nitrogen deficit yielded broadly similar findings
[7][9][11,13]. The outcome measures included the expression of several cytokines and two inflammation-associated transcription factors by mononuclear cells from diverse lymphoid organs. In particular, the deficient animals mounted an increased IL-10 response (assessed by blood cytokine concentration) following inflammatory stimulation in vivo
[7][11], and corroborating outcomes were reported on the part of splenic T cells
[9][13] and LPS-responsive mononuclear cells from the spleen
[9][13] and bone marrow
[7][11] when stimulated in vitro. Although malnutrition-associated influences were few and relevance to prepubescent malnutrition is uncertain, the findings appeared, collectively, to reflect a non-inflammatory immune character within both peripheral and primary lymphoid sites. In a concurrent investigation, splenic and nodal T cells from fasted adult mice exhibited marked depression in the production of IL-2 and INF-γ following stimulation in vitro
[21][25], although interpretation for the present purpose is compromised by the absence of data pertaining to non-inflammatory or tolerogenic cytokines.
The growing database pertaining to cytokine production and blood concentration profiles prompted an investigation to test and refine the tolerance model according to the prediction that acute prepubescent deficits of nitrogen and energy will elicit a shift toward a non-inflammatory identity within characteristically inflammatory lymphoid sites while sustaining the disposition of non-inflammatory sites
[92][40]. Using two weanling models, this investigation addressed the expression of mRNA for IL-10, TGF-β and IL-12 in the spleen, a typically inflammatory lymphoid organ, and in the small intestine, which is characteristically non-inflammatory. These models had been reported previously to exhibit non-inflammatory, tolerogenic blood cytokine profiles
[2][93][8,35], a systemic predisposition toward type 2 cytokine production
[89][90][91][36,38,39] and a type 2 effector/memory T cell character
[90][38], and the outcome of the investigation suggested that the immunological re-shaping associated with both weanling systems centers on typically inflammatory sites. In addition, the findings added TGF-β and IL-12 to the pre-existing list of cytokines (IL-10
[7][89][90][91][11,36,38,39], IL-4
[90][38] and INF-γ
[90][38]) for which evidence consistent with the tolerance model was available on the basis of information pertaining to synthesis assessed in vivo. Clearly, the outcome of the investigation refined and extended the tolerance model in a manner indicative of a controlled, systemic attempt to achieve a non-inflammatory and tolerogenic immune character. A precautionary caveat should be emphasized, however, that sustaining the non-inflammatory character of mucosal immune sites must not be overinterpreted as evidence that mature mucosal adaptive defences remain intact.
4.3. Cytokine Assay Techniques Key to the Development of the Tolerance Model
Two laboratory technologies, each applied to weanling rodents, are noteworthy at this juncture. In the first place, the application of bioassays rather than the understandably popular ELISA appears to have conferred an important benefit in terms of consistent clarity in the estimation of constitutive blood cytokine concentrations
[2][89][93][96][8,35,36,37]. Although seldom openly considered, potentially misleading limitations of the ELISA are a matter of record in relation to the assessment of cytokine concentrations in biological fluids
[112][113][114][115][115,116,117,118]. The ELISA provides estimates restricted to the unbound fraction of cytokine without reference to biological activity, and it appears that this type of assay detects only a small and unrepresentative fraction of the total quantity of biologically active cytokine accessible by bioassay. In particular, direct comparisons with the ELISA point persuasively to the bioassay as more reliable, if validated in terms of specificity, for assessing blood concentrations of IL-10
[114][117] and TGF-β
[115][118], the tolerogenic cytokines at the heart of the tolerance model
[2][8].
The second key assay technology, the in vivo cytokine capture assay
[116][119], provided information directly related to the rate of cytokine production by prepubescent animals during their response to acute forms of malnutrition. The assay revealed robust net systemic production of IL-10, constitutively, even in advanced stages of malnutrition
[89][91][36,39] and, importantly, permitted the conclusion that the high blood concentrations of this tolerogenic cytokine were not a consequence of a reduced rate of turnover (catabolism). Likewise, the same outcome emerged regarding the production of IL-4 by effector/memory T cells, whereas net production of INF-γ by this cellular compartment declined simultaneously
[90][38]—a comparison between types 1 and 2 cytokines rendered particularly interesting by an assay that permits a view of synthesis, in vivo, largely unimpeded by interference from catabolism. Thus, the in vivo capture assay provided unique direct evidence that, despite a profoundly limited amino acid supply, a non-inflammatory immune character is initiated early in the development of acute prepubescent nitrogen and energy deficits
[89][36] and is sustained into its advanced stages
[90][91][38,39]. Such an outcome is predicted by the tolerance model but cannot fit within a paradigm of unregulated immunological attrition.
Enlargement of the aforementioned database is needed both to increase the diversity of experimental systems and to expand the array of tested cytokines. In this context, however, it is relevant to note that the cited information was derived from the application of the bioassay and cytokine capture techniques to two longstanding and validated animal models. The particular merit of the models stems from their demonstrated relevance to severe forms of acute pediatric malnutrition and, in addition, from the database built up over three decades of investigation regarding their immunological characteristics. Although the critical physical and metabolic (particularly hormonal) characteristics of the two animal models have not been summarized in a single, consolidated source, they can be found recorded within the body of research based on the models, and some of these reports are cited herein
[2][3][48][49][50][51][63][64][65][66][67][68][70][88][89][90][91][92][93][96][117][8,9,35,36,37,38,39,40,58,59,60,61,72,73,74,75,76,77,79,97,120] because of their particular significance to the development of the tolerance model. A broader treatment of animal modeling for research in pediatric malnutrition
[1][7] also identifies important validating features of the two models. The use of validated and substantively characterized models enhanced the confidence with which the findings yielded by the bioassay and cytokine capture techniques could be interpreted.
4.4. Intervention Studies
Studies reporting responsiveness to endocrine hormones, cytokines and other mediators or mediator-containing fluids constituted an important part of the information base leading to the formal declaration of the model in 2006
[2][8]. Six subsequent intervention studies added to the supportive database, five reporting administration of a hormone or cytokine
[3][21][110][118][119][9,25,113,121,122] and one reporting an adoptive cell transfer strategy
[117][120].
Leptin, having featured in studies leading to the formalization of the model, continued to be of interest. T cells taken from the blood of acutely malnourished children responded to this hormone in vitro with an increase, evident both constitutively and following mitogen stimulation, in the percentage of cells expressing type 1 cytokines (IL-2 and INF-γ) and a corresponding decrease in the percentage of IL-4- and IL-10-positive elements
[110][113]. The in vitro intervention also restored the capacity of the T cells to express activation markers in response to a mitogenic stimulus. Despite the complication of concurrent infection and the limitations of an in vitro intervention strategy, the investigation stands alone as a chemically defined intervention pertaining to a pediatric population. Subsequently, leptin intervention studies were extended to demonstrate responsiveness on the part of the B cell system in the context of an adult rodent fasting model
[118][121]. In this investigation, administration of leptin either peripherally or centrally (intracerebroventricular) prevented starvation-induced attenuation in B cell development within the bone marrow, and further pharmacological interventions suggested a chain of events mediated through the central nervous system and the hypothalamic–pituitary–adrenal axis. Shortly thereafter, a report based on the same adult rodent model revealed that leptin intervention, either in vitro or in vivo, reinvigorated the production of IL-2 and INF-γ by splenic and nodal effector T cells generated in vitro
[21][25]. Although extrapolation to acute forms of prepubescent malnutrition is hazardous, these findings extended evidence of immunological responsiveness in the face of catabolic malnutrition to include the B cell system and central neuroendocrine regulation
[118][121], and suggested a direct influence on the glucose uptake of activated T cells, and, hence, ongoing responsiveness to “metabolic reprogramming”
[21][25].
Two additional interventions each probed responsiveness to a previously untested cytokine. In the first investigation
[119][122], granulocyte colony-stimulating factor elicited a substantial recovery of the blood neutrophil count of adult mice subjected to acute deficits of nitrogen and energy, although the increase in cell numbers was attenuated relative to the response generated by animals fed a complete diet ad libitum. In this piece of work, the intervention was initiated subsequent to the establishment of advanced catabolic malnutrition and evidence of depressed inflammatory competence; therefore, the findings pointed forcefully to the maintenance of immunological plasticity despite advanced wasting disease, albeit in adulthood. The second investigation centered on acutely malnourished weanling mice given exogenous fms-like tyrosine kinase 3 ligand
[3][9], a cytokine both essential and sufficient for the maintenance of dendritic cell compartments
[120][123]. In this piece of work, the intervention prevented depression in the numbers of conventional splenic dendritic cells (CD11c
+ F4/80
−/low) despite profound splenic lymphoid atrophy. Moreover, regardless of deepening catabolic disease, the exogenous cytokine supported a vigorous spleen-based cell-mediated adaptive immune response that was, otherwise, profoundly depressed. This report extended a previous piece of work using the same weanling model
[117][120] in which intervention by adoptive transfer of murine dendritic cells at an advanced stage of malnutrition restored the same spleen-based cell-mediated immune response despite ongoing and deepening catabolic disease.