Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Ziyad S. Haidar and Version 2 by Camila Xu.
Nanobubbles also known as ultra-fine bubbles or sub-micron bubbles, are extremely small gas-filled spherical bodies enclosed by an interface (gas–liquid or gas–solid) with a diameter of less than 1000 nm (i.e., ranging in size from tens to hundreds of nanometers in diameter). In other words, nanobubbles are composed of gas, such as oxygen or nitrogen, encapsulated within a liquid, typically water, and stabilized by a thin layer of molecules at the gas–liquid interface.
- oxygen
- nanobubbles
- hypoxia
- mitochondria
- metabolism
1. Introduction
Hypoxia is a medical condition characterized by an inadequate/insufficient oxygen supply to tissues and organs, leading to potential harm or dysfunction. It results from various pathological phenomena, including diminished cardiac output, reduced hemoglobin concentration, and an atypical vasculature network. Currently, there is a widely acknowledged consensus that restricted oxygen availability jeopardizes not only cell viability but also cellular phenotype and functionality [1]. Furthermore, inadequate oxygen supply (hypoxia: different levels of oxygen deprivation, from mild to severe; hypoxic stress: in cellular or molecular biology research, this specifically refers to a situation where cells or tissues are exposed to lower-than-optimal oxygen levels, causing them to adapt or respond to this oxygen deficiency, where they may activate specific molecular pathways, like the hypoxia-inducible factor or HiF pathway to adapt to the low oxygen environment) may disturb numerous biological processes, particularly those requiring high energy demands, such as tissue repair, restoration, and regeneration. Upon establishing hypoxia, it could lead to a grave metabolic crisis, especially in individuals with metabolic syndromes, cardiovascular diseases, acute traumas, chronic diseases, and cancer, to list a few [2][3][4][5][2,3,4,5].
Today, it is widely recognized that exposing human blood to free oxygen gas bubbles causes hemolysis [6]. Nonetheless, various approaches have been employed to increase oxygen levels and overcome hypoxia, including the utilization of oxygen microparticles, hydrogen peroxide solutions, hyperbaric oxygenation, and inhalation of gas mixtures such as carbogen (composed of 5% CO2 and 95% O2) [7]. However, they are considered ineffective due to clinical and logistical limitations. These approaches include limited gas solubility, cytotoxicity, instability, low gas pay-load, ill-defined gas-release profiles, and low cellular uptake, rendering them unsuitable for gas delivery. Consequently, an imminent need for novel methodologies and strategies arises that can offer and/or provide oxygen to tissues and organs in a manner that is both safe/biocompatible as well as cost-effective whilst being highly biodegradable and easily deployable or user-friendly too [7].
Herein, nanobubbles, also known as ultra-fine bubbles or sub-micron bubbles, are extremely small gas-filled spherical bodies enclosed by an interface (gas–liquid or gas–solid) with a diameter of less than 1000 nm (i.e., ranging in size from tens to hundreds of nanometers in diameter) [8][9][8,9]. In other words, nanobubbles are composed of gas, such as oxygen or nitrogen, encapsulated within a liquid, typically water, and stabilized by a thin layer of molecules at the gas–liquid interface. Nanobubbles have gained significant attention in various fields, including medicine, materials science, and environmental science due to their unique properties and potential applications. Indeed, several remarkable properties of these nanobubbles have been widely studied, such as their long-term stability and longevity [10][11][12][10,11,12], enhanced dissolution of gases, and increased gas interfacial diffusion [13][14][13,14]. In addition, nanobubbles can be biocompatible and have demonstrated interesting potential as suitable candidates for drug delivery purposes (drugs, genes, or other therapeutic agents, with improved release and delivery to specific tissues or cells) [7][15][7,15]. Given that an optimal oxygen-release material must demonstrate compatibility with biological systems, strategies for oxygen delivery, such as nanobubbles, have garnered substantial attention as robust mechanisms to alleviate the hypoxic stress response(s) [16][17][16,17], which is an exciting and growing area of research, development, and innovation in various scientific, medical, and industrial fields, in laboratories World-wide.
Notably, during the past decade, there has been a remarkable increase in scientific (and technological) interest and a surge in publications in this emerging field (Figure 1), particularly concerning oxygen nanobubbles (OnB) and OnB-mediated oxygenation.
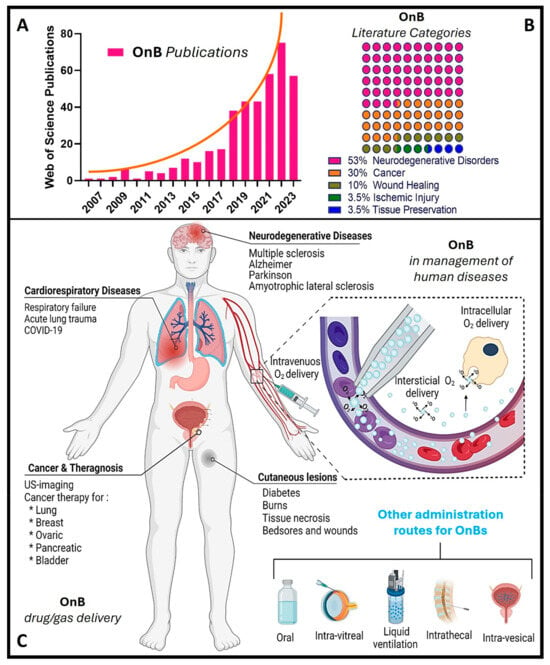 Nonetheless, it is widely accepted that metabolism and oxygen levels act as intricately interlinked factors that determine the physiological behavior of cells, the onset of pathologies, and the progression of chronic diseases. Among the advantages of developing oxygenation systems, selective metabolic reprogramming can target (1) tumor suppression in cancer by disrupting their ability to adapt to low oxygen conditions [18], (2) enhanced wound healing by stimulating angiogenesis and oxygen utilization in affected tissues [19], and (3) restoration of mitochondrial function in neurodegenerative diseases through promoting the alternative energy production pathways and reducing oxidative stress, i.e., most likely slowing disease progression and enhancing neuronal survival [20].
Nonetheless, it is widely accepted that metabolism and oxygen levels act as intricately interlinked factors that determine the physiological behavior of cells, the onset of pathologies, and the progression of chronic diseases. Among the advantages of developing oxygenation systems, selective metabolic reprogramming can target (1) tumor suppression in cancer by disrupting their ability to adapt to low oxygen conditions [18], (2) enhanced wound healing by stimulating angiogenesis and oxygen utilization in affected tissues [19], and (3) restoration of mitochondrial function in neurodegenerative diseases through promoting the alternative energy production pathways and reducing oxidative stress, i.e., most likely slowing disease progression and enhancing neuronal survival [20].

Figure 1. OnB (oxygen nanobubbles) and biomedicine research. (A) The number of articles published each year retrieved through a scientific search engine (Web of Science) using the term “oxygen nanobubble” followed by filtration/classification for application(s) in biomedicine. (B) The percentage of OnB articles published categorized by indication. (C) Illustration of drug/gas delivery based on OnB and their commonly investigated administration route(s) for the management of disease(s).
2. Hypoxia and Mitochondrial Dysfunction: Interplay and Implications in Pathology(ies)
The interplay between hypoxia (oxygen deficiency) and mitochondrial dysfunction (perturbing the cellular energy-producing organelles) is pivotal in the development and progression of various human diseases. These intertwined processes, once considered distinct, have emerged as key players in conditions spanning cancer, cardiovascular diseases, neuro-degenerative disorders, and more. Hypoxia disrupts the balance of oxygen supply to tissues, triggering a cascade of cellular responses. Simultaneously, mitochondrial dysfunction, characterized by impaired energy production and oxidative stress, contributes to cellular damage and dysfunction. The dynamic interaction between these two phenomena amplifies disease mechanisms and influences treatment outcomes. The normal partial pressure of oxygen (PaO2) in arterial blood typically falls within the range of 75 to 100 mm of mercury (mmHg) when measured at sea level and standard atmospheric pressure, exhibiting variability across various tissues. Normal tissue oxygen levels can range from 0 to around 60 mmHg, but these values can fluctuate significantly depending on the specific tissue type and its physiological conditions. Measuring PaO2 directly within cells can be challenging and typically requires specialized techniques, such as microelectrode oxygen sensors or imaging methods. These measurements are valuable for understanding tissue oxygenation in various physiological and pathological conditions and can guide treatment strategies in clinical settings. In most cases, the physiological range spans from 14 to 65 mmHg, corresponding to atmospheric oxygen levels of 2–9% [21][24]. Distinct PaO2 gradients are also evident across both superficial and deep cellular layers, displaying diverse characteristics from vascular lumen to perivascular tissues (Figure 2A) [21][22][24,25]. On the other hand, cellular hypoxia occurs when the oxygen level is in the range of 10–16 mmHg (1–2%), and it depends on the type of tissue [23][26]. Once the demand for oxygen exceeds its supply, the oxygen level decreases (herein known as hypoxia) in local tissues and/or peripherical tissues, leading to a metabolic crisis [24][25][27,28] (Figure 2B,C).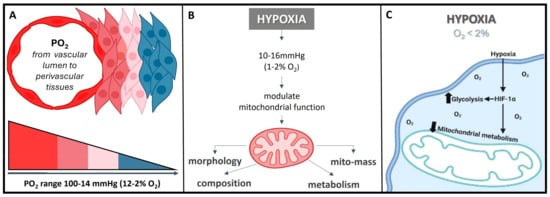
Figure 2. O2 need depends on cell niche amongst other demands. (A) Sites that are distant from major blood vessels are usually kept at a lower O2 level. However, low PO2 might still be higher than the PO2 of truly hypoxic conditions <16 mmHg (<2% O2). (B) Hypoxia acts on mitochondrial function, affecting the protein composition of the electron transport chain, mitochondrial morphology, and mitochondrial mass. (C) Hypoxia, besides playing a crucial role in influencing the cellular signaling pathways, gene expression, and the overall microenvironment, it regulates metabolic adaptation, promotes glycolysis, and decreases mitochondrial respiration, including HiF stabilization.
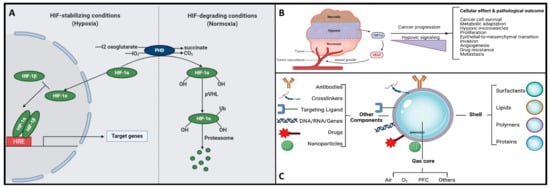
Figure 3. (A) The functional interplay of the HiF pathway in response to different oxygen concentrations. Herein, under hypoxic conditions, PHD is inhibited, and HiF-α is stabilized, which then translocates to the nucleus, dimerizes with its corresponding β-sub-unit, and recognizes the HREs motifs to induce the transcription of several target genes. On the other hand, when under normoxia, HiF1α-subunit prolyl residues are continuously hydroxylated by PHD. Following hydroxylation, pVHL binds and targets the HiF-1 α-subunit for ubiquitination and proteasomal degradation. (B) Hypoxic signaling and HiF-1α over-expression: a key hallmark in cancer progression. (C) Schematic representation of the structure and composition of engineering shelled micro/nanobubble systems.
3. Nanobubbles in Biomedicine: Bridging Basic Fundamentals to Practical Application(s)
Nanobubbles are minuscule gas-filled spherical entities enclosed by an interface (gas–liquid or gas–solid) with diameters typically in the nanometer range, spanning from tens to hundreds of nanometers [8][9][8,9]. They represent a distinct class of bubbles, significantly smaller than conventional microbubbles, and are characterized by their unique properties at the nanoscale. In the realm of nanotechnology, nanobubbles have recently garnered considerable attention, mainly attributed to their exceptional stability and longevity. This remarkable stability arises from the presence of a thin molecular layer at the gas–liquid interface, which effectively confines the gas within the liquid medium. As a result, nanobubbles exhibit prolonged lifetimes, making them pertinent for biomedicine in various scientific and technological applications, including drug delivery [41][42][41,42], gene delivery [43], cancer immunotherapy and chemotherapy [34][35][34,35], wound healing, and tissue regeneration, through the intravenous delivery of oxygen carriers such as OnB [44]. Furthermore, nanobubbles have displayed proficiency in surface modification and cleaning at the nanoscale. Their presence can facilitate the detachment of nanosized contaminants or particles from solid surfaces, offering potential applications in surface engineering, nanofabrication, and precision cleaning processes. In the field of medical imaging, nanobubbles serve as contrast agents for advanced ultrasound imaging techniques. Their nanoscale dimensions allow for improved resolution and specificity in visualizing biological tissues, vasculature, and cellular structures. Beyond the biomedical realm, it is perhaps worth mentioning to the interested reader that nanobubbles have promising environmental applications, including groundwater remediation and wastewater treatment, where their ability to facilitate gas transfer and enhance chemical reactions is leveraged to address various environmental challenges. Henceforth, the multi-faceted properties and applications of nanobubbles will continue to be further explored, fueling ongoing research endeavors and innovation across diverse scientific and technological disciplines [41][42][43][44][41,42,43,44]. Various techniques enable the synthesis of nanobubbles, including hydrodynamic cavitation, acoustic cavitation, microfluidics, intense mechanical agitation, and electrolysis, among others. What sets nanobubbles apart from other bubbles are several defining parameters, with bubble diameter being the most critical classification criterion, as outlined by the ISO/TC 281 [45] and ISO 20480-1:2017 [46] standards for terminology and definitions in the R&D&I area of fine bubble technology(ies). The growing interest in nanobubbles across numerous fields stems not only from their expansive surface area and reactivity when compared to macrobubbles and microbubbles but also from their remarkable attributes, including a heightened gas diffusion rate, enhanced cellular uptake, and robust stability against coalescence, collapse, or rupture. These qualities enable nanobubbles to persist in liquid environments for extended periods, spanning several weeks [12][47][12,47].3.1. Bubble Size and Physico-Chemico-Mechanical Properties
As mentioned previously, the size of nanobubbles, typically falling within the range of 100 to 250 nm, serves as a fundamental parameter that imparts them with inherent and advantageous physico-chemical characteristics [12][47][12,47]. This size distribution proves highly advantageous in nanobubble preparations, as it aids in overcoming infiltration limitations and facilitates the disruption of the blood–brain barrier [48]. A noteworthy feature of nanobubbles is their ability to effectively target sites of inflammation, such as those observed in cancer. This efficacy is attributed to the pore cutoff size of the fenestrated endothelium, which ranges from 380 to 780 nm. This mechanism, known as the enhanced permeability and retention effect (EPR effect basically describes the abnormal characteristics of the blood vessels associated with pathological conditions, particularly in cancer and inflammation), represents a passive targeting strategy that capitalizes on the efficient accumulation of nanobubbles within tissues featuring elevated vascular permeability [7][15][7,15]. To simplify, in the context of EPR (leaky blood vessels and the unique properties of the TME to allow therapeutic agents to accumulate preferentially in cancerous tissue while sparing healthy tissue) and nanobubbles, passive targeting means that nanobubbles, due to their size and other properties, can effectively accumulate in areas of disease, such as tumors or inflamed tissues, without the need for active targeting mechanisms. This passive accumulation can enhance the delivery of therapeutic agents or imaging agents to the specific site of interest, contributing to the effectiveness of various medical interventions. Furthermore, their mechanical properties also contribute significantly to their unique characteristics and potential applications. One notable mechanical property is the elevated internal pressure within nanobubbles. Due to their extremely small size, nanobubbles can contain gas at significantly higher pressures compared to larger bubbles. This heightened internal pressure is a result of the Laplace overpressure, which is inversely proportional to the radius of the bubble. Despite this theoretical thermos-dynamic instability, nanobubbles often exhibit impressive stability, remaining intact for extended periods. The mechanical robustness of nanobubbles, particularly their resistance to coalescence, collapse, or rupture, is another noteworthy trait. This resilience against external forces makes them suitable for various applications where durability is crucial. Indeed, understanding the stability of nanosized bubbles can be partially elucidated through their underlying physical behavior, as elaborated below. When any bubble forms within a solution, it creates an interface defined by the surface tension (γ) [49]. The existence of nanobubbles has generated extensive discussion, partly due to the predicted Laplace overpressure associated with them, which can reach several atmospheres, theoretically rendering them thermos-dynamically unstable [49]. However, in contrast to macro- or microbubbles, nanobubbles often exhibit robust persistence characterized by reduced buoyancy, enabling them to remain intact for days, weeks, or even months [12][50][12,50]. Additionally, nanobubbles exhibit unique acoustic properties, which have implications in diagnostic imaging and therapeutic ultrasound. Their response to acoustic waves differs from that of microbubbles, opening new opportunities for innovative ultrasound-based techniques. The remarkable attributes defining nanobubbles, including their diminutive size, high surface-to-volume ratio, elevated internal pressure, prolonged stability, electrostatic charge properties, acoustic characteristics, and biocompatibility, have spurred intensive research into their potential across various biomedical applications [51]. In the realm of biomedicine, the combination of nanobubbles’ mechanical attributes, such as their size, stability, and response to external forces, along with their biocompatibility, has led to intensive research into their potential across diverse applications. These mechanical properties, along with their other afore-mentioned physico-chemico-mechanical characteristics, position nanobubbles as a promising platform for advancing various fields, from drug delivery to medical imaging.3.2. Structural Composition and Electrostatic Charge of OnB Affects Gas Core and Diffusion
The gas core of a nanobubble is the central part filled with a gas, typically oxygen or nitrogen [6]. This gas core is encapsulated by a thin layer of liquid, often water, which forms the bubble’s shell. The gas core plays a crucial role in the unique properties and applications of nanobubbles. (1) High internal pressure: Nanobubbles exhibit significantly higher internal pressure compared to their larger counterparts, such as microbubbles. This increased pressure is a consequence of the Laplace overpressure, which is inversely proportional to the radius of the bubble. The small size of nanobubbles results in elevated internal pressures, which can have implications for their stability and behavior. (2) Gas diffusion: The gas core of nanobubbles allows for enhanced gas diffusion in liquids. This property is particularly relevant in applications where gas delivery, such as oxygen, to specific biological targets is essential. Nanobubbles can serve as carriers for gases, facilitating their transport to desired locations. The composition of the gas core is another critical factor influencing the stability of nanobubbles [6]. The introduction of gases like perfluorocarbons (PFCs), especially when combined with oxygen, leads to a reduced efflux from the nanobubble, resulting in a prolonged residence time [52]. In parallel research efforts, studies have explored combinations of PFCs, sulfur hexafluoride (SF6), and nitric oxide (NO) with oxygen to formulate micro-nanobubble systems [53][54][55][53,54,55]. On a different note, within the realm of nanodroplets (i.e., extremely tiny liquid droplets significantly smaller than conventional microdroplets and hence, due to their very small size, can offer more advantages such as increased surface area-to-volume ratios and the ability to penetrate biological barriers or tissues more effectively; they are increasingly being considered as valuable tools in challenging applications including drug delivery, medical imaging, and nanomedicine), liquid PFCs have found application as O2 carriers, capitalizing on their higher O2 release capability [50][56][50,56]. Nanobubbles have a unique structural composition that sets them apart from larger bubbles. While their precise composition can vary depending on the preparation method and the surrounding medium, the following is a general overview. (1) Gas core: At the center of a nanobubble is the gas core, typically composed of gases like oxygen, nitrogen, or a mixture of gases. The choice of gas can influence the bubble’s behavior and applications. (2) Liquid shell: Surrounding the gas core is a thin shell of liquid, which acts as a stabilizing layer. The liquid is often water but can include other liquids or solutions depending on the specific application. (3) Surface molecules: The interface between the gas core and the liquid shell is defined by surface molecules. These molecules play a critical role in stabilizing the nanobubble and preventing its premature collapse or coalescence. Surface molecules can be surfactants or other stabilizing agents that reduce surface tension. (4) Electrostatic charge: Nanobubbles may carry a charge on their surface due to interactions with the surrounding medium or the presence of charged molecules. This surface charge can influence behavior, stability, and interactions with other particles and surfaces. Henceforth, understanding the structural composition of nanobubbles is crucial for customizing their properties to suit specific applications. Researchers and engineers can manipulate these structural elements to craft nanobubbles with tailored characteristics, catering to a wide array of fields like medicine, materials science, and environmental science. For instance, uncoated nanobubbles, lacking a shell structure, have primarily found utility in applications like water treatment and select human applications such as oral drinks and liquid ventilation (Table 1, Table 2, Table 3 and Table 4). These uncoated nanobubbles maintain stability through charge stabilization, interacting with electrolytes in water, ultrapure water, distilled water, or saline solutions. Conversely, most engineered nanobubbles adopt a core–shell structure, where the core predominantly contains the chosen gas content strategically selected for its intended purpose. The shell component exhibits varying compositions and structures, which may include lipids, proteins, polymers, or surfactants. This core–shell configuration has traditionally been employed in the realm of biomedical applications, serving as a contrast agent for ultrasound or as a platform for oxygenation [57]. The outer shell of nanobubbles functions as a protective layer surrounding the gas core, exerting control over crucial characteristics such as stiffness, elasticity, half-life, and the rate of gas exchange. By encapsulating the gas core, the shell effectively prevents direct contact between the gas and the surrounding solvent, minimizing premature interfacial gas diffusion [50][58][50,58]. In earlier studies, it was argued that a shell structure was not necessarily a fundamental requirement for bubble formation [11][59][11,59]. Consequently, both uncoated and coated nanobubbles or unshelled and shelled nanobubbles have been utilized in the development of therapeutic strategies for various medical applications. However, increased attention has been directed toward coated nanobubbles, primarily because of their ability to incorporate a versatile array of core and shell materials, manipulation options, and bio-conjugation. This versatility extends beyond gases and drugs, allowing for the inclusion of a diverse range of molecules such as proteins, DNA, and ligands [60], enabling precise in vivo targeted delivery (Figure 3C). It is perhaps worth mentioning for the reader herein that shelled micro/nanobubbles (tiny gas-filled bubbles with a protective thin outer shell or coating that is typically composed of various materials, including lipids, proteins, polymers, surfactants, or other biocompatible substances) exhibit a more gradual external diffusion of internal gases [7][58][61][62][63][7,58,61,62,63], contributing their unique characteristics.Table 1.
OnB application in cancer studies.
| Clinical Indication |
Study Type |
Oxygen Release Strategy | Delivery | Main Results and Conclusions |
|---|---|---|---|---|
| Study | Type |
Oxygen Release Strategy |
Delivery | Main Results and Conclusions |
| ). | ||||
Table 3.
OnB application in cardio–respiratory studies.
| Clinical Indication |
Study Type |
Oxygen Release Strategy | Delivery | Main Results and Conclusions | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lung cancer | in vitro | Diffusion | Cell Culture Media | |||||||||
| Amyotrophic Lateral Sclerosis (ALS) | Uncoated OnB reduce hypoxia-induced resistance in cancer cells | in vitro/in vivo | [ | 64 | Diffusion | ]. | ||||||
| Intraperitoneal | Diabetes, Burns, Tissue necrosis, Bedsores, and Wounds | in vitro/in vivo | RNS60 | protects neurons, decreasing ALS progression [ | US-mediated | 37 | Cell Culture Media | Dextran and chitosan nanobubbles might be proposed for the delivery of oxygen, which is enhanced by US with a frequency of 45 kHz in hypoxic-related diseases [2]][87][2[74][] and demonstrating the feasibility, safety, and tolerability of long-term administration of RNS60 in patients with ALS [75]. | ||||
| Respiratory Failure and Acute Lung Trauma/Injury | in vitro/in vivo | Diffusion | Intraperitoneal Intravenous |
Peritoneal microbubble oxygenation (PMO) provides extrapulmonary ventilation after complete tracheal occlusion [79]. PMO is also a promising strategy for other pulmonary diseases [6] as it oxygenates blood within 4 sec and does not cause hemolysis or complement activation in hypoxic rabbits [80,87]. | ||||||||
| Pancreatic cancer |
in vitro/in vivo | Clinical TrialUltraSound | ||||||||||
| Clinical Trial (recruiting) | mediated | Oral | Surfactant-stabilized OnB reduce the transcriptional and protein levels of HIF1α | Diffusion | [ | Intravenous | Diffusion | The effect of RNS60 treatment on selected pharmacodynamic biomarkers in ALS patients was concurrently treated with riluzole (65 | ||||
| ] | . | |||||||||||
| ] | . | NCT03456882 | ). | ]. Lipid-stabilized oxygen micro-bubbles and US reveal a 45% reduction in tumor volume five days after treatment | Irrigation | [66]. | ||||||
| Ovarian cancer | ||||||||||||
| Parkinson’s disease (PD) | in vitro/in vivo | Diffusion | Intraperitoneal | RNS60 enhance mitochondrial bio-genesis via PI3K/CREB and PGC1alpha in PD model [78]. Moreover, RNS60 inhibited the activation of NF-κB in the SNpc of MPTP-intoxicated mice | ||||||||
| Blood Oxygenation |
in vitro/in vivo | Diffusion | Cell Culture Media |
This micro-/nano-bubble solution is suggested as an irrigation solution to improve wound oxygenation in ischemic tissues (NCT05169814).Oxygen microbubble-containing dextran solutions were effective for improving blood oxygenation [81]. | in vivo | UltraSound mediated |
Intraperitoneal | Oxygen and Paclitaxel (PTX)-loaded lipid micro-bubbles downregulate HiF-1α and increase PTX effectivity | Multiple Sclerosis (MS) | in vitro/in vivo | Diffusion | |
| Diabetic Retinopathy | Intraperitoneal and | Nebulization | [ | RNS60 | in vitro/in vivo | induced the activation of PI3K, promoting myelin gene transcription in oligodendrocytes (OL) and glial cells | Diffusion | [ | Cell Culture Intravitreal | 76]. RNS60 enhanced OL spare respiratory capacity (SRC) in response to metabolic stress (glucose-nutrient deprivation) | Dextran-OnB release 74.06 µg of O2 after 12 h at 37 °C and mitigate hypoxia during ischemic conditions in the eye upon timely administration [88]. | |
| . | ||||||||||||
| Tissue Cutaneous Lesions |
in vitro/in vivo | US-mediated | Cell Culture Media/Gel Formulation Topically Applied | Oxygen-loaded nano-droplets (OLNDs) are more effective than former oxygen-loaded nanobubbles enhancing oxy-hemoglobin levels by photoacoustic [89]. US-activated chitosan-shelled/DFP-cored OLNDs might be a novel, suitable, and cost-effective way to treat several hypoxia-associated pathologies of the cutaneous tissues [3] | PLA-combined Fe3O4-GO-ASA nanobubbles improve the anti-thrombin parameters and significantly inhibit thrombosis within rabbit blood [84]. | |||||||
| MicroFluidic Device for Hypoxemia | in vitro/in vivo | Diffusion | Intravascular | OnB from the device was infused into the femoral vein, in vivo, wherein ∼20% of baseline VO2 can be delivered intravenously in real time [85]. | ||||||||
| Ischemic Stroke Re-Perfusion |
in vivo | US-mediated | Intravascular | OnB with US stimulation provide sono-perfusion and local oxygen for the reduction in brain infarct size and neuroprotection after stroke re-perfusion [86]. |
Table 4.
OnB application in metabolic diseases, regenerative medicine, and molecular imaging studies.
| Clinical Indication |
Study Type |
Oxygen Release Strategy | Delivery | Main Results and Conclusions |
|---|---|---|---|---|
| Glioma | ||||
| in vivo | Diffusion | 67 | ] | . |
| in vitro/in vivo | ||||
| Photodynamic (PDT) | ||||
| Intravenous | OnB, stimulated by function as an oxygen self-supplement agent, enhance the survival rate in the glioma-bearing mice model | [ | 30 | ][33]. |
Table 2.
OnB application in neuroscience studies.
| Clinical Indication |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 37 | , | 74 | |||||||||
| [ | |||||||||||
| 5 | ] | . | RNS60 | led to the enrichment of anti-autoimmune regulatory T cells (Tregs) suppression of autoimmune Th17 cells [77]. | |||||||
| Intravenous | Oxygen microbubbles are safe and effective in delivering more oxygen than human red blood cells (per gram) after being injected in vivo | [ | 82]. | Breast cancer | in vitro | Diffusion | Cell Culture Media | Alzheimer’s disease (AD) | OnB revert hypoxia, downregulates HiF-1a, and improve cellular conditions, leading to further medical applications [ | in vivo | 7]. |
| Diffusion | Intraperitoneal | RNS60 | |||||||||
| suppressed the hippocampus neuronal apoptosis and attenuated Tau phosphorylation and the burden of Ab | |||||||||||
| ExtraCorporeal Membrane Oxygenation (ECMO) | [ | 33 | in vitro | ][23]. RNS60 upregulated the plasticity-related proteins (PSD95 and NR2A) and NMDA-dependent hippocampal calcium influx [ | Diffusion | 31][ | De-oxygenated PBS | 21 | Protein-encapsulated oxygen microbubbles rapidly equilibrate hypoxia by releasing their oxygen core into an oxygen-depleted saline solution [83].. | ||
| in vivo | UltraSound mediated |
Intraperitoneal | |||||||||
| Transdermal Drug Release |
in vitro/in vivo | Oxygen micro-bubbles strongly enhance echo intensity in tumor and significantly enhance PO | 2 after US irradiation [53]. | ||||||||
| [ | 78 | ] | . | Chorio- | |||||||
| Spinal Cord Diseases, Injuries, and Compression | |||||||||||
| Anti-Thrombotic Effect | in vitro | US-mediated | US-mediatedIntravascular | Transdermal Micro-needle Gel | The nanobubbles added into a micro-needle patch and used in addition to US cause better penetration and diffusion of drugs [90]. | carcinoma |
in vitro | ||||
| Cytocompatibility of OnB | in vitro | Diffusion | Cell Culture Media | Human JEG-3 cells showed a reduction (2-fold decrease—50%) in HiF-1α transcript levels at 3% O2 incubation | Clinical Trial | [ | US Contrast | 68]. | |||
| Intrathecal | US-mediated | Cell Culture Media | The use of OnB and US improves the identification of discrete areas of perfusion changes in the spinal cord in subjects undergoing spinal cord decompression ( | NCT05530798 | Lipid-shelled/coated OnB show US imaging-responsiveness and enhance cell viability in several cell lines [15]. | NasoPharyngeal carcinoma | in vitro/in vivo | pH-responsive | |||
| Drug Delivery in Fluids | in vitro | US-mediated | Intravenous | pH-responsive OnB increase the intra-tumoral oxygen concentration six-fold, suggesting great potential for overcoming hypoxia-induced resistance | Hypoxic | [69]. | |||||
| Solution | Oxygen release from polysaccharides–peptides, Pingxiao, and chitosan is 94.6%, 75.1%, and 40.2% ( | respectively | ) higher than water, especially under the US stimulus | [91]. | Bladder/Colon Tumor Cell lines | in vitro/in vivo | UltraSound mediated |
Intravesical Intravascular |
OnB are a promising multi-modal and multifunctional strategy for imaging and targeting the hypoxic tumoral micro-environment [70][71][70,71]. The bladder tumoral PO2 increased by around 140% after the injection of OnB | ||
| On-chip Contrast Agent for US Imaging | [ | in vitro | US-mediated | Microfluidic | 72]. Biogenic nanobubbles (gas vesicles) enhance US contrast signal when compared to/with synthetic nanobubbles, enhancing tumor penetration with a range size of 2–200 nm [73] | Micron-sized lipid shell-based perfluorocarbon gas microbubbles enhance the gas composition for US contrast agents with new shell materials [92]. |
