Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Mirjam Bonanno.
In the neurorehabilitation field, robot-aided motion analysis (R-AMA) could be helpful for two main reasons: (1) it allows the registration and monitoring of patients’ motion parameters in a more accurate way than clinical scales (clinical purpose), and (2) the multitude of data produced using R-AMA can be used to build machine learning algorithms, detecting prognostic and predictive factors for better motor outcomes (research purpose).
- robot-aided motion analysis
- objective motor assessment
- biomechanics
1. Introduction
In the field of neurorehabilitation, innovative technologies, such as robotic devices, have been widely used to treat and evaluate patients affected by motor impairment due to different neurological disorders (e.g., stroke, multiple sclerosis (MS), and spinal cord injury (SCI)) [1]. Compared with conventional rehabilitation approaches, robotic-assisted therapy (RAT) may have some advantages, including (i) guaranteeing repetitive, intensive, and task-oriented rehabilitation; (ii) reducing the physical burden on clinical therapists, giving them the possibility to treat more patients simultaneously; and (iii) quantitatively and objectively assessing patients’ motor performance over time [2,3][2][3]. In particular, objective assessment of motor performance is a fundamental issue in neurorehabilitation [4]. In fact, clinical scales are still widely used in hospital settings, despite their validity and reliability being under debate. Robot-aided motion analysis (R-AMA) could be helpful for two main reasons: (i) it allows the registration and monitoring of patients’ motion parameters in a more accurate way than clinical scales (clinical purpose), and (ii) the multitude of data produced using R-AMA can be used to build machine learning algorithms, detecting prognostic and predictive factors for better motor outcomes (research purpose). Specifically, motion analysis refers to the recording of three-dimensional movements of human body segments and the subsequent computation of meaningful parameters that describe human movement from raw kinematic parameters [5,6][5][6]. Motion analysis is commonly carried out through wearable and non-wearable sensors that are able to detect biomechanical parameters of movements [7]. Similarly, robotic devices, both end effectors and exoskeletons, through specific sensors, could allow the detection of passive or active range of motion, movement accuracy, and planning [8]. For example, Maggioni et al. [9] examined the possibilities of assessing lower extremity function using robots, with parameters such as range of motion (RoM), muscle strength, and proprioception. In fact, the Lokomat (which is a tethered exoskeleton) was used to assess joint position sense (i.e., proprioception) in patients with incomplete spinal cord injury. Despite their potential in clinical settings, robotic assessment tools have not gained widespread clinical acceptance. Some barriers to and doubts about their clinical adoption remain, such as their reliability and validity compared to the existing standardized scales and motion analysis.
2. Motion Analysis and Its Biomechanical Contribution to Accuracy Prediction
Motion analysis involves registering the three-dimensional movements of human body segments and then calculating biomechanical parameters that describe human movement [11][10]. The modeling of human motion can be studied from different perspectives. For this purpose, various approaches are used to derive mathematical expressions that describe human motion. Newton’s equations of motion are the fundamental tools for understanding the cause–effect relationship between the forces acting on a system and the resulting motion [12][11]. However, applying them to complex systems, such as human locomotion, which involve a large number of degrees of freedom, requires formulating and solving multiple equations, leading to high computational costs. The Euler–Lagrange method is used in multibody systems because it analyzes the entire system without studying the reaction and contact forces between the elements that comprise the system. This equation allows for the study of human motion by focusing solely on the mechanical energy of the system. The knowledge of motion equations allows researchers to identify problems and design mechanisms that seek to recognize or recover human movements [13][12]. Nowadays, motion analysis has evolved substantially in parallel with technological advancements, encompassing various applications, such as clinical gait analysis and 3D biomechanical modeling [14][13]. Biomechanical motion analysis is generally based on two types of models: the multibody model and the finite element model. The first type consists of a set of rigid or flexible bodies connected by joints, while the second type of motion analysis reconstructs internal strain, stress, or deformation in flexible bodies based on continuum mechanics theories [15,16][14][15]. Within a rehabilitation setting, quantitative analysis of human body kinematics is a powerful tool that has been used to understand the different biomechanical patterns of both healthy and pathological individuals [17][16]. Recently, biomechanical tools have also been developed, ranging from simple manual annotation of images to marker-based optical trackers and inertial sensor-based systems. Nowadays, motion analysis can be performed using marker-less systems that use sophisticated human body models, computer vision, and machine learning algorithms [17][16]. Biomechanical parameters that are considered during motion analysis include kinematic and kinetic parameters [18,19][17][18]. In particular, kinematic parameters [20][19] include the spatial and temporal aspects of movement. These parameters describe (a) the “static” direction during point-to-point movements; (b) the continuous change of position, speed, and acceleration, which can be further subdivided into its amplitude and direction components; or (c) combinations of these, such as movement trajectories.3. Robotic Devices for Upper Limb Measurement
Kinematic (e.g., position, velocity, and acceleration) and kinetic (e.g., force, joint torque, and muscle activity) data are acquired from sensors affixed to robotic and passive mechanical devices to measure biomechanical aspects of upper extremities [21,22,23,24,25,26,27,28][20][21][22][23][24][25][26][27] (see more in Table 1).Table 1.
Studies about upper limb robotic-aided motion analysis performed in neurological disorders.
| Reference No. | Robotic Device | Description | Usefulness of Robot-Aided Motion Analysis |
|---|---|---|---|
| [29][28] | Armeo®Power (Hocoma AG, Switzerland) | The Armeo®Power is a 6-degrees-of-freedom exoskeleton for upper limb rehabilitation. | Useful tool for the objective evaluation of upper limbs in post-stroke patients. The kinetic parameters of the motion analysis included kinetic parameters of the shoulder (flexion–extension, abduction and adduction, internal and external rotation), of the elbow (flexion–extension, prone–supination), of the wrist (flexion–extension), and of the hand (opening and closing). The values deriving from the valuation of the articular range were expressed in degrees; the values deriving from the evaluation of the force were expressed in Newton meters (Nm). |
| [30][29] | Armeo®Spring (Hocoma AG, Switzerland) |
The Armeo®Spring device is an exoskeleton for upper limb rehabilitation. It is equipped with 7 goniometers and 1 pressure sensor, which permits free 3D arm movement. At the end of the robotic arm, there is a handle, which contains a pressure sensor, measuring the grip force. | The authors used the Armeo®Spring device to conduct a quantitative assessment of the precision, speed, and smoothness of upper limb motion. Among the several measures, the hand path ratio is the ratio between the actual path in the horizontal plane and the shortest-possible path, which reflects movement efficiency. The mean velocity and the number of peaks in the velocity profile were also assessed. Additionally, the normalized jerk (Norm Jerk), a measure of trajectory smoothness, was analyzed. |
| [31][30] | Armeo®Spring (Hocoma AG, Switzerland) |
As described before | The Armeo®Spring was used to assess movement accuracy by measuring the hand path ratio, the mean velocity, and the number of peaks in the velocity profile. The authors concluded that the device should be integrated into the clinical evaluation of upper limb functions in post-stroke patients. |
| [32][31] | InMotion 2.0 (Bionik Laboratories, Watertown, MA, USA) |
The InMotion 2.0 device is an end effector in which the subject moves their arm from a central target to 8 peripheral targets. | The authors assessed kinematic parameters of the upper limb, including elbow extension and shoulder flexion, abduction and external rotation of the shoulder, elbow flexion and shoulder extension, and adduction and internal rotation of the shoulder. These parameters, calculated at baseline, can assist clinicians in defining a rehabilitation program for post-stroke patients. |
| [33][32] | Gloreha Sinfonia (Idrogenet, Lumezzane BS, Italy –) |
Gloreha Sinfonia is a robotic glove for hand rehabilitation to maintain range of motion (i.e., the flexion angle excursion of the finger metacarpophalangeal joints) of the patient’s hand. | The authors objectively evaluated hand movements using the Gloreha Sinfonia glove in order to customize rehabilitation sessions according to patients’ motor abilities. The angular values of the joints were assessed using bending sensors embedded in the glove. |
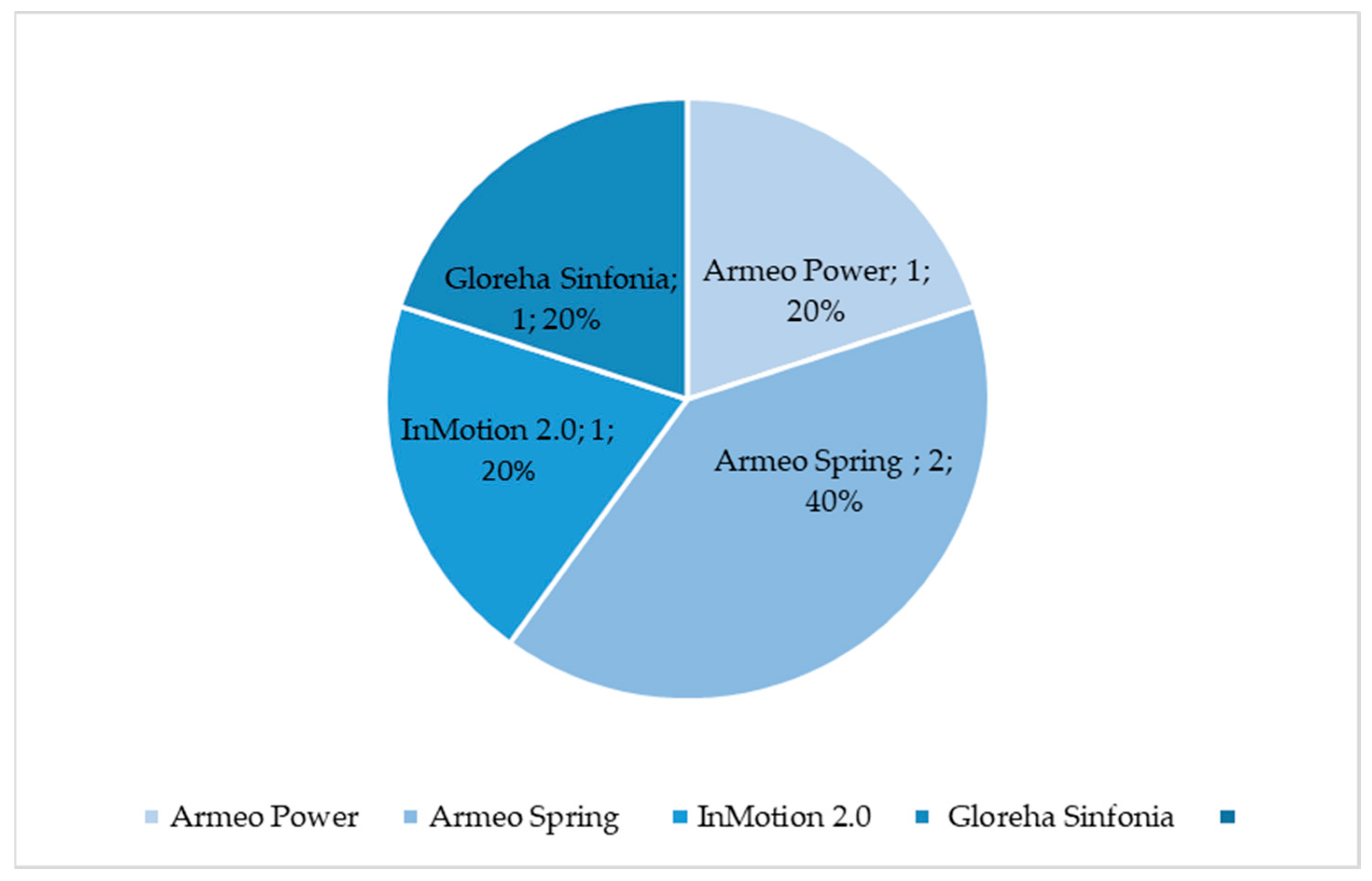
Figure 1.
Percentage of selected articles reported in
Table 1
dealing with upper limb robotic-aided motion analysis.
4. Robotic Device for Lower Limb Assessment
Walking recovery in neurological patients is one of the most important goals planned by therapists [45][44]. In order to maximize the recovery of the walking function, it is important to define a personalized rehabilitation treatment, in addition to an accurate assessment to monitor patients’ progress. In fact, both clinical and instrumental tools already exist to perform an accurate analysis of motion [45][44]. However, if the assessment protocol takes too much time to perform, clinicians and therapists may be reluctant to adopt them. A possible solution could involve the use of robotic devices in which the patient would undergo both training and assessment. In this study, Imoto et al. [46][45] used a novel gait training robot known as WelWalk WW-2000. This robot enables the adjustment of various gait parameters (such as time and mechanical assistance load) during the training session. The robot is equipped with sensors and a markerless motion capture system to detect altered gait patterns in stroke patients. This system can evaluate individuals’ gait patterns and provide tailored rehabilitation gait training [46][45]. Generally, the objective assessment of the lower limb should consider the simultaneous measurement of joint angles, spatial and temporal parameters of gait, muscle strength, proprioception, and spasticity and/or muscle stiffness [47][46] (see Table 2).Table 2.
Studies about lower limb robotic-aided motion analysis performed on neurological patients.
| Reference No. | Robotic Device | Description | Usefulness of Robot-Aided Motion Analysis |
|---|---|---|---|
| [46][45] | WelWalk (WW-2000, Toyota Motor Corporation, Aichi, Japan) |
Knee-ankle-foot robot, low floor treadmill, safety suspension device for body weight support, monitor for patient use, 3D sensor, and control panel | Three-dimensional joint positions, lower limb tilt, and knee joint angle were recorded during a task using a 3D sensor, an inertial sensor, and a knee angle sensor. Two-dimensional joint positions collected using skeletal tracking software (VisionPose®, NEXT-SYSTEM Co., Ltd., Fukuoka, Japan) and depth data from the 3D sensor were used to estimate the three-dimensional coordinates of the joint positions. Bilateral hip, knee, ankle, and shoulder joints, as well as the midpoints of the shoulder and hip joints, were the predicted locations of the 3D joints. This objective gait analysis can be useful for individuals with hemiparetic stroke, as it provides individually tailored gait training based on these assessments. |
| [48][47] | Ekso (Ekso Bionics, San Rafael, CA 94901, USA) |
Ekso a wearable unthethered exoskeleton. Motors power the hip and knee joints and all motion are started either through specific patient actions or the use of an external controller. | The authors conducted a comprehensive assessment by utilizing both kinematic and kinetic parameters, as well as EEG registrations, in patients with Parkinson’s disease. In this way, clinicians can personalize the rehabilitation treatment with a device that could increase the treatment intensity and dose without burdening therapists. |
| [49][48] | Ekso (Ekso Bionics, San Rafael, CA 94901, USA) |
As described before | Muscle synergies and activation profiles were extracted using non-negative matrix factorization. The authors’ findings provided insights into the potential underlying mechanism for improving gait functions through exoskeleton-assisted locomotor training. |
| [50][49] | Lokomat (Hocoma AG, Switzerland) |
The Lokomat is a robotic tethered exoskeleton with active hip–knee actuation and passive ankle control during the swing phase, in addition to a variable level of assistance. | The Lokomat was used to assess proprioception, which provides information about static position and movement sense, using custom software to measure joint position sense in the hip and knee. The authors demonstrated the usefulness of the Lokomat in measuring proprioception in SCI patients. |
| [51][50] | Lokomat (Hocoma AG, Switzerland) |
As described before | The authors proved the Lokomat’s usefulness in objectively assessing proprioception at the hip and knee in people with SCI. |
| [52][51] | Lokomat (Hocoma AG, Switzerland) |
As described before | Since lower limb kinesthesia deficits are common in SCI patients, the authors demonstrated that the Lokomat can serve as a valid and reliable robotic device for monitoring sensory function. Kinesthesia was evaluated using angular encoders of the hip and knee. During the analysis, a score was generated based on the difference between the initial angle and the final angle. |
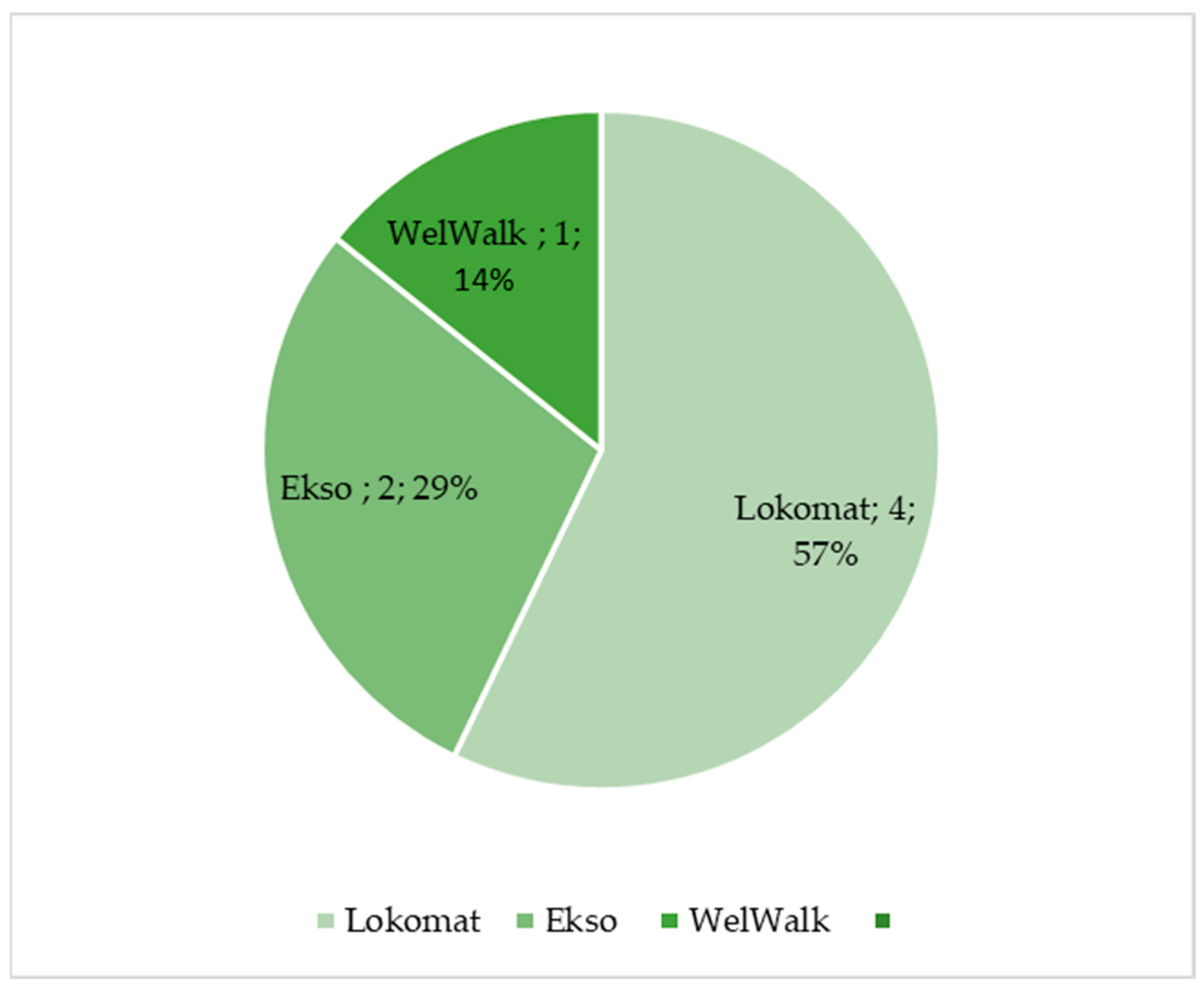
Figure 2.
Percentage of selected articles reported in
Table 2
dealing with lower limb robotic-aided motion analysis.
References
- Iandolo, R.; Marini, F.; Semprini, M.; Laffranchi, M.; Mugnosso, M.; Cherif, A.; De Michieli, L.; Chiappalone, M.; Zenzeri, J. Perspectives and Challenges in Robotic Neurorehabilitation. Appl. Sci. 2019, 9, 3183.
- Meng, W.; Liu, Q.; Zhou, Z.; Ai, Q.; Sheng, B.; Xie, S. Recent development of mechanisms and control strategies for robot-assisted lower limb rehabilitation. Mechatronics 2015, 31, 132–145.
- Rehmat, N.; Zuo, J.; Meng, W.; Liu, Q.; Xie, S.Q.; Liang, H. Upper limb rehabilitation using robotic exoskeleton systems: A systematic review. Int. J. Intell. Robot. Appl. 2018, 2, 283–295.
- Shishov, N.; Melzer, I.; Bar-Haim, S. Parameters and Measures in Assessment of Motor Learning in Neurorehabilitation; A Systematic Review of the Literature. Front. Hum. Neurosci. 2017, 11, 82.
- Roggio, F.; Ravalli, S.; Maugeri, G.; Bianco, A.; Palma, A.; Di Rosa, M.; Musumeci, G. Technological advancements in the analysis of human motion and posture management through digital devices. World J. Orthop. 2021, 12, 467–484.
- Crenna, F.; Rossi, G.B.; Berardengo, M. Filtering Biomechanical Signals in Movement Analysis. Sensors 2021, 21, 4580.
- Muro-de-la-Herran, A.; Garcia-Zapirain, B.; Mendez-Zorrilla, A. Gait analysis methods: An overview of wearable and non-wearable systems, highlighting clinical applications. Sensors 2014, 14, 3362–3394.
- Laut, J.; Porfiri, M.; Raghavan, P. The Present and Future of Robotic Technology in Rehabilitation. Curr. Phys. Med. Rehabil. Rep. 2016, 4, 312–319.
- Maggioni, S.; Melendez-Calderon, A.; van Asseldonk, E.; Klamroth-Marganska, V.; Lünenburger, L.; Riener, R.; van der Kooij, H. Robot-aided assessment of lower extremity functions: A review. J. Neuroeng. Rehabil. 2016, 13, 72.
- Ma, C.Z.-H.; Li, Z.; He, C. Advances in Biomechanics-Based Motion Analysis. Bioengineering 2023, 10, 677.
- Grimmer, M.; Zeiss, J.; Weigand, F.; Zhao, G.; Lamm, S.; Steil, M.; Heller, A. Lower limb joint biomechanics-based identification of gait transitions in between level walking and stair ambulation. PLoS ONE 2020, 15, e0239148.
- Sugihara, T.; Fujimoto, Y. Dynamics Analysis: Equations of Motion. In Humanoid Robotics: A Reference; Goswami, A., Vadakkepat, P., Eds.; Springer: Dordrecht, The Netherlands, 2017.
- Yeadon, M.; Pain, M. Fifty years of performance-related sports biomechanics research. J. Biomech. 2023, 155, 111666.
- Lerchl, T.; Nispel, K.; Baum, T.; Bodden, J.; Senner, V.; Kirschke, J.S. Multibody Models of the Thoracolumbar Spine: A Review on Applications, Limitations, and Challenges. Bioengineering 2023, 10, 202.
- Parashar, S.K.; Sharma, J.K. A review on application of finite element modelling in bone biomechanics. Perspect. Sci. 2016, 8, 696–698.
- Colyer, S.L.; Evans, M.; Cosker, D.P.; Salo, A.I.T. A Review of the Evolution of Vision-Based Motion Analysis and the Integration of Advanced Computer Vision Methods towards Developing a Markerless System. Sports Med.-Open 2018, 4, 24.
- Kwon, C.-W.; Yun, S.-H.; Koo, D.-K.; Kwon, J.-W. Kinetic and Kinematic Analysis of Gait Termination: A Comparison between Planned and Unplanned Conditions. Appl. Sci. 2023, 13, 7323.
- Aprile, I.; Rabuffetti, M.; Padua, L.; Di Sipio, E.; Simbolotti, C.; Ferrarin, M. Kinematic Analysis of the Upper Limb Motor Strategies in Stroke Patients as a Tool towards Advanced Neurorehabilitation Strategies: A Preliminary Study. BioMed Res. Int. 2014, 2014, 636123.
- Brihmat, N.; Loubinoux, I.; Castel-Lacanal, E.; Marque, P.; Gasq, D. Kinematic parameters obtained with the ArmeoSpring for upper-limb assessment after stroke: A reliability and learning effect study for guiding parameter use. J. Neuroeng. Rehabil. 2020, 17, 130.
- Branco, M.P.; de Boer, L.M.; Ramsey, N.F.; Vansteensel, M.J. Encoding of kinetic and kinematic movement parameters in the sensorimotor cortex: A Brain-Computer Interface perspective. Eur. J. Neurosci. 2019, 50, 2755–2772.
- Al-Mulla, M.R.; Sepulveda, F.; Colley, M. A Review of Non-Invasive Techniques to Detect and Predict Localised Muscle Fatigue. Sensors 2011, 11, 3545–3594.
- Schaefer, L.V.; Bittmann, F.N. Are there two forms of isometric muscle action? Results of the experimental study support a distinction between a holding and a pushing isometric muscle function. BMC Sports Sci. Med. Rehabil. 2017, 9, 11.
- Halilaj, E.; Rajagopal, A.; Fiterau, M.; Hicks, J.L.; Hastie, T.J.; Delp, S.L. Machine learning in human movement biomechanics: Best practices, common pitfalls, and new opportunities. J. Biomech. 2018, 81, 1–11.
- Giarmatzis, G.; Zacharaki, E.I.; Moustakas, K. Real-Time Prediction of Joint Forces by Motion Capture and Machine Learning. Sensors 2020, 20, 6933.
- Mundt, M.; Koeppe, A.; Bamer, F.; David, S.; Markert, B. Artificial Neural Networks in Motion Analysis—Applications of Unsupervised and Heuristic Feature Selection Techniques. Sensors 2020, 20, 4581.
- Ai, Q.; Liu, Z.; Meng, W.; Liu, Q.; Xie, S.Q. Machine Learning in Robot Assisted Upper Limb Rehabilitation: A Focused Review. IEEE Trans. Cogn. Dev. Syst. 2021.
- Maura, R.M.; Parra, S.R.; Stevens, R.E.; Weeks, D.L.; Wolbrecht, E.T.; Perry, J.C. Literature review of stroke assessment for upper-extremity physical function via EEG, EMG, kinematic, and kinetic measurements and their reliability. J. Neuroeng. Rehabil. 2023, 20, 21.
- Galeoto, G.; Berardi, A.; Mangone, M.; Tufo, L.; Silvani, M.; González-Bernal, J.; Seco-Calvo, J. Assessment Capacity of the Armeo® Power: Cross-Sectional Study. Technologies 2023, 11, 125.
- Merlo, A.; Longhi, M.; Giannotti, E.; Prati, P.; Giacobbi, M.; Ruscelli, E.; Mancini, A.; Ottaviani, M.; Montanari, L.; Mazzoli, D. Upper limb evaluation with robotic exoskeleton. Normative values for indices of accuracy, speed and smoothness. NeuroRehabilitation 2013, 33, 523–530.
- Longhi, M.; Merlo, A.; Prati, P.; Giacobbi, M.; Mazzoli, D. Instrumental indices for upper limb function assessment in stroke patients: A validation study. J. Neuroeng. Rehabil. 2016, 13, 52.
- Goffredo, M.; Pournajaf, S.; Proietti, S.; Gison, A.; Posteraro, F.; Franceschini, M. Retrospective Robot-Measured Upper Limb Kinematic Data From Stroke Patients Are Novel Biomarkers. Front. Neurol. 2021, 12, 803901.
- Cordella, F.; Scotto, D.; Luzio, F.; Bravi, M.; Santacaterina, F.; Bressi, F.; Zollo, L. Hand motion analysis during robot-aided rehabilitation in chronic stroke. J. Biol. Regul. Homeost. Agents 2020, 34 (Suppl. S3), 45–52.
- Calabrò, R.S.; Russo, M.; Naro, A.; Milardi, D.; Balletta, T.; Leo, A.; Filoni, S.; Bramanti, P. Who May Benefit From Armeo Power Treatment? A Neurophysiological Approach to Predict Neurorehabilitation Outcomes. PM&R 2016, 8, 971–978.
- Calabrò, R.S.; Naro, A.; Russo, M.; Milardi, D.; Leo, A.; Filoni, S.; Trinchera, A.; Bramanti, P. Is two better than one? Muscle vibration plus robotic rehabilitation to improve upper limb spasticity and function: A pilot randomized controlled trial. PLoS ONE 2017, 12, e0185936.
- Palermo, E.; Hayes, D.R.; Russo, E.F.; Calabrò, R.S.; Pacilli, A.; Filoni, S. Translational effects of robot-mediated therapy in subacute stroke patients: An experimental evaluation of upper limb motor recovery. PeerJ 2018, 6, e5544.
- Santisteban, L.; Térémetz, M.; Bleton, J.-P.; Baron, J.-C.; Maier, M.A.; Lindberg, P.G. Upper Limb Outcome Measures Used in Stroke Rehabilitation Studies: A Systematic Literature Review. PLoS ONE 2016, 11, e0154792.
- De-La-Torre, R.; Oña, E.D.; Balaguer, C.; Jardón, A. Robot-Aided Systems for Improving the Assessment of Upper Limb Spasticity: A Systematic Review. Sensors 2020, 20, 5251.
- Bonanno, M.; De Luca, R.; Torregrossa, W.; Tonin, P.; Calabrò, R.S. Moving toward Appropriate Motor Assessment Tools in People Affected by Severe Acquired Brain Injury: A Scoping Review with Clinical Advices. Healthcare 2022, 10, 1115.
- Kung, P.-C.; Lin, C.-C.K.; Ju, M.-S. Neuro-rehabilitation robot-assisted assessments of synergy patterns of forearm, elbow and shoulder joints in chronic stroke patients. Clin. Biomech. 2010, 25, 647–654.
- Zhao, K.; Zhang, Z.; Wen, H.; Liu, B.; Li, J.; D’avella, A.; Scano, A. Muscle synergies for evaluating upper limb in clinical applications: A systematic review. Heliyon 2023, 9, e16202.
- Safavynia, S.A.; Torres-Oviedo, G.; Ting, L.H. Muscle Synergies: Implications for Clinical Evaluation and Rehabilitation of Movement. Top. Spinal Cord Inj. Rehabil. 2011, 17, 16–24.
- Toigo, M.; Flück, M.; Riener, R.; Klamroth-Marganska, V. Robot-assisted assessment of muscle strength. J. Neuroeng. Rehabil. 2017, 14, 103.
- Tiboni, M.; Borboni, A.; Vérité, F.; Bregoli, C.; Amici, C. Sensors and Actuation Technologies in Exoskeletons: A Review. Sensors 2022, 22, 884.
- Bonanno, M.; De Nunzio, A.M.; Quartarone, A.; Militi, A.; Petralito, F.; Calabrò, R.S. Gait Analysis in Neurorehabilitation: From Research to Clinical Practice. Bioengineering 2023, 10, 785.
- Imoto, D.; Hirano, S.; Mukaino, M.; Saitoh, E.; Otaka, Y. A novel gait analysis system for detecting abnormal hemiparetic gait patterns during robot-assisted gait training: A criterion validity study among healthy adults. Front. Neurorobot. 2022, 16, 1047376.
- Boudarham, J.; Hameau, S.; Zory, R.; Hardy, A.; Bensmail, D.; Roche, N. Coactivation of Lower Limb Muscles during Gait in Patients with Multiple Sclerosis. PLoS ONE 2016, 11, e0158267.
- Romanato, M.; Spolaor, F.; Beretta, C.; Fichera, F.; Bertoldo, A.; Volpe, D.; Sawacha, Z. Quantitative assessment of training effects using EksoGT® exoskeleton in Parkinson’s disease patients: A randomized single blind clinical trial. Contemp. Clin. Trials Commun. 2022, 28, 100926.
- Afzal, T.; Zhu, F.; Tseng, S.-C.; Lincoln, J.A.; Francisco, G.E.; Su, H.; Chang, S.-H. Evaluation of Muscle Synergy During Exoskeleton-Assisted Walking in Persons With Multiple Sclerosis. IEEE Trans. Biomed. Eng. 2022, 69, 3265–3274.
- Domingo, A.; Marriott, E.; de Grave, R.B.; Lam, T. Quantifying lower limb joint position sense using a robotic exoskeleton: A pilot study. In Proceedings of the 2011 IEEE 12th International Conference on Rehabilitation Robotics: Reaching Users & the Community (ICORR 2011), Zurich, Switzerland, 29 June–1 July 2011; pp. 1–6.
- Domingo, A.; Lam, T. Reliability and validity of using the Lokomat to assess lower limb joint position sense in people with incomplete spinal cord injury. J. Neuroeng. Rehabil. 2014, 11, 167.
- Chisholm, A.E.; Domingo, A.; Jeyasurya, J.; Lam, T. Quantification of Lower Extremity Kinesthesia Deficits Using a Robotic Exoskeleton in People With a Spinal Cord Injury. Neurorehabilit. Neural Repair 2016, 30, 199–208.
- Moeller, T.; Moehler, F.; Krell-Roesch, J.; Dežman, M.; Marquardt, C.; Asfour, T.; Stein, T.; Woll, A. Use of Lower Limb Exoskeletons as an Assessment Tool for Human Motor Performance: A Systematic Review. Sensors 2023, 23, 3032.
- Maggioni, S.; Lunenburger, L.; Riener, R.; Melendez-Calderon, A. Robot-aided assessment of walking function based on an adaptive algorithm. In Proceedings of the 2015 IEEE International Conference on Rehabilitation Robotics (ICORR 2015), Singapore, 11–14 August 2015; Yu, H., Ed.; IEEE: Piscataway, NJ, USA, 2015; pp. 804–809, ISBN 978-1-4799-1808-9.
- Mercado, L.; Alvarado, L.; Quiroz-Compean, G.; Romo-Vazquez, R.; Vélez-Pérez, H.; Platas-Garza, M.; González-Garrido, A.A.; Gómez-Correa, J.; Morales, J.A.; Rodriguez-Liñan, A.; et al. Decoding the torque of lower limb joints from EEG recordings of pre-gait movements using a machine learning scheme. Neurocomputing 2021, 446, 118–129.
- El Yaakoubi, N.A.; McDonald, C.; Lennon, O. Prediction of Gait Kinematics and Kinetics: A Systematic Review of EMG and EEG Signal Use and Their Contribution to Prediction Accuracy. Bioengineering 2023, 10, 1162.
More
