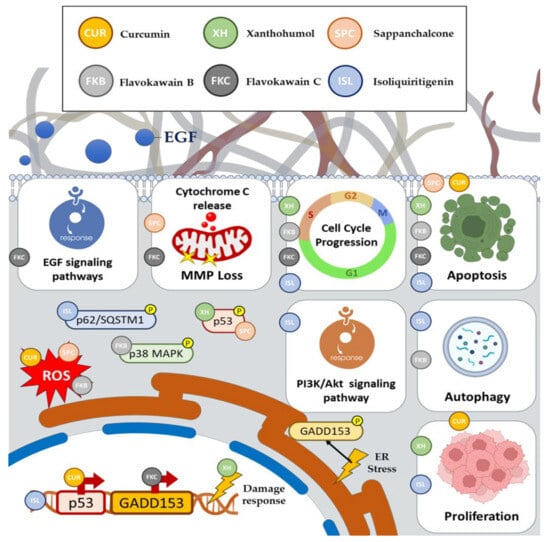
Previous research has examined the impact of XH on CRC inhibition or eradication. In a study by Liu et al., XH demonstrated a significant anti-tumor effect on CRC by reducing HK2 expression and glycolysis. XH effectively inhibited CRC cell growth in both in vitro and in vivo models
[10][11]. Additionally, XH treatment stimulated cytochrome C release and activated the intrinsic apoptosis pathway
[10][11][11,12]. Furthermore, the study findings indicated that XH downregulated the EGFR-Akt signaling pathway. When constitutively activated Akt1 was overexpressed exogenously, it notably compromised XH-induced glycolysis suppression and apoptosis induction
[10][11].
2.3. Sappanchalcone
Sappanchalcone (SPC) is a natural compound derived from the heartwood of the Sappan tree (
Caesalpinia sappan), which is native to Southeast Asia
[12][19]. This phytochemical has emerged as a subject of interest in cancer research due to its cytotoxic effects on various cancer cell lines, including colon cancer (
Figure 23).
Studies have explored the cytotoxic effects of SPC on colon cancer cells, particularly HCT116 and SW480 cells with different p53 statuses
[13][20]. The study demonstrates that SPC inhibits the growth of both cell lines, with HCT116 cells being more sensitive
[13][20]. It induces apoptosis in both cell lines via the caspase-dependent and caspase-independent pathways
[13][20]. SPC disrupts the mitochondrial membrane potential, regulates Bcl-2 family proteins, and increases ROS production, leading to apoptosis. In HCT116 cells, SPC activates p53, suggesting a p53-associated apoptotic mechanism, whereas this effect is absent in SW480 cells, due to the lack of significant changes in cleaved caspase expression
[13][20].
2.4. Isoliquiritigenin
Isoliquiritigenin (ISL) is a natural compound with a simple chalcone structure that belongs to the flavonoid group. It is known for its various potential health benefits and is found in a variety of plant sources, primarily in the roots of licorice (
Glycyrrhiza glabra) and some other plants
[14][15][21,22]. Various in vitro studies have explored its anticancer activity, suggesting that ISL may have the potential to inhibit the growth of cancer cells and induce apoptosis, making it a subject of interest in cancer research
[16][23].
In fact, ISL was found to induce G2 cell cycle arrest
[17][24], and to have an effect on death-associated protein kinase 1 (DAPK1) promoter methylation in the colon cancer cell line, indicating its role in influencing the epigenetic regulation of genes associated with cancer
[15][22].
2.5. Flavokawains
2.5.1. Flavokawain B
Flavokawain B (FKB) is a naturally occurring compound derived from the roots of
Alpinia pricei, a plant native to specific regions, including Taiwan
[18][19][27,28]. This chalcone compound is part of the flavonoid family and is known for its bioactive properties and potential as an anticancer agent
[19][28]. It is one of the constituents found in the extracts from the rhizomes of this plant, which has gained attention for its medicinal properties, particularly its ability to inhibit the growth of cancer cells and induce various cellular processes related to cancer treatment
[18][19][20][27,28,29] (
Figure 23).
2.5.2. Flavokawain C
Flavokawain C (FKC) is a bioactive compound with a fascinating origin that is deeply rooted in nature. This natural compound is primarily found in the kava plant (
Piper methysticum), which is native to the South Pacific region. Kava has a long history of traditional use in this region for its calming and stress-reducing effects when consumed as a beverage
[21][30] (
Figure 23).
Recent studies have explored its interesting anticancer activity. Researchers investigated the growth-inhibitory and apoptosis-inducing effects of FKC on human cancer cell lines, particularly HCT116 carcinoma cells, while it showed minimal cytotoxicity toward normal colon cells
[22][31]. The study also examined a structurally related compound, gymnogrammene (GMM), for comparison, revealing that FKC exerted pronounced cytotoxicity against HCT116 cells, while GMM had no such effect. This underscored the importance of structural variations in these compounds and their cytotoxicity. The molecular mechanisms of FKC-induced apoptosis were explored, involving the intrinsic and extrinsic pathways. FKC influenced the intrinsic pathway by modifying the expression of Bcl-2 family proteins, Bak and Bax, resulting in mitochondrial membrane permeabilization and the release of apoptogenic proteins such as cytochrome C, Smac/DIABLO, and apoptosis-inducing factor (AIF)
[22][31]. Extrinsic pathway activation was mediated by FKC through increased death receptor levels (DR4 and DR5) and the downregulation of c-FLIP
L, along with the activation of caspase-8, caspase-9, and caspase-3
[22][31]. FKC also disrupted the cell cycle by regulating proteins such as CDK2, CDK4, p21Cip, and p27Kip, causing S-phase arrest. Additionally, FKC-induced ER stress was evident from the elevated CHOP levels
[22][31].
2.6. Derricin and Derricidin
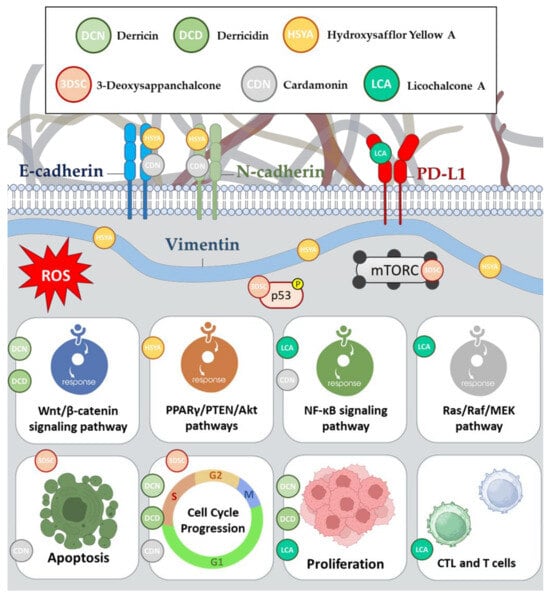
Derricin (DCN) and derricidin (DCD) are flavonoids belonging to the chalcone subclass
[23][33]. These compounds are natural plant-derived chemicals with similar chemical structures. They have been studied for their potential therapeutic properties, particularly in the context of cancer research (
Figure 34).
Figure 34. Anticancer mechanisms of action of derricin, derricidin, hydroxysafflor yellow A, 3-deoxysappanchalcone, cardamonin, and licochalcone A.
2.7. Hydroxysafflor Yellow A
Hydroxysafflor Yellow A (HSYA) is a natural compound found in
Carthamus tinctorius and has gained attention for its potential therapeutic applications, particularly in cancer treatment (
Figure 34).
The anticancer potential of HSYA in CRC was investigated in vitro, focusing on the underlying molecular mechanisms. HSYA demonstrated concentration-dependent inhibitory effects on CRC cell proliferation, migration, and invasion while promoting apoptosis
[24][34]. These actions were associated with regulating the EMT markers, such as the upregulation of E-cadherin and the downregulation of N-cadherin and vimentin. Additionally, HSYA was found to activate the PPARγ/PTEN/Akt signaling pathway, with increased expression of PPARγ and PTEN and decreased phosphorylation of Akt in CRC cells
[24][34]. The role of PPARγ in mediating PTEN expression and subsequently inhibiting the PI3K/Akt pathway was highlighted. The study also revealed that inhibiting PPARγ with GW9662 or the PPARγ knockdown reversed the anticancer effects of HSYA on CRC cells, implicating PPARγ as a key player in HSYA’s therapeutic action
[24][34].
2.8. The 3-deoxysappanchalcone Compound
The compound 3-DSC, which is short for 3-deoxysappanchalcone, is a natural compound derived from
Caesalpinia sappan L.
[25][35]. This compound has gained attention due to its potential therapeutic properties, particularly in the context of cancer treatment (
Figure 34).
An in vitro study conducted by Zhao et al. focused on the compound’s potential anticancer properties against CRC
[26][36]. The abnormal signaling of T-LAK cell-originated protein kinase (TOPK) is associated with various cancers, including CRC, and has been considered as a therapeutic target
[27][37]. Although previous TOPK inhibitors had several limitations, 3-DSC was identified as a promising candidate
[26][36]. The research demonstrated that 3-DSC specifically inhibits TOPK activity, inhibiting CRC cell growth, cell cycle arrest, and apoptosis. Importantly, 3-DSC showed selectivity for cancer cells, sparing the normal colon cells. This specificity is linked to its ability to induce apoptosis in CRC cells with wild-type p53 while sparing those with mutant p53
[26][36].
2.9. Cardamonin
Cardamonin (CDN), a compound derived from traditional Chinese medicine that is primarily found in the seeds of black cardamom (
Amomum subulatum), exhibits promising effects in the treatment of chemotherapy-resistant colon cancer
[28][38] (
Figure 34).
Studies have shown that CDN significantly reduces cell viability and induces apoptosis in resistant cancer cells, potentially overcoming chemotherapy resistance
[29][39]. Furthermore, CDN suppresses the expression of the key proteins associated with cancer growth and proliferation, including c-Myc and Oct4. Additionally, it inhibits the NF-κB signaling pathway, which is linked to oncogenesis and chemotherapy resistance
[29][39].
2.10. Licochalcone A
Licochalcone A (LCA) is a bioactive compound that is naturally found in certain plants, particularly in the roots of licorice plants (
Glycyrrhiza species).
Glycyrrhiza uralensis Fisch. ex DC, commonly known as licorice, is a primary source of LCA. This compound has garnered attention for its potential medicinal properties and has been the subject of research in various fields, including its use in cancer therapy and anti-inflammatory applications
[30][31][32][41,42,43] (
Figure 34).
Researchers evaluated the effects of LCA on specific proteins and pathways
[33][44]. The results, both in vitro and in vivo in a xenograft mouse model, showed the ability of LCA to significantly suppress PD-L1 expression, a vital immune checkpoint molecule often upregulated in various human tumor cells. Moreover, LCA inhibited the NF-κB signaling pathway, which is crucial in cancer cell survival, inflammation, and immunity, and affected the Ras/Raf/MEK pathway
[33][44], which is known for its role in cell growth and cancer
[34][45].
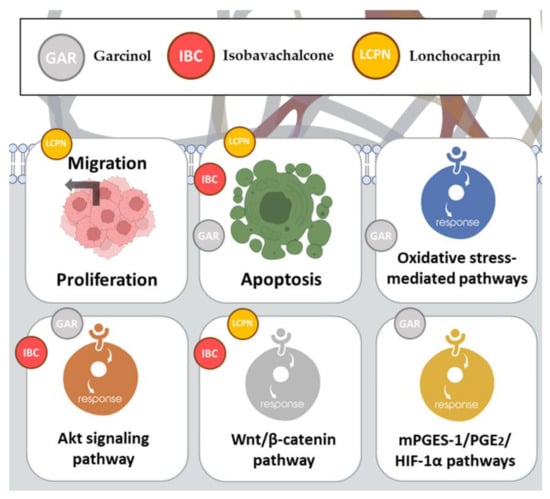
2.11. Garcinol
Garcinol (GAR), a chalcone derivative, is a natural compound renowned for its anti-inflammatory and anti-carcinogenic properties. It has been the focus of recent studies investigating its effects on cell growth in colon cancer cells and immortalized intestinal cells
[35][36][46,47] (
Figure 45).
Figure 45.
Anticancer mechanisms of action of garcinol, isobavachalcone, and lonchocarpin.
2.12. Isobavachalcone
Isobavachalcone (IBC) is a bioactive molecule derived from , a well-known traditional Chinese medicinal herb. IBC has garnered significant attention for its potential anticancer properties and ability to modulate various cellular pathways involved in cancer progression (Figure 4). In a duration- and dose-dependent manner, IBC demonstrated significant cytotoxicity against CRC cell lines, including SW480 and HCT116 [37]. Morphological changes and decreased cell viability were observed in IBC-treated cells, which was consistent with previous findings showing IBC’s inhibitory effects on tumor cell growth [37].
2.13. Lonchocarpin
5). In a duration- and dose-dependent manner, IBC demonstrated significant cytotoxicity against CRC cell lines, including SW480 and HCT116 [50]. Morphological changes and decreased cell viability were observed in IBC-treated cells, which was consistent with previous findings showing IBC’s inhibitory effects on tumor cell growth [50].
2.13. Lonchocarpin
Lonchocarpin (LCPN), a chalcone compound, is derived from the
Lonchocarpus sericeus plant, often referred to as the “Lancepod” or “Yopo” tree, which is native to various regions in Central and South America
[38][51]. LCPN has displayed significant potential in several research studies, particularly regarding its role as a negative modulator of the Wnt/β-catenin pathway and its prospects as an anticancer agent
[39][52].
3. Challenges Related to Chalcones Administration
3.1. Poor Solubility
The poor solubility of chalcones presents a significant challenge when it comes to their administration as potential therapeutic agents
[40][41][54,55]. One of the primary obstacles posed by poor chalcone solubility is the limited rate and extent of their dissolution in the gastrointestinal tract
[42][56]. This leads to inadequate absorption in the body, resulting in lower plasma concentrations and reduced bioactivity
[41][55]. As a result, higher doses may be required to achieve the desired therapeutic effect, potentially increasing the risks of toxicity and adverse effects. Overcoming the poor solubility of chalcones is essential for harnessing their therapeutic potential and incorporating them into effective pharmaceutical formulations.
3.2. Therapeutic Window
The efficacy of chalcones in various therapeutic contexts often hinges on the timing and dosing regimens employed. Achieving the optimal sequencing and dosage is a challenging aspect of chalcone research, as it depends on the specific disease target and the pharmacokinetic properties of the chalcone in question
[43][57].
Determining the therapeutic window for chalcone-based therapies is critical to balance efficacy and safety
[43][44][57,58]. The therapeutic window represents the range of doses at which a chalcone exerts its desired effects without causing unacceptable toxicity
[45][59].
3.3. Resistance Mechanisms
The cancer resistance mechanism involves the overexpression of efflux pumps in cancer cells
[46][47][48,61]. Efflux pumps can actively remove drugs from the intracellular environment, reducing their intracellular concentrations
[48][62]. Chalcones have garnered significant attention for their ability to sensitize cancer cells to chemotherapy and improve the pharmacokinetics of poorly absorbed cancer drugs. Numerous studies have investigated the potential of chalcones as modulators of resistance to conventional chemotherapy drugs, particularly by targeting multidrug efflux transporters such as P-glycoprotein
[49][50][51][63,64,65], multidrug resistance-associated protein 1
[52][53][66,67], and breast cancer resistance protein
[54][55][68,69]. These transporters play a crucial role in drug accumulation within cancer cells and contribute to multidrug resistance (MDR)
[47][61].
3.4. Combination Therapies
Chalcones are often explored as a possible part of combination therapies with other drugs or treatments
[29][36][56][57][58][59][15,16,39,47,70,71]. This strategy aims to enhance their efficacy while minimizing toxicity
[56][57][60][15,16,72]. The optimal sequencing and dosage of chalcones in combination therapies depend on multiple factors, including the mechanism of action of the co-administered agents and the potential drug–drug interactions.
4. Nanoparticle-Based Delivery Systems for Chalcones
4.1. Advantages of Nanoparticles for Chalcone Delivery
4.1.1. Enhanced Drug Stability
One of the most significant advantages of NPs is their ability to enhance drug stability
[61][75]. Conventional drugs often degrade rapidly, making it challenging to maintain their efficacy. NPs can encapsulate drugs, protecting them from environmental factors such as oxidation, light, temperature, moisture, and chemical reactions
[62][63][76,77]. This preservation of drug integrity extends the shelf life and ensures consistent therapeutic effects.
4.1.2. Prolonged Circulation Time
NPs possess the unique ability to extend the circulation time of drugs within the body
[62][76]. Their small size allows them to evade rapid clearance mechanisms, such as renal filtration, enabling drugs to remain in the bloodstream longer
[64][78]. This prolonged circulation time enhances drug bioavailability and reduces the need for frequent dosing, ultimately improving patient compliance.
4.1.3. Enhanced Cellular Uptake
NPs facilitate the delivery of therapeutic agents to target cells and tissues
[65][79]. Their small size and customizable surface properties enable them to interact favorably with cell membranes, promoting cellular uptake
[65][66][79,80]. This targeted delivery minimizes off-target effects and enhances the therapeutic efficacy of drugs.
4.1.4. Controlled Release of Therapeutic Agents
Controlling the release of therapeutic agents is crucial to achieving optimal drug efficacy while minimizing side effects. NPs can be engineered to release drugs in a controlled and sustained manner
[64][67][78,81]. This precise control ensures that therapeutic concentrations are maintained over an extended period, reducing the need for frequent dosing and mitigating adverse reactions
[67][81].
4.2. Chalcone-Based NPs for CRC Treatment and Pre-Clinical Studies
One of the primary derivatives of chalcones explored in the context of colon cancer treatment is CUR. Notably, several NP-based formulations, including liposomes, micelles, nanogels, chitosan, and polymeric NPs, have been developed, demonstrating their effectiveness in combatting colon cancer in both in vitro and in vivo studies
[68][69][70][71][72][73][74][75][76][82,83,84,85,86,87,88,89,90]. Additionally, innovative delivery systems have emerged. For instance, Ndong Ntoutoume et al. developed CUR-cyclodextrin/cellulose nanocrystal complexes (CUR-CD/CNCx), which have exhibited promising in vitro results, demonstrating lower IC
50 values and a more significant anti-proliferative effect against HT-29 colon cancer cell lines
[77][91].
5. Conclusions
Natural chalcones and derivatives are promising candidates for colon cancer treatment. Their potential to modulate crucial signaling pathways in colon cancer development and progression makes them valuable as targeted therapeutics. However, it is essential to acknowledge that while chalcones show promise, more pre-clinical studies are needed to validate their efficacy and safety further. Additionally, the integration of NP-based drug delivery systems presents a novel avenue by which to enhance the effectiveness of chalcones in treating colon cancer. While researchers have made strides in developing encapsulated synthetic chalcones that target various cancer cell lines
[78][79][80][92,93,94], their specific applicability in the context of colon cancer and their mechanisms of action remain relatively unexplored.
In essence, the success of colon cancer treatment is multifaceted, and future research endeavors should strive to unravel the complexities of this promising therapeutic approach. By delving deeper into these aspects, we can pave the way for more targeted, effective, and safe treatments for colon cancer in the years to come.