Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Lindsay Dong and Version 1 by DHRUVI PATEL.
Polymeric-based nanomaterials have exhibited wonderful properties due to their fabrication ability using various technologies. These have been of immense interest as nanocarriers in the design of pharmaceuticals and in the refining of drug delivery systems; therefore, they offer a suitable tool for the release of hydrophobic drugs and bioactive agents.
- self-assembly
- block copolymers (BCPs)
- polymeric micelles (PMs)
- polymersomes
- polymerization-induced self-assembly (PISA)
- drug delivery
1. Introduction
As with surface-active agents, which possess dual moieties that behave differently in water and thus adsorb onto interfaces and self-assemble to form nanoscale micelles of different sizes and shapes, block and graft copolymers contain incompatible polymer-size moieties that behave differently in selective solvents and form core–shell micelles and polymersomes. Depending on the polymeric blocks, which have a varied structure and chemical composition and a wide range of polar and non-polar entities linked together, these block copolymers (BCPs) impart unique solid-state and solution properties [1,2][1][2].
Self-assembly can be induced in molecularly dissolved polymers with different hydrophilic groups that have one block that is responsive to a stimulus which transforms it and makes it hydrophobic. Furthermore, such copolymers with a hydrophilic polyelectrolyte block may also assemble into ion complex micelles in the presence of an oppositely charged polymer. Also, self-assembly can take place during polymerization.
The most common BCPs, which have been commercially available for several decades, are ethylene oxide (EO)-propylene oxide (PO)-based BCPs. These have been sold under their BASF trade names as linear triblock copolymers (Pluronics®) and star-block copolymers (Tetronics®). These nano-ionic amphiphilic BCPs are available with varying molecular characteristics and demonstrate superior surface activity and micelle formation and are analogous to poly(ethylene oxide) (PEO, also called polyoxyethylene and poly(ethylene glycol)) condensate-type conventional nonionic surfactants with different nonpolar (lipophilic) parts, such as commercial Triton®, Brij®, Tween®, Soluplus®, Solutol® HS15, Cremophor® EL, Gelucire®, Akypo®, et cetera [8,9][3][4].
2. Types of BCPs
2.1. Hydrophilic BCPs
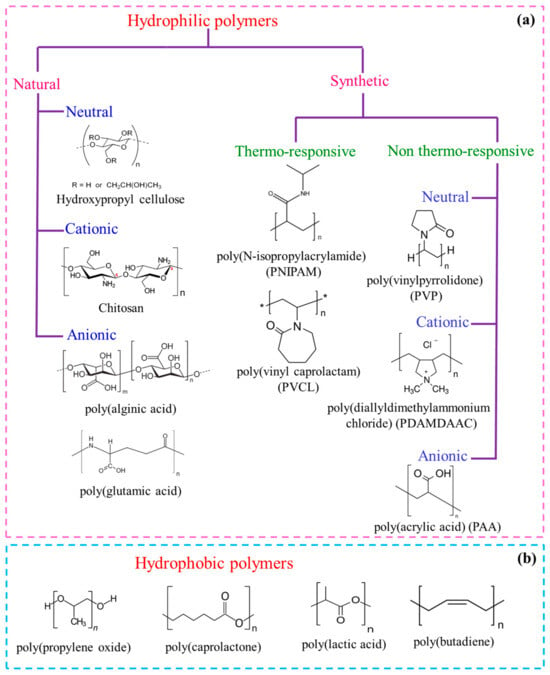
Hydrophilic polymers are easily dissolved in water and cannot form micelles easily due to a water-loving tendency. Double-hydrophilic block copolymers (DHBCs) represent a new class of switchable water-soluble amphiphiles and have been of great interest due to their propensity to undergo self-assembly into varied micellar morphologies when one of the blocks is switched from hydrophobic to hydrophilic because of stimuli such as temperature, pH, or the presence of some additives [29,30][5][6]. DHBCs are made of neutral–neutral, neutral–polyelectrolyte, or polyelectrolyte–polyelectrolyte blocks and remain molecularly dissolved in aqueous media. This enables them to provide a new scope of applications in drug carrier systems, gene therapy, crystal growth, colloid synthesis, and desalination membranes [31,32,33][7][8][9]. A few examples of hydrophilic blocks based on several water-soluble polymers, both neutral and charged and from natural sources or synthetically produced, are mentioned below (Figure 21a). (i) The naturally sourced blocks are, for example, hydroxypropyl cellulose, chitosan, alginate, sulfated polysaccharides, and poly(amino acids) (or polypeptides), such as poly(L-glutamic acid), poly(L-aspartic acid), poly(L-lysine), and poly(L-histidine). (ii) The synthetic neutral polymers are poly(ethylene oxide), polyvinylpyrrolidone, polyvinyl alcohol, poly(N-isopropylacrylamide), polyvinylcaprolactam, and various polyelectrolytes (anionic, cationic or zwitterionic), such as polyacrylates, poly(vinylpyridinium chloride), poly(diallyldimethylammonium chloride), poly(styrene sulfonate), et cetera.
Figure 21. Examples of some (a) water–soluble homopolymers from natural or synthetic origin (charged or uncharged), and (b) hydrophobic polymers that may undergo polymerization to form BCPs.
2.2. Hydrophobic BCPs
Hydrophobic polymers, being nonpolar, are a bit too rigid to dissolve in the aqueous medium. They form micelles at a very low temperature and have a tendency to undergo phase separation [12,34,35,36][10][11][12][13]. A few examples of hydrophobic blocks are poly(propylene oxide) (PPO), poly(butylene oxide) (PBO), poly(lactic acid) (PAA), poly(caprolactone) (PCL), poly(butadiene) (PB), poly(styrene) (PS), poly(methylacrylate) (PMA), poly(dimethyl siloxane), poly(vinyl pyridine), et cetera (Figure 21b).
The DHBCs with two distinct hydrophilic blocks remain molecularly dissolved in water. Conversely, if one of the blocks is responsive to any external stimulus, then that block becomes hydrophobic in nature and the DHBC turns to amphiphilic BCP, which would then self-assemble to form core–shell polymer micelles. Such DHBCs can form “stimuli-responsive” or “smart” schizophrenic copolymeric micelles (with a reverse core and shell arrangement), based on which the two blocks in the BCPs have to be hydrophobic (incompatible with water or a given solvent), as demonstrated in Figure 32.
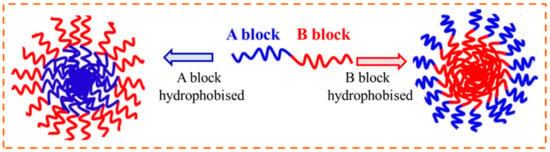

Figure 32.
Formation of schizophrenic micelles in AB type DHBC.
Thus, in diluted aqueous solution, AB type DHBC molecules can self-assemble to form two different micelles, one with a core of the hydrophobized A block and a shell of the hydrophilic B block, and the other with a core of the hydrophobized B block and a shell of the hydrophilic A block. The schizophrenic micelles can be switched from conventional to the reverse and vice versa by changing the temperature, solution pH, ionic strength, or solvent composition or in the presence of additives. This type of self-assembly leads to schizophrenic morphological features, as illustrated in Figure 32.
Due to their structural fragility, the practical applicability of such micelles is virtually never acknowledged. By utilizing a bifunctional cross-linker and the reactive functional groups of polymer chains, the micellar core and corona are cross-linked to increase their stability. This modulation is represented well in Figure 43.
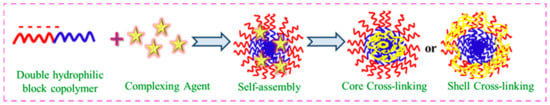

Figure 43.
Self-assembly of core–shell cross-linking using DHBC.
2.3. Stimuli-Responsive Block Copolymers (SRPs)
“Stimuli-responsive”, “smart”, or “intelligent” polymers are water-soluble smart macromolecules that respond to varied stimuli, such as (i) physical, viz., temperature, solvents, light, magnet, and ultrasound; (ii) chemical, viz., reactants, redox conditions, and pH; and (iii) biological, viz., enzymes and glucose (Figure 54). These are of immense interest in biomedical applications such as drug delivery, biosensors, tissue engineering, and self-heating materials [37,38][14][15].
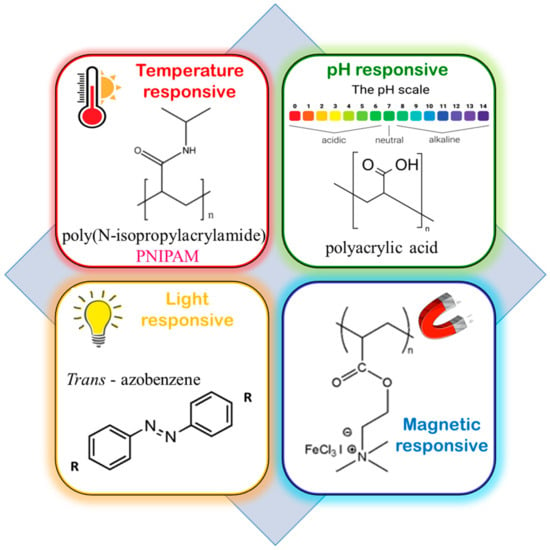

Figure 54.
Some common examples of stimuli-responsive blocks in BCPs.
Investigations of SRPs have advanced in the past two decades [39,40,41][16][17][18]. These polymers can undergo phase transitions or exhibit configurational tuning in response to external stimuli such as pH, temperature, light, electric field, chemicals, magnetism, ionic strength, et cetera. These can cause certain physical or chemical changes in these polymers, which can modify their solubility, surface properties, sol–gel transition, and other properties. Noncovalent forces like electrostatic interactions, hydrophobicity, or hydrogen bonding are frequently implicated in the characteristics of solutions. Such responsive polymers are extensively used in separation science, water treatment, water-borne coatings, recyclable catalysis, and oil recovery. In the case of BCPs, one or more blocks is responsive to any of the stimuli and can behave either hydrophilically or hydrophobically. Several studies have presented the use of such stimuli-responsive polymeric micelles for nano-cargos in drug delivery. Additionally, reports have shown the significance of the dual responsiveness or even the multi-responsiveness of BCPs [37,38,39,40,41,42,43][14][15][16][17][18][19][20].
3. Physicochemical Features of the Self-Assembly in BCPs
3.1. Polymeric Micelles (PMs)
Unlike classic conventional micelles, the critical micelle concentration (CMC) of BCPs is often very low (<10 mg/L) and not accurately determined. They form kinetically stable nanoscale core–shell architectures that exist in nanoscale sizes (~10–100 nm) and have a fairly narrow size distribution, which enables them to accommodate nonpolar/weakly polar bioactive substances. Furthermore, PMs are less expected to break down and expedite the efficient drug administration over a long period of time. As a consequence, PMs have grabbed most of the research interest as potential cargo for therapeutic applications [20,59,60][21][22][23]. However, the solubilizate site relies on the chemical nature/structure of the solubilizate as well as that of the BCP and its micelle size/shape. As mentioned above, the core signifies the “cargo” for the encapsulation of various therapeutic reagents, where the hydrophilic shell confirms that the micelles remain in a discrete state, thereby minimizing the unwanted drug interactions with cells. Thus, PMs provide promising vectors for the carrying and delivery of drugs and allow the formation of multipurpose roles for the expansion of innovative therapies for incurable diseases [2,61,62][2][24][25].
Additionally, PMs can attain different features; with these features, they can be transformed into varied nanoparticles that remain undissociated in extreme dilutions via cross-linking of the core or shell, as shown in Figure 95. Cross-linking instigates covalent/non-covalent bonding and produces dynamic micellar systems [63,64,65][26][27][28]. Cross-linking raises the solubility by several orders of magnitude without diminishing the drug-loading capability. Furthermore, cross-linking has an effect on the permeability of the shell, which changes the pace at which the drug is released over time.
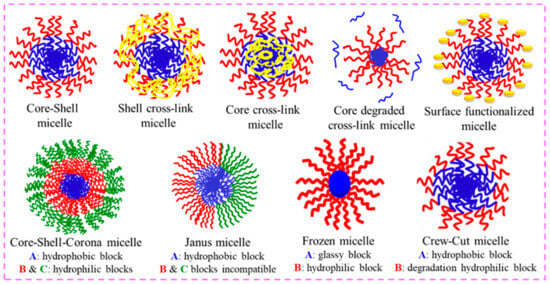

Figure 95. Alteration in the PMs via cross-linking of the core, shell, and surface functionalization. Also, other possible PMs structures, viz., core–shell–corona, Janus micelles, frozen micelles, and crew-cut micelles, are schematically shown.
An overall methodology aims to introduce some functional groups/substituents in the BCPs which ease the cross-linking of the core or shell. Also, the shell permeability can be tuned by changing the degree of cross-linking, which determines the drug loading and the release mechanism of the polymeric micelles.
3.2. Polymersomes
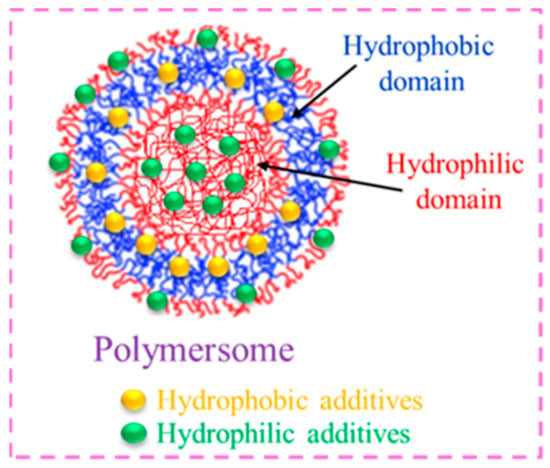
Usually, amphiphilic BCPs aggregate to form core–shell micelles of different microstructural features (spheroidal, rod- or wormlike structures). In addition, they exhibit artificial analogues of liposomes (bi-layered vesicles from low molecular weight phospholipids), which are often referred to as polymersomes. [78,79][29][30] These soft nanoparticles comprise a spherical aqueous environment enclosed by a bilayer membrane that is composed of a hydrated hydrophilic inner corona and an outer shield made of the hydrophobic middle part. These entities are well known due to their excellent robustness, high stability, longer blood circulation time, structural design, chemical versatility, and ease of surface functionalization, which make them attractive candidates in pharmaceuticals and the medicinal field [80,81][31][32]. Drugs, enzymes, proteins, peptides, and fragments of nucleic acids can all be solubilized by polymersomes inside their aqueous core area or membrane region [82,83][33][34]. Because of this, polymersomes have been developed into highly intriguing materials that can be used as nano-reservoirs in drug delivery systems (Figure 106).
Figure 106.
Hydrophobic/hydrophilic molecules entrapped in polymersomes.
3.3. Ethylene Oxide (EO)-Propylene Oxide (PO)-Based BCP Micelles
BCP has two or more different monomeric blocks in diverse structures and compositions, which give them distinctive solid-state and solution properties and allow them to show their usability in applications involving catalysis, detergency, dispersants, emulsifiers, pharmaceutical vehicles, et cetera. Among the amphiphilic BCPs, Pluronics
®
(poloxamers) and Tetronics
® (poloxamines), the commercially available FDA-approved EO-PO block copolymers, have been extensively examined [86,87,88]. These polymeric surfactants are marketed as triblock and 4-armed branched structures with varying PPO mol wt and %PEO. Pluronics
®
are poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) (PEO-PPO-PEO)-type amphiphilic triblock block copolymers, whereas Tetronics
®
are star-shaped poly(ethylene oxide) (EO)-poly(propylene oxide) (PO)-
based
amphiphilic block copolymers (BCPs); they have ethylene diamine as the central unit, to which four PO blocks are attached to nitrogen atoms, while the other end of the PO is linked to the EO block. Thus, their self-assembly leads to different sizes/shapes of core–shell micelles and polymersomes, liquid crystalline structures, and thermo-reversible gels, depending on their concentration. Furthermore, the self-assembly is strongly dependent on temperature and the presence of kosmotropic salts, ionic liquids, surfactants, and polar/nonpolar additives, which have been shown to have a profound effect on the formation properties and behavior of these nanoaggregates. Additionally, these copolymers can be functionalized at the end hydroxyl groups with drugs, bioimaging agents, and other functional polymers, which impart useful and stimuli-responsive properties. There have been several excellent reviews that describe the advances in aggregation and rheological aspects using a variety of experimental techniques, such as scattering and thermal techniques in particular, which detail the size/microstructures of the nanoaggregates and thermodynamics of their assemblies. Pluronics
®
and Tetronics
®
are chemically altered or functionalized to utilize various synthetic techniques, giving them improved and desirable qualities as vectors for delivery systems that enhance their properties. At the PEO chains of Pluronics
®
and Tetronics
®
, the covalent attachment of any small molecule, polymer block, ligand, or stimuli-responsive molecule changes the micellization, surface activity, solubilization power, and rheological behavior, which can be used to fine-tune the desired features in micelles. Medical diagnostics, imaging, and other applications have all taken advantage of these chemical changes in Pluronics
®
and Tetronics
® [36,89,90,91]. The core–shell micelles with an outer hydrophilic and extremely polar and flexible PEO shell provide stability and stealth properties to these micelles and prevent aggregation and interactions with mononuclear phagocytic systems that lead to the removal of micelles from systemic circulation. These micelles show a high drug-loading capacity and possess superior thermodynamic/kinetic properties and stability, which can be easily tuned further using mixed systems of two copolymers in a suitably designed concentration/mixing ratio. Mixed Pluronic (L61/F127) micelles with loaded doxorubicin have already become the first Pluronic
[13][38][39][40]. The core–shell micelles with an outer hydrophilic and extremely polar and flexible PEO shell provide stability and stealth properties to these micelles and prevent aggregation and interactions with mononuclear phagocytic systems that lead to the removal of micelles from systemic circulation. These micelles show a high drug-loading capacity and possess superior thermodynamic/kinetic properties and stability, which can be easily tuned further using mixed systems of two copolymers in a suitably designed concentration/mixing ratio. Mixed Pluronic (L61/F127) micelles with loaded doxorubicin have already become the first Pluronic
®
-
based micellar formulation to enter clinical trials as SP1049C (Supratek Pharma Inc., Dorval, QC, Canada). The therapeutic efficiency of these polymeric micelles relies on how these overcome biological barriers and deliver and release the drug at the target site [88,89,90,91,92].
micellar formulation to enter clinical trials as SP1049C (Supratek Pharma Inc., Dorval, QC, Canada). The therapeutic efficiency of these polymeric micelles relies on how these overcome biological barriers and deliver and release the drug at the target site [37][38][39][40][41].
3.4. Polyion Complex Micelles (PICMs)
Polyion complex micelles (PICMs) form when aqueous solutions of two oppositely charged polyelectrolytes are mixed. In addition, strong electrostatic interaction increases entropy
because of the counter ion release from the macroions; several other interactions,
like hydrogen bonding and hydrophobic interactions, could also contribute. Additionally, stoichiometrically mixing two polyelectrolytes with opposing charges leads to the spontaneous formation of PICMs/polymersomes in water (insoluble in other solvents) due to electrostatic attraction. Due to the reciprocal neutralization of the two diametrically opposed charges, PICMs/polymersomes have no charge. Polyelectrolytes with opposing charges neutralize each other, which causes them to
drop their colloidal stability and precipitate out of the solution. If polyelectrolytes can be connected to any hydrophilic and nonionic unit, the precipitation of PICMs/polymersomes can be prevented. The complexes can be water-soluble or insoluble (as coacervates or stable colloidal dispersions); their nature in terms of charged groups, molecular weight, flexibility, et cetera depends on the polyelectrolytes used and the stoichiometric mixing,
in addition to the solution conditions (pH, temperature, and the presence of salt or other additives). The effects of ionic strength and pH are
significant in the formation of PICs.
PICs have multiple applications in textiles, ink, and paper industries as binders, in coatings, and as flocculants for water purification, to name a few. In 1949, Fuoss et al. first reported that two oppositely charged polymers formed insoluble precipitates, but it was not until 1965 [100] that Michaels et al., after mixing two polyelectrolytes with opposing charges, studied stable nanosized spherical complexes by adjusting the solution pH, temperature, salt content, and other parameters. By successively adsorbing polyelectrolytes from solution onto a surface, layer-b-layer (LbL) films of interacting polyelectrolytes can be created [101,102,103]. The polyelectrolyte complexes formed in solution and multilayers on surfaces are extensively investigated using a variety of polyelectrolytes with several experimental techniques. PICs have multiple applications in textiles, ink, and paper industries as binders, in coatings, and as flocculants for water purification, to name a few. In 1949, Fuoss et al. first reported that two oppositely charged polymers formed insoluble precipitates, but it was not until 1965 [42] that Michaels et al., after mixing two polyelectrolytes with opposing charges, studied stable nanosized spherical complexes by adjusting the solution pH, temperature, salt content, and other parameters. By successively adsorbing polyelectrolytes from solution onto a surface, layer-b-layer (LbL) films of interacting polyelectrolytes can be created [43][44][45]. The polyelectrolyte complexes formed in solution and multilayers on surfaces are extensively investigated using a variety of polyelectrolytes with several experimental techniques.
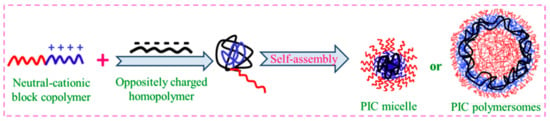
A polyelectrolyte complex can self-assemble into PICMs when it becomes amphiphilic, which is similar to the way that amphiphilic BCPs behave. DHBCs made of a hydrophilic neutral block–polyelectrolyte block are molecularly dissolved in water. Nevertheless, if the solution of an oppositely charged polymer is added to it, self-assembly happens as a consequence of electrostatic attraction, where a core of complexed oppositely charged polymers is formed. The formation of micelles, viz., polyion complex micelles or polyion complex polymersomes and their morphological features, can be finely tuned based on the charge densities of complexing polyelectrolytes, their mixing composition, and their solution conditions, which enable them to be employed in drug and protein delivery [104,105,106,107,108]. The micelles formed with a complexed core and hydrated shells are PICMs (A polyelectrolyte complex can self-assemble into PICMs when it becomes amphiphilic, which is similar to the way that amphiphilic BCPs behave. DHBCs made of a hydrophilic neutral block–polyelectrolyte block are molecularly dissolved in water. Nevertheless, if the solution of an oppositely charged polymer is added to it, self-assembly happens as a consequence of electrostatic attraction, where a core of complexed oppositely charged polymers is formed. The formation of micelles, viz., polyion complex micelles or polyion complex polymersomes and their morphological features, can be finely tuned based on the charge densities of complexing polyelectrolytes, their mixing composition, and their solution conditions, which enable them to be employed in drug and protein delivery [46][47][48][49][50]. The micelles formed with a complexed core and hydrated shells are PICMs (
Figure 11).7).

Figure 117. Polyion complex (PIC) micelles and polymersomes.
3.5. Polymerization-Induced Self-Assembly (PISA)
Polymerization-induced self-assembly (PISA) is a versatile approach that associates polymerization and self-assembly in a more concentrated solution for the rational production of concentrated BCP assemblies in a high yield using controlled/living polymerization techniques. It is a straightforward one-pot process with convenient scalability and economic viability. [108,109,110] The synthesis of linear as well as nonlinear (e.g., star-shaped, or branched) BCPs can be performed by PISA. PISA mediated by RAFT using suitable chain transfer agents and a water-miscible monomer has become a powerful technique that provides BCP micelles with a defined morphology, a controlled size, and surface functionality [111,112,113]. In contrast to the conventional approach, PISA effectively produces a higher concentration of nano-assemblies without adding a non-solvent to induce micellization. The first polymeric block and the second monomer that are polymerized in PISA must both be soluble in the same solvent. When the second block is sufficiently lengthy, its solubility is reduced, allowing the resultant BCP to self-assemble in place and produce nanostructures. PISA involves the aqueous dispersion polymerization of a water-miscible monomer till a critical degree of polymerization (DP) transforms it into a water insoluble polymer, which then self-assembles into a core–shell micelle of different morphologies, depending on the molecular weight and nature/composition of the blocks (
Polymerization-induced self-assembly (PISA) is a versatile approach that associates polymerization and self-assembly in a more concentrated solution for the rational production of concentrated BCP assemblies in a high yield using controlled/living polymerization techniques. It is a straightforward one-pot process with convenient scalability and economic viability. [50][51][52] The synthesis of linear as well as nonlinear (e.g., star-shaped, or branched) BCPs can be performed by PISA. PISA mediated by RAFT using suitable chain transfer agents and a water-miscible monomer has become a powerful technique that provides BCP micelles with a defined morphology, a controlled size, and surface functionality [53][54][55]. In contrast to the conventional approach, PISA effectively produces a higher concentration of nano-assemblies without adding a non-solvent to induce micellization. The first polymeric block and the second monomer that are polymerized in PISA must both be soluble in the same solvent. When the second block is sufficiently lengthy, its solubility is reduced, allowing the resultant BCP to self-assemble in place and produce nanostructures. PISA involves the aqueous dispersion polymerization of a water-miscible monomer till a critical degree of polymerization (DP) transforms it into a water insoluble polymer, which then self-assembles into a core–shell micelle of different morphologies, depending on the molecular weight and nature/composition of the blocks (
Figure 12).
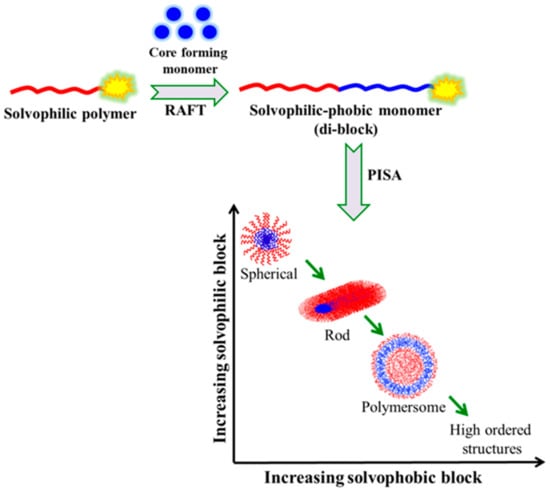
8).

Figure 128. Schematic representation of PISA in solution.
3.6. Crystallization-Driven Self-Assembly (CDSA)
Crystallization-driven self-assembly (CDSA) represents a handy methodology for the production of 1D and 2D anisotropic nanostructures with a high level of dimensional control and a tunable core–shell micelle in solution. This approach has shown that semicrystalline BCPs are promising for the development of materials with complex architectures [117,118,119]. This propensity is caused by the core-forming block crystallizing after being heated over the glass transition temperature and cooled in a weak solvent. As a result, polymers that are easily accessible by coupled ring-opening and RAFT polymerizations can be used to create nanostructures with anisotropic morphologies and excellent solution stability. The self-assembly process is facilitated when one block of the BCP is crystallizable.
Crystallization-driven self-assembly (CDSA) represents a handy methodology for the production of 1D and 2D anisotropic nanostructures with a high level of dimensional control and a tunable core–shell micelle in solution. This approach has shown that semicrystalline BCPs are promising for the development of materials with complex architectures [56][57][58]. This propensity is caused by the core-forming block crystallizing after being heated over the glass transition temperature and cooled in a weak solvent. As a result, polymers that are easily accessible by coupled ring-opening and RAFT polymerizations can be used to create nanostructures with anisotropic morphologies and excellent solution stability. The self-assembly process is facilitated when one block of the BCP is crystallizable.
4. Applications of BCPs
The applications of BCP-derived nanopatterns in real-life technologies are of great significance in several areas. BCPs are extensively used in the advancing field of nanopatterning, including next-generation lithography for semiconductors. The capabilities constituting the BCP toolbox have grown over the ages and have delivered numerous extraordinary achievements. However, a few challenges remain to be overcome in ensuring its integrity in the development process. The nanoscale self-assembly of BCPs like micelles and vesicles has received great attention in the field of drug delivery applications due to its effective control of morphology, surface chemistry, and responsiveness [126,127,128,129,130,131,132,133,134,135,136,137,138][59][60][61][62][63][64][65][66][67][68][69][70][71]. Organic dyes are only partly water soluble, which is a major challenge for dye and paint industries. BCPs tend to solubilize such hydrophobic moieties in their micellar core in a manner which depends on the hydrophobicity of the BCPs. Several studies have reported the ease in which dye solubilization can be influenced in the presence of external stimuli or additives [139,140,141,142][72][73][74][75]. Due to their ideal drug loading and release features, lengthy shelf life, and low toxicity, smart BCPs are particularly successful in controlled drug delivery applications, as well as for treatment and diagnostics. In particular, polymersomes serve as successful carriers for drug delivery systems [143,144,145,146][76][77][78][79]. Consequently, due to their high functionality, these BCPs might be used in a variety of medical devices, including nanoreactors, “semi-artificial” enzymes, and biodevices with blood-compatible surface treatments [147,148,149,150][80][81][82][83].
Polymeric micelles have emerged as promising nanocarriers due to their ability to control the drug release profile and the release at the target site; they have enhanced permeability and retention and enhance the solubility of hydrophobic bioactive substances, particularly anticancer drugs. They can build up in the tumor microenvironment because of the increased permeability and retention impact of their nanoscale size. These micelles from biocompatible and nontoxic amphiphilic polymers are anticipated to be a fruitful treatment option for drug and gene delivery (DNA/siRNA) in various cancer therapeutic strategies, like the crossing of the blood–brain barrier, photothermal therapy, gene therapy, and immunotherapy. The majority of polymeric micelles are undergoing clinical trials for various cancer therapies. Here is a description of both the commercially available polymeric micelles and the micelles presently undergoing clinical trials. The paclitaxel (PTX)-loaded polymeric micelles (NK105) have improved clinical efficacy against ovarian, lung, neck, breast, and brain cancers.
Pharmaceutical formulations are generally very complex and not completely understood. An enormous number of BCP-based drug delivery systems are widely explored to understand the drug release from the dosage, with the aim of developing the formulations that ensure safety and efficacy for patients [161,162,163,164,165][84][85][86][87][88]. Several different strategies have been engaged to improve the solubility and bioavailability of hydrophobic drugs. The nanoscale self-assemblies (polymer micelles and polymersomes) have been of great interest in recent years as these offer several advantages. The use of cosolvents, hydrotropes, micronization, and supramolecular complexes and the altering of the crystal structure have been adopted for the initial processes. Hydrogels, solid lipid nanoparticles, self-emulsifying dispersions, nano- and microemulsions, and liposomes have been developed over time.
The application of BCPs in the sensing field is also gaining momentum significantly. In an effort to create sensors with high sensitivity and selectivity, novel transduction methods and manufacturing techniques have been disclosed [167][89]. Metal oxides, non-oxide inorganics, and carbon-based ordered porous materials exhibit aligned structures and uniform cavities with micro- to meso- to macropore sizes, which lead to a very high surface area. Such features enable their utilization in energy conversion and storage, catalysis, gas capture, and water purification. Apart from the above-mentioned applications of these BCPs, their extensive use in separation and surface coating is anticipated [139,140,141,142,143][72][73][74][75][76].
References
- Riess, G.; Hurtrej, G.; Bahadur, P. Block Copolymers, Encyclopedia of Polymer Science Engineering. Wiley 1985, 2, 324–434.
- Riess, G. Micellization of block copolymers. Prog. Polym. Sci. 2003, 28, 1107–1170.
- Alexandridis, P.; Lindman, B. Amphiphilic Block Copolymers: Self-Assembly and Applications; Elsevier: Amsterdam, The Netherlands, 2000.
- Lu, Y.; Lin, J.; Wang, L.; Zhang, L.; Cai, C. Self-Assembly of Copolymer Micelles: Higher-Level Assembly for Constructing Hierarchical Structure. Chem. Rev. 2020, 120, 4111–4140.
- Nabiyan, A.; Max, J.B.; Schacher, F.H. Double hydrophilic copolymers-synthetic approaches, architectural variety, and current application fields. Chem. Soc. Rev. 2022, 51, 995–1044.
- Nadal, C.; Gineste, S.; Coutelier, O.; Marty, J.D.; Destarac, M. A deeper insight into the dual temperature- and pH-responsiveness of poly(vinylamine)-b-poly(N-isopropylacrylamide) double hydrophilic block copolymers. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128502–128513.
- Schmidt, B.V.K.J. Double Hydrophilic Block Copolymer Self-Assembly in Aqueous Solution. Macromol. Chem. Phys. 2018, 219, 1700494–1700508.
- Butun, V.; Armes, S.; Billingham, N.; Tuzar, Z.; Rankin, A.; Eastoe, J.; Heenan, R. Synthesis and aqueous solution properties of a well-defined thermo-responsive schizophrenic diblock copolymer. Macromolecules 2001, 34, 1503.
- Liu, S.; Billingham, N.C.; Armes, S.P. A Schizophrenic Water-Soluble Diblock Copolymer. Angew. Chem. Int. Ed. 2001, 40, 2328–2332.
- Kuperkar, K.; Patel, D.; Atanase, L.; Bahadur, P. Amphiphilic Block Copolymers: Their Structures, and Self-Assembly to Polymeric Micelles and Polymersomes as Drug Delivery Vehicles. Polymers 2022, 14, 4072.
- Liu, S.; Armes, S.P. Micelle Formation and Inversion Kinetics of a Schizophrenic Diblock Copolymer. Langmuir 2002, 19, 4432.
- Papadakis, C.M.; Müller-Buschbaum, P.; Laschewsky, A. Switch it inside-out: “Schizophrenic” behavior of all thermoresponsive UCST-LCST diblock copolymers. Langmuir 2019, 35, 9660–9676.
- Alam, M.; Keiko, H.; Yusa, S.; Nakashima, K. Schizophrenic micelle of a water-soluble diblock polymer and its application to a thermo-optical device. Colloid Polym. Sci. 2014, 292, 1611–1617.
- Guragain, S.; Bastakoti, B.; Malgras, V.; Nakashima, K.; Yamauchi, Y. Multi-Stimuli-Responsive Polymeric Materials. Chem.-A Eur. J. 2021, 21, 13164–13174.
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003.
- Stuart, M.A.C.; Huck, W.T.S.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, T.; Urban, M.; Winnik, F.; et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113.
- Mizoue, Y.; Takahashi, R.; Sakurai, K.; Yusa, S. A Thermo-Responsive Polymer Micelle with a Liquid Crystalline Core. Polymers 2023, 15, 770.
- Bhattacharya, D.; Behera, B.; Sahu, S.; Ananthakrishnan, R.; Maiti, T.; Pramanik, P. Design of Dual Stimuli Responsive Polymer Modified Magnetic Nanoparticles for Targeted Anti-Cancer Drug Delivery and Enhanced MR Imaging. New J. Chem. 2016, 40, 545–557.
- Appold, M.; Mari, C.; Lederle, C.; Elbert, J.; Schmidt, C.; Ott, I.; Stühn, B.; Gasser, G.; Gallei, M. Multi-stimuli responsive block copolymers as a smart release platform for a polypyridyl ruthenium complex. Polym. Chem. 2016, 8, 890–900.
- Fleige, E.; Quadir, M.; Haag, R. Stimuli-responsive polymeric nanocarriers for the controlled transport of active compounds: Concepts and applications. Adv. Drug. Deliv. Rev. 2012, 64, 866–884.
- Kataoka, K.; Harada, A.; Nagasaki, Y. Block copolymer micelles for drug delivery: Design, characterization and biological significance. Adv. Drug Deliv. Rev. 2001, 47, 113–131.
- Mai, Y.; Eisenberg, A. Self-assembly of block copolymers. Chem. Soc. Rev. 2012, 41, 5969–5985.
- Procházka, K.; Limpouchová, Z.; Štěpánek, M.; Šindelka, K.; Lísal, M. DPD Modelling of the Self-and Co-Assembly of Polymers and Polyelectrolytes in Aqueous Media: Impact on Polymer Science. Polymers 2022, 14, 404.
- Tuzar, Z.; Kratochvil, P. Block and graft copolymer micelles in solution. Adv. Colloid Interface Sci. 1976, 6, 201–232.
- Aqeel, R.; Srivastava, N.; Kushwaha, P. Micelles in Cancer Therapy: An Update on Preclinical and Clinical Status. Recent Pat. Nanotechnol. 2022, 16, 283–294.
- Wang, H.; Fliedel, C.; Manoury, E.; Poli, R. Core-crosslinked micelles with a poly-anionic poly(styrene sulfonate)-based outer shell made by RAFT polymerization. Polymer 2022, 243, 124640–124649.
- Xiong, D.; Yao, N.; Gu, H.; Wang, J.; Zhang, L. Stimuli-responsive shell cross-linked micelles from amphiphilic four-arm star copolymers as potential nanocarriers for “pH/redox-triggered” anticancer drug release. Polymer 2017, 114, 161–172.
- Bai, J.; Wang, J.; Feng, Y.; Yao, Y.; Zhao, X. Stability-tunable core-crosslinked polymeric micelles based on an imidazole-bearing block polymer for pH-responsive drug delivery. Colloids Surf. A 2022, 639, 128353–128361.
- Chen, W.; Meng, F.; Cheng, R.; Zhong, Z. pH-Sensitive degradable polymersomes for triggered release of anticancer drugs: A comparative study with micelles. J. Control. Release 2010, 142, 40–46.
- Discher, D.E.; Eisenberg, A. Polymer vesicles. Science 2002, 297, 967–973.
- Discher, D.E.; Ahmed, F. Polymersomes. Annu. Rev. Biomed. Eng. 2006, 8, 323–341.
- Letchford, K.; Burt, H. A review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: Micelles, nanospheres, nanocapsules and polymersomes. Eur. J. Pharm. Biopharm. 2007, 65, 259–269.
- Robertson, J.; Yealland, G.; Avila-Olias, M.; Chierico, L.; Bandmann, O.; Renshaw, S.; Battaglia, G. pH-sensitive tubular polymersomes: Formation and applications in cellular delivery. ACS Nano 2014, 8, 4650–4661.
- Pawar, P.; Gohil, S.; Jain, J.; Kumar, N. Functionalized polymersomes for biomedical applications. Polym. Chem. 2013, 4, 3160–3176.
- Pitto-Barry, A.; Barry, N.P.E. Pluronic® block-copolymers in medicine: From chemical and biological versatility to rationalisation and clinical advances. Polym. Chem. 2014, 5, 3291–3297.
- Singla, P.; Garg, S.; McClements, J.; Jamieson, O.; Peeters, M.; Mahajan, R. Advances in the therapeutic delivery and applications of functionalized Pluronics: A critical review. Adv. Colloid Interface Sci. 2022, 299, 102563–102594.
- Chiappetta, D.A.; Sosnik, A. Poly(ethylene oxide)-poly(propylene oxide) block copolymer micelles as drug delivery agents: Improved hydrosolubility, stability and bioavailability of drugs. Eur. J. Pharm. Biopharm. 2007, 66, 303–317.
- Zlotnikov, I.D.; Ezhov, A.A.; Ferberg, A.S.; Krylov, S.S.; Semenova, M.N.; Semenov, V.V.; Kudryashova, E.V. Polymeric Micelles Formulation of Combretastatin Derivatives with Enhanced Solubility, Cytostatic Activity and Selectivity against Cancer Cells. Pharmaceutics 2023, 15, 1613.
- Jundi, A.E.; Buwalda, S.J.; Bakkour, Y.; Garric, X.; Nottelet, B. Double hydrophilic block copolymers self-assemblies in biomedical applications. Adv. Colloid Int. Sci. 2020, 283, 102213–102279.
- Giaouzi, D.; Pispas, S. PNIPAM-b-PDMAEA double stimuli responsive copolymers: Effects of composition, end groups and chemical modification on solution self-assembly. Eur. Polym. J. 2020, 135, 109867.
- Bhowmik, S.; Pham, T.; Takahashi, R.; Kim, D.; Matsuoka, H.; Ishihara, K.; Yusa, S. Preparation of Water-Soluble Polyion Complex (PIC) Micelles with Random Copolymers Containing Pendant Quaternary Ammonium and Sulfonate Groups. Langmuir 2023, 39, 8120–8129.
- Nakai, K.; Nishiuchi, M.; Inoue, M.; Ishihara, K.; Sanada, Y.; Sakurai, K.; Yusa, S. Preparation and characterization of polyion complex micelles with phosphobetaine shells. Langmuir 2013, 29, 9651–9661.
- Damsongsang, P.; Yusa, S.; Hoven, V. Zwitterionic nano-objects having functionalizable hydrophobic core: Formation via polymerization-induced self-assembly and their morphology. Eur. Polym. J. 2022, 179, 111536.
- Ohno, S.; Ishihara, K.; Yusa, S. Formation of Polyion Complex (PIC) Micelles and Vesicles with Anionic pH-Responsive Unimer Micelles and Cationic Diblock Copolymers in Water. Langmuir 2016, 32, 3945–3953.
- Yusa, S.; Yokoyama, Y.; Morishima, Y. Synthesis of oppositely charged block copolymers of polyethylene glycol via reversible addition-fragmentation chain transfer radical polymerization and characterization of their polyion complex micelles in water. Macromolecules 2009, 42, 376–383.
- Chen, F.; Stenzel, M. Polyion Complex Micelles for Protein Delivery. Aust. J. Chem. 2018, 71, 768–780.
- Harada, A.; Kataoka, K. Polyion complex micelle formation from double-hydrophilic block copolymers composed of charged and non-charged segments in aqueous media. Polym. J. 2018, 50, 95–100.
- Nakamura, N.; Mochida, Y.; Toh, K.; Anraku, Y.; Cabral, H. Effect of mixing ratio of oppositely charged block copolymers on polyion complex micelles for in vivo application. Polymers 2021, 13, 5.
- Charleux, B.; Delaittre, G.; Rieger, J.; D’Agosto, F. Polymerization-Induced Self-Assembly: From Soluble Macromolecules to Block Copolymer Nano-Objects in One Step. Macromolecules 2012, 45, 6753–6765.
- Derry, M.; Fielding, L.; Armes, S. Polymerization-induced self-assembly of block copolymer nanoparticles via RAFT non-aqueous dispersion polymerization. Prog. Polym. Sci. 2016, 52, 1–18.
- Penfold, N.; Yeow, J.; Boyer, C.; Armes, S. Emerging Trends in Polymerization-Induced Self-Assembly. ACS Macro Lett. 2019, 8, 1029–1054.
- Warren, N.; Armes, S. Polymerization-Induced Self-Assembly of Block Copolymer Nano-objects via RAFT Aqueous Dispersion Polymerization. J. Am. Chem. Soc. 2014, 136, 10174–10185.
- Pei, Y.; Lowe, A. Polymerization-induced self-assembly: Ethanolic RAFT dispersion polymerization of 2-phenylethyl methacrylate. Polym. Chem. 2014, 5, 2342–2351.
- Zhang, W.; Kadirkhanov, J.; Wang, C.; Ding, S.; Hong, C.; Wang, F.; You, Y. Polymerization-induced self-assembly for the fabrication of polymeric nano-objects with enhanced structural stability by cross-linking. Polym. Chem. 2020, 11, 3654–3672.
- Cao, J.; Tan, Y.; Chen, Y.; Zhang, L.; Tan, J. Expanding the Scope of Polymerization-Induced Self-Assembly: Recent Advances and New Horizons. Macromol. Rapid Comm. 2021, 42, 2100498–2100510.
- Inam, M.; Cambridge, G.; Pitto-Barry, A.; Laker, Z.L.; Wilson, N.R.; Mathers, R.T.; Dove, A.P.; O’Reilly, R.K. 1D vs. 2D shape selectivity in the crystallization-driven self-assembly of polylactide block copolymers. Chem. Sci. 2017, 8, 4223–4230.
- Gädt, T.; Ieong, N.; Cambridge, G.; Winnik, M.; Manners, I. Complex and hierarchical micelle architectures from diblock copolymers using living, crystallization-driven polymerizations. Nat. Mater. 2009, 8, 144–150.
- Ganda, S.; Stenzel, M. Concepts, fabrication methods and applications of living crystallization-driven self-assembly of block copolymers. Prog. Polym. Sci. 2020, 101, 101195–101205.
- Modena, M.; Rühle, B.; Burg, T.; Wuttke, S. Nanoparticle Characterization: What to Measure. Adv. Mater. 2019, 31, 1901556–1901580.
- Mourdikoudis, S.; Pallares, R.; Thanh, N. Characterization Techniques for Nanoparticles: Comparison and Complementarity upon Studying Nanoparticle Properties. Nanoscale 2018, 10, 12871–12934.
- Xu, L.; Zhang, Z.; Wang, F.; Xie, D.; Yang, S.; Wang, T.; Feng, L.; Chu, C. Synthesis, characterization, and self-assembly of linear poly(ethyleneoxide)-block–poly(propylene oxide)-block–poly(e-caprolactone) (PEO–PPO–PCL) copolymers. J. Colloid Interf. Sci. 2013, 393, 174–181.
- Jung, Y.; Park, W.; Park, H.; Lee, D.; Na, K. Thermo-sensitive injectable hydrogel based on the physical mixing of hyaluronic acid and Pluronic F-127 for sustained NSAID delivery. Carbohydr. Polym. 2017, 156, 403–408.
- Patel, D.; Ray, D.; Aswal, V.; Kuperkar, K.; Bahadur, P. Temperature stimulated self-association and micellar transition for star shaped normal and reverse EO-PO block copolymers and their mixed systems as potential use for anticancer drug solubilization. Soft Matter. 2022, 18, 4543–4553.
- Patel, D.; Jana, R.; Lin, M.; Kuperkar, K.; Seth, D.; Chen, L.; Bahadur, P. Revisiting the salt-triggered self-assembly in very hydrophilic triblock copolymer Pluronic® F88 using multitechnique approach. Colloid Polym. Sci. 2021, 299, 229–239.
- Lunagariya, J.; Sivakumar, N.; Asif, M.; Dhar, A.; Vekariya, R. Dependency of Anion and Chain Length of Imidazolium Based Ionic Liquid on Micellization of the Block Copolymer F127 in Aqueous Solution: An Experimental Deep Insight. Polymers 2017, 9, 285.
- Patel, D.; Ray, D.; Kuperkar, K.; Pal, H.; Aswal, V.; Bahadur, P. Solubilization, micellar transition and biocidal assay of loaded multifunctional antioxidants in Tetronic® 1304 micelles. Polym. Int. 2020, 69, 1097–1104.
- Patel, D.; Ray, D.; Kuperkar, K.; Aswal, V.; Bahadur, P. Parabens induced spherical micelle to polymersome transition in thermo-responsive amphiphilic linear and star-shaped EO-PO block copolymers. J. Mol. Liq. 2020, 316, 113897–113908.
- Singla, P.; Singh, O.; Chabba, S.; Aswal, V.; Mahajan, R. Sodium deoxycholate mediated enhanced solubilization and stability of hydrophobic drug Clozapine in pluronic micelles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 15, 143–154.
- Kim, H.; Park, S.; Hinsberg, W. Block copolymer-based nanostructures: Materials, processes, and applications to electronics. Chem. Rev. 2010, 110, 146–177.
- Kim, J.; Lee, J. Preparation and Properties of Poly(l-lactide)-block-poly(trimethylenecarbonate) as Biodegradable Thermoplastic Elastomer. Polym. J. 2002, 34, 203–208.
- Chowdhry, B.; Snowdena, M.; Leharne, S. A scanning calorimetric investigation of phase transitions in a PPO-PEO-PPO block copolymer. Eur. Polym. J. 1999, 35, 273–278.
- Sarolia, J.; Kumar, D.; Shah, S.; Bahadur, P.; Tiwari, S. Thermodynamics of pluronic 103 micellization in mannitol solution: Analyses based on isothermal titration calorimetry. Colloid Surf. A 2022, 648, 129240–129244.
- Santiago, A.; Vargas, J.; Jorge, V.; Cruz-Morales, J.; Mikhail, T.; Gavino, R.; Malkanduev, Y.; Sivov, N. Synthesis of New Polymer Ionomers via Ring-Opening Metathesis Polymerization. Open J. Organic Polym. Mat. 2014, 4, 84–91.
- Patel, D.; Vaswani, P.; Sengupta, S.; Ray, D.; Bhatia, D.; Choudhury, S.D.; Aswal, V.K.; Kuperkar, K.; Bahadur, P. Thermoresponsive phase behavior and nanoscale self-assembly generation in normal and reverse Pluronics®. Colloid Polym. Sci. 2023, 301, 75–92.
- Akhlaghi, S.; Ribeiro, I.; Boyd, B.; Loh, W. Impact of preparation method and variables on the internal structure, morphology, and presence of liposomes in phytantriol-Pluronic® F127cubosomes. Colloids Surf. B 2016, 145, 845–853.
- Rodrigues, E.; Morales, M.; Medeiros, S.; Suguihiro, M.; Baggio-Saitovitch, E. Pluronics coated sterically stabilized magnetite nanoparticles for hyperthermia applications. J. Magn. Magn. Mater. 2016, 416, 434–440.
- Kanga, E.; Sharker, S.; Inc, I.; Park, S. Pluronic mimicking fluorescent carbon nanoparticles conjugated with doxorubicin via acid-cleavable linkage for tumor-targeted drug delivery and bioimaging. J. Ind. Eng. Chem. 2016, 43, 150–157.
- Bhattacharjee, A.; Kumar, K.; Arora, A.; Katti, D. Fabrication and characterization of Pluronic modified poly(hydroxybutyrate) fibers for potential wound dressing applications. Mat. Sci. Eng. C 2016, 63, 266–273.
- Pellosi, D.; Calori, I.; Paula, L.; Hioka, N.; Quaglia, F.; Tedesco, A. Multifunctional theranostic Pluronic mixed micelles improve targeted photoactivity of Verteporfin in cancer cells. Mat. Sci. Eng. C 2017, 71, 1–9.
- Yap, L.; Yang, M. Evaluation of hydrogel composing of Pluronic F127 and carboxymethyl hexanoyl chitosan as injectable scaffold for tissue engineering applications. Colloids Surf. B 2016, 146, 204–211.
- Negut, I.; Bita, B. Polymeric Micellar Systems—A Special Emphasis on “Smart” Drug Delivery. Pharmaceutics 2023, 15, 976.
- Talelli, M.; Barz, M.; Rijcken, C.; Kiessling, F.; Hennink, W.; Lammers, T. Core-crosslinked polymeric micelles: Principles, preparation, biomedical applications and clinical translation. Nano Today 2015, 10, 93–117.
- Colfen, H. Double-Hydrophilic Block Copolymers: Synthesis and Application as Novel Surfactants and Crystal Growth Modifiers. Macromol. Rapid Commun. 2001, 4, 220–252.
- Zlotnikov, I.; Streltsov, D.; Ezhov, A.; Kudryashova, E. Smart pH- and Temperature-Sensitive Micelles Based on Chitosan Grafted with Fatty Acids to Increase the Efficiency and Selectivity of Doxorubicin and Its Adjuvant Regarding the Tumor Cells. Pharmaceutics 2023, 15, 1135.
- Szewczyk-Łagodzińska, M.; Plichta, A.; Dębowski, M.; Kowalczyk, S.; Iuliano, A.; Florjańczyk, Z. Recent Advances in the Application of ATRP in the Synthesis of Drug Delivery Systems. Polymers 2023, 15, 1234.
- Movassaghian, S.; Merkel, O.M.; Torchilin, V.P. Applications of polymer micelles for imaging and drug delivery. Wiley Interdiscip. Rev. Nanomed. 2015, 7, 691–707.
- Varela-Moreira, A.; Yang, S.; Marcel, H.F.; Twan, L.; Wim, E.H.; Raymond, M.S. Clinical application of polymeric micelles for the treatment of cancer. Mater. Chem. Front. 2017, 1, 1485–1501.
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Favero, E.D.; Cantù, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release. 2021, 332, 312–336.
- Junnuthula, V.; Kolimi, P.; Nyavanandi, D.; Sampathi, S.; Vora, L.K.; Dyawanapelly, S. Polymeric Micelles for Breast Cancer Therapy: Recent Updates, Clinical Translation and Regulatory Considerations. Pharmaceutics 2022, 4, 1860.
More
