Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Yasameen Etami.
Trauma in childhood and adolescence has long-term negative consequences in brain development and behavior and increases the risk for psychiatric disorders. Among them, post-traumatic stress disorder (PTSD) during adolescence illustrates the connection between trauma and substance misuse, as adolescents may utilize substances to cope with PTSD.
- adolescence
- SUDs
- PTSD
- epigenetics
- memory
- neuroimaging
1. Introduction
As PTSD can result in changes in the neural pathways of adolescents, it raises the question of potential connections to developing an SUD. During adolescence, physical and emotional changes occur that shape an individual’s development [1]. Different brain regions develop at various rates—including some that do not fully develop until the mid-20s—which have consequences for behavior [2]. The plasticity of the brain makes it susceptible to various internal and external influences until the mid-twenties [3]. Since adolescence is a period of high vulnerability for the emergence of mental illness, understanding brain developmental trajectories can provide explanations for a range of important behaviors including academic performance, sociability, and potential criminal justice involvement.
Trauma is also more likely to occur during childhood and adolescence than at any other time of life for individuals with SUD, and 24–30% of adolescents with PTSD have comorbid SUDs [4]. Adolescents with SUD reported a threefold higher rate of traumatic events and a fivefold higher prevalence of PTSD following traumatic events, compared to the general adolescent population [5]. SUDs can emerge as a coping mechanism for PTSD, and on their own have the potential to create complications in the physical and mental wellbeing of an individual.
2. Risk Factors
Post-traumatic stress disorder (PTSD) is a condition that can develop after a stressful, traumatic, or overwhelming event that may involve actual or potential injury or death. It is a complex disorder that cannot be defined strictly as a fear response, but rather as a variety of determinants that culminate after the traumatic event that maintains the disorder. Symptoms of PTSD include experiencing the event in the form of nightmares, avoidance, emotional numbing, or a high level of arousal. There are some defining clinical features of PTSD, as it is associated with stress, however, most individuals who are placed in extremely stressful situations will not develop PTSD. PTSD is similar to various other psychiatric disorders as its onset occurs after a stressor and its manifestation is even more likely in vulnerable individuals [35][6]. Childhood maltreatment is one of the most common causes of PTSD in adolescents [36][7].
When considering risk factors for developing PTSD, there are three main categories, pre-trauma, peri-trauma, and post-trauma. Not all individuals develop PTSD after experiencing a traumatic event, making these risk factors crucial for understanding the development of psychopathology. Pre-trauma factors can include age, gender, and race/ethnicity, peri-trauma factors include duration/severity of trauma experience, and post-trauma factors can include access to resources and social support following the traumatic event [37][8]. There are also factors that raise the chance of experiencing a traumatizing event and factors that raise the possibility of symptom development after the traumatic event. Through a diathesis-stress model, vulnerability factors and environmental stressors are considered together. Violence exposure can maintain PTSD symptoms over time, and boys and older youth are more likely to experience violence than girls and younger children. Sexual violence is another risk factor that becomes more common in late adolescence. Racial and ethnic differences associated with social disadvantages and a higher likelihood of exposure to adverse environments may serve as risk factors. For example, African American adolescents have a greater likelihood of experiencing violence, even though they are less likely to meet the criteria for PTSD, compared to youth from other racial groups. Previous history of violence and associating with deviant peers can serve as perpetuating risk factors for PTSD [38][9]. Social problems are another risk factor that can be a factor for developing PTSD in various ways, such as attachment insecurity, low social support, or social conflict [39,40,41][10][11][12].
3. Effects on the Limbic System
Studies have found that PTSD is associated with alterations to the fronto-limbic circuitry, specifically the hippocampus, amygdala, cingulate cortex, and prefrontal cortex [42][13]. For a summary of these findings, see Figure 1. Adversity faced early in life critically impacts the developing hippocampus [43][14]. In support, adolescents with childhood trauma show lower hippocampal gray matter compared to those without a history of trauma [44][15]. As previously mentioned, higher gray matter volume can indicate a malfunction in necessary synaptic pruning processes [16,17][16][17]. However, other researchers have interpreted this differently, finding that decreases in gray matter correlate with decreases in cognitive functioning, suggesting this may be a consequence of disorders triggered by environmental causes [45][18]. Many studies have examined memory impairments in adolescents with PTSD. Adolescents who have experienced bereavement show greater autobiographical memory impairments than those who have not [46][19]. Similarly, PTSD youth had lower scores on memory tests compared to healthy controls, indicating the influence of early trauma exposure at a young age on memory [47][20]. Children who were exposed to physical abuse and who came from households with low socioeconomic status were found to have smaller brain volumes in the hippocampus compared to those who did not have these experiences [48][21]. Another study found heightened activity of the hippocampus when adolescents were read trauma-related scripts [49][22]. With the hippocampus being a hub for memory, alterations in this essential brain region can signal long-lasting changes to neural circuitry, producing PTSD symptoms such as flashbacks.
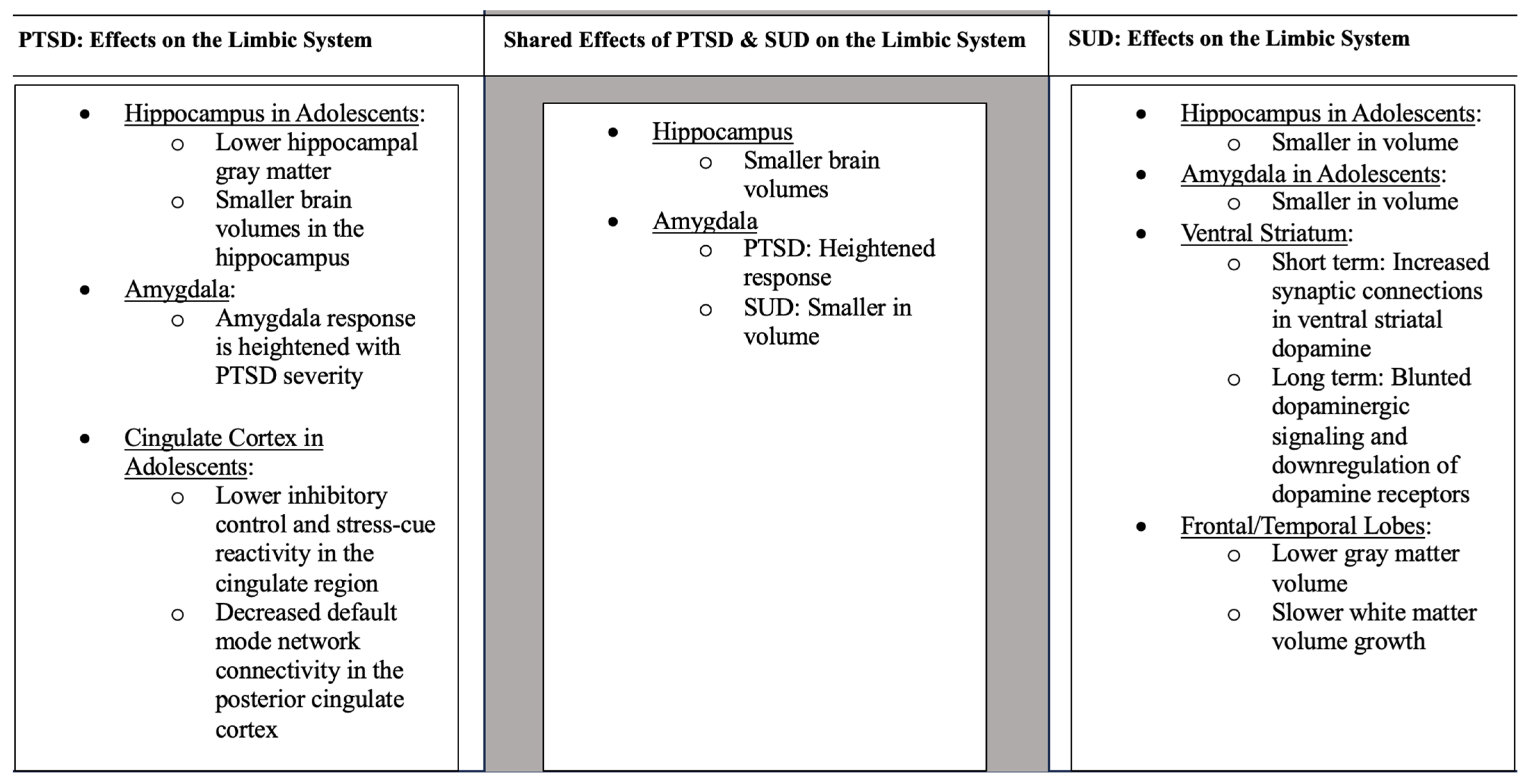
Figure 1. Unless otherwise stated, the information applies to adults.
Studies have also found that the amygdala is affected in adolescents with PTSD. One study found that compared to those without PTSD, children aged 10–16 who were diagnosed with PTSD after an earthquake were found to have higher concentrations of the following neurochemicals in the right amygdala compared to controls: N-acetylaspartate, myo-inositol, choline compounds, as well as creatine and phosphocreatine [50][23]. In an fMRI study, participants were shown happy, sad, neutral, and no-face primes and reported whether they produced positive or negative feelings. Results supported the idea that childhood adversity is associated with exaggerated amygdala response to negative facial stimuli [51][24]. However, since the traumatic experiences faced by the participants were self-reported, it could be that those who had stronger memories of traumatic events in their childhood were more likely to have a stronger amygdala response to a negative event. Additionally, it is important to note that the participants from this study were adults and were administered the Childhood Trauma Questionnaire to understand each individual’s retrospective trauma. Further, children aged 9–14 who were physically abused, faced negligence, and came from low socioeconomic households were shown to have smaller amygdala volumes than controls, similar to results for the hippocampus [48][21]. However, these results were from a single MRI scan, so the causal direction of effects is not yet established. Among college-aged individuals, amygdala volume in specific subregions linked to fear extinction and memory, including the centrocorticomedial complex (CMA) and the basolateral complex (BLA), were also correlated with PTSD symptomatology [52][25]. Additionally, listening to a description of past traumas resulted in higher amygdala activity compared to controls [49][22]. This finding supports previous research that the amygdala response is heightened as PTSD severity increases [53,54,55,56,57][26][27][28][29][30].
The cingulate cortex is essential for inhibitory control and stress responses [58][31]. Childhood trauma is associated with lower inhibitory control and diminished stress-cue reactivity in the cingulate region as found in a study with adolescents aged 14–17 [59][32]. Decreased inhibitory abilities provide a possible explanation for why traumatized youth act more impulsively than the general population. In adult participants affected by PTSD, there were significantly lower fractional anisotropy (FA) values in the cingulum than controls [60][33]. Alterations to this circuit in early adolescence correlate with poor cognitive and emotional functioning, potentially leading to heightened vulnerability to external stressors [61,62,63][34][35][36]. In another small fMRI study, participants aged 13–19 were asked to listen to a script of either a positive or negative event individualized to their past traumas. Elevated activity in the dorsal anterior cingulate cortex (ACC) was found in the individualized conditions compared to the generalized positive and negative scripts that were given as a baseline to each participant [49][22]. Interestingly, in a study examining adult PTSD, researchers have found gray matter decreases in the rostral ACC compared to healthy adults [60][33].
Other brain regions also appear to be implicated in trauma. A study exposing 74 healthy female subjects aged 18–36 to traumatic films found that more early intrusive memories correlated with lower volumes of the left insula, a common area affected in those with PTSD. Further, larger volumes of the left lingual gyrus/cerebellum and right inferior frontal gyrus/precentral gyrus correlated with greater amounts of intrusions [64][37]. These diverse findings point to the potentially diffuse nature of trauma pathophysiology.
4. Brain Network Connectivity
Many recent studies have also looked at the connectivity patterns in adolescents with PTSD. Supporting the hypothesis that adolescents with PTSD display similar network dysfunction as adults with PTSD, adolescents had increased connectivity within the default mode network (DMN) and decreased connectivity between the DMN and salience network (SN) and central executive network (CEN) than controls [65][38]. Since the DMN contributes to episodic memory and autobiographical memory, impaired DMN function may underlie some of the cognitive symptoms of PTSD. Other studies have also found disrupted nodal centrality, a measure of the significance of a node within a network, in the DMN, SN, and CEN [66][39]. The authors suggested that this decrease in DMN connectivity compared to controls may explain the flashbacks commonly experienced by PTSD victims, whereas the increased connectivity between the DMN and SN compared to controls may explain the exaggerated neural response during episodic memory recall in those with PTSD [65][38]. They also found positive and negative correlations between DMN and CEN connectivity strength, which may explain the disruptions of autobiographical memories during recollection of episodic memories.
Adolescents with PTSD also show decreased connectivity in limbic system regions [67][40]. Decreased DMN connectivity was found in the posterior cingulate cortex in adolescents with PTSD, a region well-studied for its functioning in visual mental imagery and autobiographical memory compared to controls [65,67][38][40]. In addition, compared to controls, adolescents aged 11–18 affected by interpersonal violence exposure and PTSD showed increases in intraparietal sulcus (IPS) cortical thickness, a crucial component of the frontoparietal cognitive control network necessary for learning and emotional processing compared to controls [68,69,70][41][42][43]. However, one recent study has found conflicting results. In 2020, Rinne-Albers et al., utilized MRI to investigate cortical thickness, surface area, and volume in adolescents with PTSD and a group of healthy controls [71][44]. Despite their initial hypothesis, there were no significant differences between the two groups on any cortical measures [71][44]. It is possible that since this study only examined women with PTSD after childhood sexual abuse, the conclusions may be specific to this population.
A history of adversities can predict the risk for the first onset of PTSD [72][45]. Different dimensions of adversity can impact brain development. Connectivity between most brain networks tends to decrease throughout adolescence, whereas youth exposed to adversity had stable connectivity over time [73][46]. Stability in functional brain networks could contribute to the internalization of symptoms from adolescence to adulthood [73][46].
Differences in the brain structural covariance network centrality of the ACC, posterior cingulate cortex (PCC), inferior frontal cortex/insula (IFC), and frontal pole (FP) are also affected in youth with PTSD [74][47]. With the findings of large centrality value for PCC, a key region within the episodic memory network, it could be theorized that kids with exposure to abuse continuously experience the memories of the events that caused their PTSD [67,74][40][47]. In each of these studies, the traumatic events faced by youth that result in PTSD diagnosis are associated with functional connectivity abnormalities in regions supporting memory function.
References
- Arain, M.; Haque, M.; Johal, L.; Mathur, P.; Nel, W.; Rais, A.; Sandhu, R.; Sharma, S. Maturation of the adolescent brain. Neuropsychiatr. Dis. Treat. 2013, 9, 449–461.
- Pujol, J.; Vendrell, P.; Junqué, C.; Martí-Vilalta, J.L.; Capdevila, A. When does human brain development end? Evidence of corpus callosum growth up to adulthood. Ann. Neurol. 1993, 34, 71–75.
- Gogtay, N.; Giedd, J.N.; Lusk, L.; Hayashi, K.M.; Greenstein, D.; Vaituzis, A.C.; Nugent, T.F.; Herman, D.H.; Clasen, L.S.; Toga, A.W.; et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. USA 2004, 101, 8174–8179.
- Simmons, S.; Suárez, L. Substance Abuse and Trauma. Child Adolesc. Psychiatr. Clin. N. Am. 2016, 25, 723–734.
- Basedow, L.A.; Kuitunen-Paul, S.; Roessner, V.; Golub, Y. Traumatic Events and Substance Use Disorders in Adolescents. Front. Psychiatry 2020, 11, 559.
- Brewin, C.R. What is it that a neurobiological model of PTSD must explain? In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2007; Volume 167, pp. 217–228.
- Bremner, J.D.; Randall, P.; Vermetten, E.; Staib, L.; Bronen, R.A.; Mazure, C.; Capelli, S.; McCarthy, G.; Innis, R.B.; Charney, D.S. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse—A preliminary report. Biol. Psychiatry 1997, 41, 23–32.
- Sayed, S.; Iacoviello, B.M.; Charney, D.S. Risk Factors for the Development of Psychopathology Following Trauma. Curr. Psychiatry Rep. 2015, 17, 70.
- Milan, S.; Zona, K.; Acker, J.; Turcios-Cotto, V. Prospective Risk Factors for Adolescent PTSD: Sources of Differential Exposure and Differential Vulnerability. J. Abnorm. Child Psychol. 2013, 41, 339–353.
- Benoit, M.; Bouthillier, D.; Moss, E.; Rousseau, C.; Brunet, A. Emotion regulation strategies as mediators of the association between level of attachment security and PTSD symptoms following trauma in adulthood. Anxiety Stress Coping 2010, 23, 101–118.
- Daviss, W.B.; Mooney, D.; Racusin, R.; Ford, J.D.; Fleischer, A.; McHUGO, G.J. Predicting Posttraumatic Stress After Hospitalization for Pediatric Injury. J. Am. Acad. Child Adolesc. Psychiatry 2000, 39, 576–583.
- Thrasher, S.; Power, M.; Morant, N.; Marks, I.; Dalgleish, T. Social Support Moderates Outcome in a Randomized Controlled Trial of Exposure Therapy and (or) Cognitive Restructuring for Chronic Posttraumatic Stress Disorder. Can. J. Psychiatry 2010, 55, 187–190.
- Herringa, R.J. Trauma, PTSD and the Developing Brain. Curr. Psychiatry Rep. 2017, 19, 69.
- Brooks, S.J.; Dalvie, S.; Cuzen, N.L.; Cardenas, V.; Fein, G.; Stein, D.J. Childhood adversity is linked to differential brain volumes in adolescents with alcohol use disorder: A voxel-based morphometry study. Metab. Brain Dis. 2014, 29, 311–321.
- Paquola, C.; Bennett, M.R.; Lagopoulos, J. Understanding heterogeneity in grey matter research of adults with childhood maltreatment-A meta-analysis and review. Neurosci. Biobehav. Rev. 2016, 69, 299–312.
- Huttenlocher, P.R.; Dabholkar, A.S. Regional differences in synaptogenesis in human cerebral cortex. J. Comp. Neurol. 1997, 387, 167–178.
- Paus, T.; Keshavan, M.; Giedd, J.N. Why do many psychiatric disorders emerge during adolescence? Nat. Rev. Neurosci. 2008, 9, 947–957.
- Luby, J.L.; Belden, A.C.; Jackson, J.J.; Lessov-Schlaggar, C.N.; Harms, M.P.; Tillman, R.; Botteron, K.; Whalen, D.; Barch, D.M. Early Childhood Depression and Alterations in the Trajectory of Gray Matter Maturation in Middle Childhood and Early Adolescence. JAMA Psychiatry 2016, 73, 31–38.
- Neshat Doost, H.T.; Yule, W.; Kalantari, M.; Rezvani, S.R.; Dyregrov, A.; Jobson, L. Reduced autobiographical memory specificity in bereaved Afghan adolescents. Memory 2014, 22, 700–709.
- Yasik, A.E.; Saigh, P.A.; Oberfield, R.A.; Halamandaris, P.V. Posttraumatic stress disorder: Memory and learning performance in children and adolescents. Biol. Psychiatry 2007, 61, 382–388.
- Hanson, J.L.; Nacewicz, B.M.; Sutterer, M.J.; Cayo, A.A.; Schaefer, S.M.; Rudolph, K.D.; Shirtcliff, E.A.; Pollak, S.D.; Davidson, R.J. Behavior Problems after Early Life Stress: Contributions of the Hippocampus and Amygdala. Biol. Psychiatry 2015, 77, 314–323.
- Malejko, K.; Tumani, V.; Rau, V.; Neumann, F.; Plener, P.L.; Fegert, J.M.; Abler, B.; Straub, J. Neural correlates of script-driven imagery in adolescents with interpersonal traumatic experiences: A pilot study. Psychiatry Res. Neuroimaging 2020, 303, 111131.
- Wang, W.; Sun, H.; Su, X.; Tan, Q.; Zhang, S.; Xia, C.; Li, L.; Kemp, G.J.; Yue, Q.; Gong, Q. Increased right amygdala metabolite concentrations in the absence of atrophy in children and adolescents with PTSD. Eur. Child Adolesc. Psychiatry 2019, 28, 807–817.
- Dannlowski, U.; Kugel, H.; Huber, F.; Stuhrmann, A.; Redlich, R.; Grotegerd, D.; Dohm, K.; Sehlmeyer, C.; Konrad, C.; Baune, B.T.; et al. Childhood maltreatment is associated with an automatic negative emotion processing bias in the amygdala. Hum. Brain Mapp. 2012, 34, 2899–2909.
- Ousdal, O.T.; Milde, A.M.; Hafstad, G.S.; Hodneland, E.; Dyb, G.; Craven, A.R.; Melinder, A.; Endestad, T.; Hugdahl, K. The association of PTSD symptom severity with amygdala nuclei volumes in traumatized youths. Transl. Psychiatry 2020, 10, 288.
- Brunetti, M.; Sepede, G.; Mingoia, G.; Catani, C.; Ferretti, A.; Merla, A.; Del Gratta, C.; Romani, G.L.; Babiloni, C. Elevated response of human amygdala to neutral stimuli in mild post traumatic stress disorder: Neural correlates of generalized emotional response. Neuroscience 2010, 168, 670–679.
- Dickie, E.W.; Brunet, A.; Akerib, V.; Armony, J.L. An fMRI investigation of memory encoding in PTSD: Influence of symptom severity. Neuropsychologia 2008, 46, 1522–1531.
- Dickie, E.W.; Brunet, A.; Akerib, V.; Armony, J.L. Neural correlates of recovery from post-traumatic stress disorder: A longitudinal fMRI investigation of memory encoding. Neuropsychologia 2011, 49, 1771–1778.
- Shin, L.M.; Rauch, S.L.; Pitman, R.K. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann. N. Y. Acad. Sci. 2006, 1071, 67–79.
- Zhong, Y.; Zhang, R.; Li, K.; Qi, R.; Zhang, Z.; Huang, Q.; Lu, G. Altered cortical and subcortical local coherence in PTSD: Evidence from resting-state fMRI. Acta Radiol. 2015, 56, 746–753.
- Bush, G.; Luu, P.; Posner, M.I. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 2000, 4, 215–222.
- Zhai, Z.W.; Yip, S.W.; Lacadie, C.M.; Sinha, R.; Mayes, L.C.; Potenza, M.N. Childhood trauma moderates inhibitory control and anterior cingulate cortex activation during stress. NeuroImage 2019, 185, 111–118.
- O’Doherty DC, M.; Ryder, W.; Paquola, C.; Tickell, A.; Chan, C.; Hermens, D.F.; Bennett, M.R.; Lagopoulos, J. White matter integrity alterations in post-traumatic stress disorder. Hum. Brain Mapp. 2018, 39, 1327–1338.
- Fields, R.D. Neuroscience. Change in the brain’s white matter. Science 2010, 330, 768–769.
- Fornari, E.; Knyazeva, M.G.; Meuli, R.; Maeder, P. Myelination shapes functional activity in the developing brain. NeuroImage 2007, 38, 511–518.
- Luna, B.; Garver, K.E.; Urban, T.A.; Lazar, N.A.; Sweeney, J.A. Maturation of cognitive processes from late childhood to adulthood. Child Dev. 2004, 75, 1357–1372.
- Gvozdanovic, G.; Stämpfli, P.; Seifritz, E.; Rasch, B. Structural brain differences predict early traumatic memory processing. Psychophysiology 2020, 57, e13354.
- Viard, A.; Mutlu, J.; Chanraud, S.; Guenolé, F.; Egler, P.-J.; Gérardin, P.; Baleyte, J.-M.; Dayan, J.; Eustache, F.; Guillery-Girard, B. Altered default mode network connectivity in adolescents with post-traumatic stress disorder. NeuroImage. Clin. 2019, 22, 101731.
- Niu, R.; Lei, D.; Chen, F.; Chen, Y.; Suo, X.; Li, L.; Lui, S.; Huang, X.; Sweeney, J.A.; Gong, Q. Disrupted grey matter network morphology in pediatric posttraumatic stress disorder. NeuroImage. Clin. 2018, 18, 943–951.
- Mo, X.; He, M.; Zhou, L.; Liu, Y.; Zhu, H.; Huang, X.; Zeng, G.; Zhang, J.; Li, L. Mapping structural covariance networks in children and adolescents with post-traumatic stress disorder after earthquake. Front. Psychiatry 2022, 13, 923572.
- Buhle, J.T.; Silvers, J.A.; Wager, T.D.; Lopez, R.; Onyemekwu, C.; Kober, H.; Weber, J.; Ochsner, K.N. Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies. Cereb. Cortex 2014, 24, 2981–2990.
- Peters, S.; Van Duijvenvoorde AC, K.; Koolschijn PC, M.P.; Crone, E.A. Longitudinal development of frontoparietal activity during feedback learning: Contributions of age, performance, working memory and cortical thickness. Dev. Cogn. Neurosci. 2016, 19, 211–222.
- Ross, M.C.; Sartin-Tarm, A.S.; Letkiewicz, A.M.; Crombie, K.M.; Cisler, J.M. Distinct cortical thickness correlates of early life trauma exposure and posttraumatic stress disorder are shared among adolescent and adult females with interpersonal violence exposure. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2021, 46, 741–749.
- Rinne-Albers, M.A.; Boateng, C.P.; Van Der Werff, S.J.; Lamers-Winkelman, F.; Rombouts, S.A.; Vermeiren, R.R.; Van Der Wee, N.J. Preserved cortical thickness, surface area and volume in adolescents with PTSD after childhood sexual abuse. Sci. Rep. 2020, 10, 3266.
- Lloyd, D.A.; Turner, R.J. Cumulative Adversity and Posttraumatic Stress Disorder: Evidence from a Diverse Community Sample of Young Adults. Am. J. Orthopsychiatry 2003, 73, 381–391.
- Chahal, R.; Miller, J.G.; Yuan, J.P.; Buthmann, J.L.; Gotlib, I.H. An exploration of dimensions of early adversity and the development of functional brain network connectivity during adolescence: Implications for trajectories of internalizing symptoms. Dev. Psychopathol. 2022, 34, 557–571.
- Sun, D.; Haswell, C.C.; Morey, R.A.; De Bellis, M.D. Brain structural covariance network centrality in maltreated youth with PTSD and in maltreated youth resilient to PTSD. Dev. Psychopathol. 2019, 31, 557–571.
More
