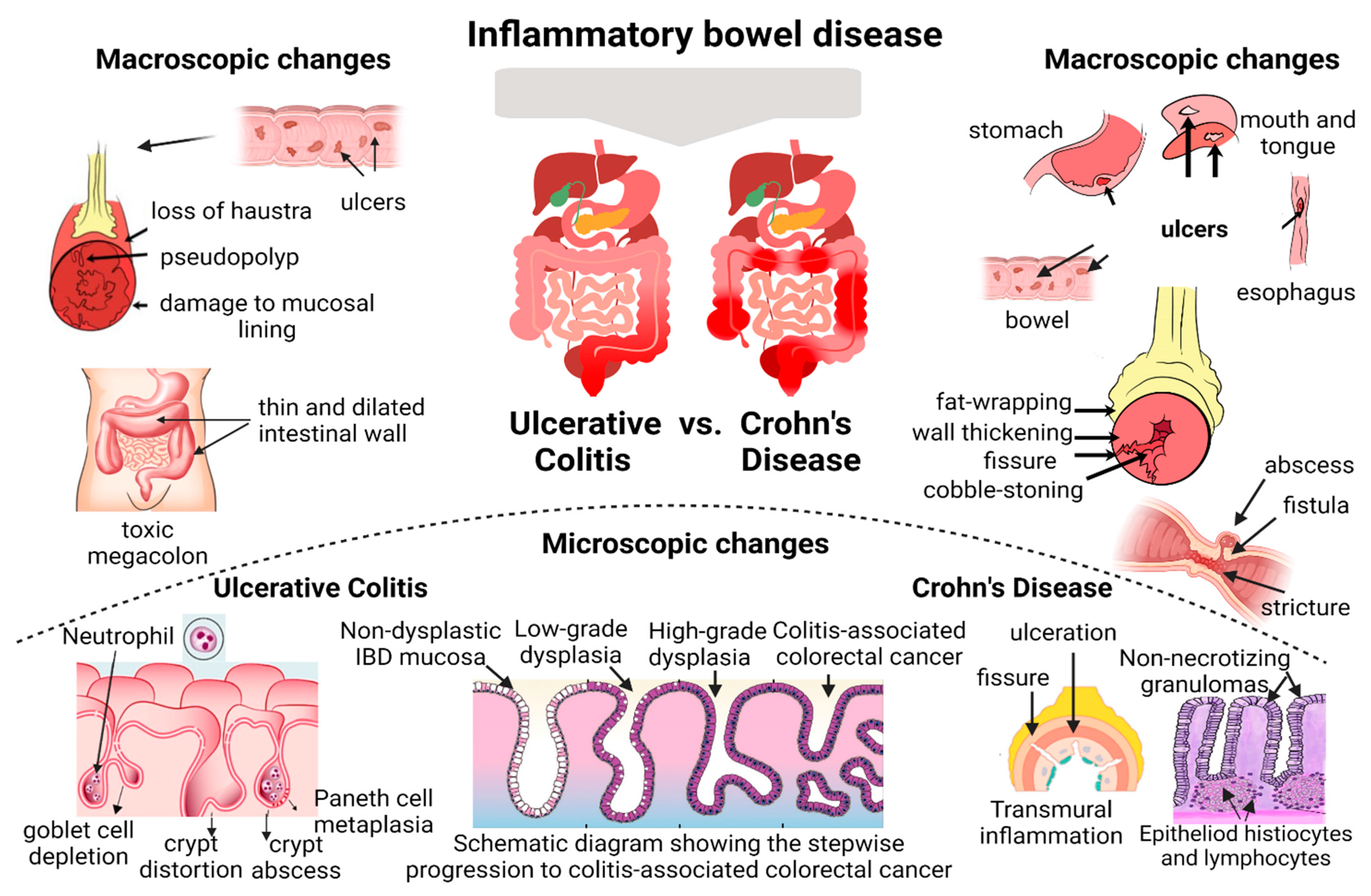
Inflammatory bowel disease (IBD) comprises two types of chronic intestinal disorders: Crohn’s disease and ulcerative colitis. In long-standing ulcerative colitis disease activity, histological persistent inflammation has been linked to an increased risk of relapse, and long-term corticosteroid use, even when endoscopic remission is reached. In Crohn’s disease, the discontinuous nature of lesions and transmural inflammation have limited the standardized histological assessment. The current evidence from research proposes that besides clinical and endoscopic healing, the achievement of histological healing constitutes an endpoint to assess disease activity and remission in IBD patients concerning better long-term disease outcomes. Histological alterations may persist even in the absence of endoscopic lesions. For these reasons, new advanced techniques promise to revolutionize the field of IBD by improving the endoscopic and histologic assessment, disease characterization, and ultimately patient care, with an established role in daily practice for objective assessment of lesions.
- Crohn’s disease
- histological healing
- inflammatory bowel disease
1. Introduction
2. Histological Healing—Current Concept and Clinical Relevance
| Study | Type of Study | Disease | N Patients | Endoscopic Activity | Histological Index | Outcome |
|---|---|---|---|---|---|---|
| Park et al. [21] | Systematic review and meta-analysis | UC | 1360 patients | Endoscopic remission | Truelove and Richards index; Riley index; Geboes score. Histological remission- present in 964 patients (71%). |
52% relative risk reduction in relapse/exacerbation for UC patients with histologic remission compared to histologic activity. |
| Narang et al. [22] | Prospective observational study | UC | 76 patients in clinical remission for at least 6 months. 46 patients with endoscopic remission included (Mayo score ≤ 1; 46/76, 60.5%), 1 year of follow-up. |
Endoscopic remission | Geboes score; Histological remission in 67.3% (31/46) of patients, while 32.7% (15/46) with histologically active disease. |
87.1% (27/31) of patients with histological remission remained asymptomatic, while 12.9% (4/31) had relapsed. Among histologically active patients, 46.6% (7/15) sustained clinical remission, while 53.3% (8/15) had relapsed. (87.1% vs. 46.6%, p = 0.006). |
| Ozaki et al. [23] | Prospective study | UC | 194 patients, 20 months of follow-up. |
Endoscopic remission | NHI was significantly higher in MES 1 than in MES 0 [1.11 ± 0.09 vs. 0.41 ± 0.07, p < 0.0001]. | 67 patients relapsed during the follow-up period; risk of relapse (HR- 2.18 [1.16–5.82]; p = 0.03). |
| Bryant et al. [24] | Prospective study | UC | 91 patients, 6 years of follow-up. |
Endoscopic remission | 24% of patients had persistent inflammation. | Histological remission predicted lower rates of corticosteroid use and acute severe colitis requiring hospitalization during follow-up (HR 0.42 (0.2 to 0.9), p = 0.02; HR 0.21 (0.1 to 0.7), p = 0.02, respectively). |
| Bessissow et al. [25] | Cohort study | UC | 75 patients, 12 months of follow-up |
Endoscopic remission | Geboes score ≥3.1 in 40% and basal plasmacytosis in 21% of patients. | The presence of basal plasmacytosis, predictive of CR; OR = 5.13 (95% CI: 1.32–19.99), p = 0.019. |
| Calafat et al. [26] | Retrospective observational study | UC | 113 patients underwent dysplasia surveillance colonoscopy between January 2005 and October 2015; follow-up of 12 months was included. The median time of follow-up—2.5 years. |
Endoscopic remission | 62 patients (57%) presented NQHA, 33 (30%) presented CHA, and 22 (20%) presented AHA. Basal plasmacytosis- present in 9 patients (8%), six of them in association with AHA (5%). | 9 patients (8%) relapsed within the first year of follow-up and 37 patients (33%) relapsed during the whole follow-up period. The presence of AHA is a risk factor for clinical relapse. |
| Christensen et al. [27] | Retrospective study | CD | 101 patients, follow-up for a median of 21 months. |
63% of patients with endoscopic remission. | 55% of patients achieved histologic remission. | CR occurred in 42% (n = 42) of patients Histologic healing was associated with a decreased risk of CR (HR- 2.05; 95% CI, 1.07–3.94; p = 0.031). |
| Brennan et al. [28] | Retrospective cohort study | CD | 62 patients, follow-up for at least 6 months. A total of 103 patients with CD underwent elective colonoscopies during clinical remission. |
55 patients (53%) in endoscopic healing, 48 patients (47%) with active disease. |
A semiqualitative score (0 to 3) was assigned for the histologic characteristics in each of the biopsy samples. | At 12 months, the rate of relapse was 25.5% in patients with histologic activity, compared with only 2.4% of patients without histologic activity at baseline. The presence of histological activity was associated with higher flare rates (p < 0.05). |
References
- Zhang, Y.; Si, X.; Yang, L.; Wang, H.; Sun, Y.; Liu, N. Association between intestinal microbiota and inflammatory bowel disease. Anim. Models Exp. Med. 2022, 5, 311–322.
- Fabian, O.; Bajer, L. Histopathological assessment of the microscopic activity in inflammatory bowel diseases: What are we looking for? World J. Gastroenterol. 2022, 28, 5300–5312.
- Chateau, T.; Feakins, R.; Marchal-Bressenot, A.; Magro, F.; Danese, S.; Peyrin-Biroulet, L. Histological Remission in Ulcerative Colitis: Under the Microscope Is the Cure. Am. J. Gastroenterol. 2020, 115, 179–189.
- Ma, C.; Sedano, R.; Almradi, A.; Vande Casteele, N.; Parker, C.E.; Guizzetti, L.; Schaeffer, D.F.; Riddell, R.H.; Pai, R.K.; Battat, R.; et al. An International Consensus to Standardize Integration of Histopathology in Ulcerative Colitis Clinical Trials. Gastroenterology 2021, 160, 2291–2302.
- Danese, S.; Roda, G.; Peyrin-Biroulet, L. Evolving therapeutic goals in ulcerative colitis: Towards disease clearance. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 1–2.
- Colombel, J.F.; D’haens, G.; Lee, W.J.; Petersson, J.; Panaccione, R. Outcomes and Strategies to Support a Treat-to-target Approach in Inflammatory Bowel Disease: A Systematic Review. J. Crohn’s Colitis 2020, 14, 254–266.
- Turner, D.; Ricciuto, A.; Lewis, A.; D’Amico, F.; Dhaliwal, J.; Griffiths, A.M.; Bettenworth, D.; Sandborn, W.J.; Sands, B.E.; Reinisch, W.; et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology 2021, 160, 1570–1583.
- Kellermann, L.; Riis, L.B. A close view on histopathological changes in inflammatory bowel disease, a narrative review. Dig. Med. Res. 2021, 4, 3.
- Magro, F.; Gionchetti, P.; Eliakim, R.; Ardizzone, S.; Armuzzi, A.; Barreiro-de Acosta, M.; Burisch, J.; Gecse, K.B.; Hart, A.L.; Hindryckx, P.; et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: Definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J. Crohn’s Colitis 2017, 11, 649–670.
- Agrawal, M.; Colombel, J.F. Treat-to-Target in Inflammatory Bowel Diseases, What Is the Target and How Do We Treat? Gastrointest. Endosc. Clin. N. Am. 2019, 29, 421–436.
- Kim, K.O. Endoscopic activity in inflammatory bowel disease: Clinical significance and application in practice. Clin. Endosc. 2022, 55, 480–488.
- Rath, T.; Atreya, R.; Neurath, M.F. Is histological healing a feasible endpoint in ulcerative colitis? Expert Rev. Gastroenterol. Hepatol. 2021, 15, 665–674.
- Ponte, A.; Pinho, R.; Fernandes, S.; Rodrigues, A.; Alberto, L.; Silva, J.C.; Silva, J.; Rodrigues, J.; Sousa, M.; Silva, A.P.; et al. Impact of histological and endoscopic remissions on clinical recurrence and recurrence-free time in ulcerative colitis. Inflamm. Bowel Dis. 2017, 23, 2238–2244.
- Pai, R.K.; Hartman, D.J.; Rivers, C.R.; Regueiro, M.; Schwartz, M.; Binion, D.G.; Pai, R.K. Complete resolution of mucosal neutrophils associates with improved long-term clinical outcomes of patients with ulcerative colitis. Clin. Gastroenterol. Hepatol. 2020, 18, 2510–2517.e5.
- Cushing, K.C.; Tan, W.; Alpers, D.H.; Deshpande, V.; Ananthakrishnan, A.N. Complete histologic normalisation is associated with reduced risk of relapse among patients with ulcerative colitis in complete endoscopic remission. Aliment. Pharmacol. Ther. 2020, 51, 347–355.
- Kevans, D.; Kirsch, R.; Dargavel, C.; Kabakchiev, B.; Riddell, R.; Silverberg, M.S. Histological markers of clinical relapse in endoscopically quiescent ulcerative colitis. Inflamm. Bowel Dis. 2020, 26, 1722–1729.
- Lobatón, T.; Bessissow, T.; Ruiz-Cerulla, A.; De Hertogh, G.; Bisschops, R.; Guardiola, J.; Van Assche, G.; Vermeire, S.; Ferrante, M. Prognostic value of histological activity in patients with ulcerative colitis in deep remission: A prospective multicenter study. United Eur. Gastroenterol. J. 2018, 6, 765–772.
- Pai, R.K.; Jairath, V. What is the role of histopathology in the evaluation of disease activity in Crohn’s disease? Best Pract. Res. Clin. Gastroenterol. 2019, 38–39, 101601.
- Hu, A.B.; Tan, W.; Deshpande, V.; Ananthakrishnan, A.N. Ileal or Colonic Histologic Activity Is Not Associated with Clinical Relapse in Patients with Crohn’s Disease in Endoscopic Remission. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2021, 19, 1226–1233.e1.
- Villanacci, V.; Baert, F.; Cornillie, F.; De Hertogh, G.; Panés, J. Challenges Faced by Cross-sectional Imaging and Histological Endpoints in Clinical Trials. J. Crohn’s Colitis 2017, 11 (Suppl. S2), S586–S592.
- Park, S.; Abdi, T.; Gentry, M.; Laine, L. Histological Disease Activity as a Predictor of Clinical Relapse Among Patients with Ulcerative Colitis: Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2016, 111, 1692–1701.
- Narang, V.; Kaur, R.; Garg, B.; Mahajan, R.; Midha, V.; Sood, N.; Sood, A. Association of endoscopic and histological remission with clinical course in patients of ulcerative colitis. Intest. Res. 2018, 16, 55–61.
- Ozaki, R.; Kobayashi, T.; Okabayashi, S.; Nakano, M.; Morinaga, S.; Hara, A.; Ohbu, M.; Matsuoka, K.; Toyonaga, T.; Saito, E.; et al. Histological Risk Factors to Predict Clinical Relapse in Ulcerative Colitis with Endoscopically Normal Mucosa. J. Crohn’s Colitis 2018, 12, 1288–1294.
- Bryant, R.V.; Burger, D.C.; Delo, J.; Walsh, A.J.; Thomas, S.; von Herbay, A.; Buchel, O.C.; White, L.; Brain, O.; Keshav, S.; et al. Beyond endoscopic mucosal healing in UC: Histological remission better predicts corticosteroid use and hospitalisation over 6 years of follow-up. Gut 2016, 65, 408–414.
- Bessissow, T.; Lemmens, B.; Ferrante, M.; Bisschops, R.; Van Steen, K.; Geboes, K.; Van Assche, G.; Vermeire, S.; Rutgeerts, P.; De Hertogh, G. Prognostic value of serologic and histologic markers on clinical relapse in ulcerative colitis patients with mucosal healing. Am. J. Gastroenterol. 2012, 107, 1684–1692.
- Calafat, M.; Lobatón, T.; Hernández-Gallego, A.; Mañosa, M.; Torres, P.; Cañete, F.; Cabré, E.; Ojanguren, I.; Domènech, E. Acute histological inflammatory activity is associated with clinical relapse in patients with ulcerative colitis in clinical and endoscopic remission. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2017, 49, 1327–1331.
- Christensen, B.; Erlich, J.; Gibson, P.R.; Turner, J.R.; Hart, J.; Rubin, D.T. Histologic Healing Is More Strongly Associated with Clinical Outcomes in Ileal Crohn’s Disease than Endoscopic Healing. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2020, 18, 2518–2525.e1.
- Brennan, G.T.; Melton, S.D.; Spechler, S.J.; Feagins, L.A. Clinical Implications of Histologic Abnormalities in Ileocolonic Biopsies of Patients with Crohn’s Disease in Remission. J. Clin. Gastroenterol. 2017, 51, 43–48.
- Flores, B.M.; O’Connor, A.; Moss, A.C. Impact of mucosal inflammation on risk of colorectal neoplasia in patients with ulcerative colitis: A systematic review and meta-analysis. Gastrointest. Endosc. 2017, 86, 1006–1111.e8.
