Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Haikun Zhang and Version 2 by Peter Tang.
Quinone compounds are a class of substances that contain cyclic unsaturated diketones, especially carbocyclic compounds and their derivatives with quinone groups. These compounds contain either q-quinone or o-quinone groups and can be divided into benzoquinone, naphthoquinone, anthraquinone, and phenanthraquinone according to their structural characteristics. Structurally, all compounds containing quinone groups have the potential to act as redox mediators to accelerate electron transfer.
- quinoid compounds
- redox mediators
- natural substances
- artificially synthesized quinone compounds
- small molecules
- QRMs
1. Introduction
Quinone compounds are a class of substances that contain cyclic unsaturated diketones, especially carbocyclic compounds and their derivatives with quinone groups. These compounds contain either q-quinone or o-quinone groups and can be divided into benzoquinone, naphthoquinone, anthraquinone, and phenanthraquinone according to their structural characteristics (Figure 1). More broadly, quinoid compounds also include a variety of non-carbocyclic quinone-like substances, such as riboflavin, flavin mononucleotide, flavin adenine dinucleotide, and humic substances. They can be found in the pigments of plants (mainly Polygonaceae, Rubiaceae, Rheaceae, Liliaceae, and Leguminaceae), a few animals (Cochineal), and bacteria, as well as in the metabolites of some lower plant lichens and fungi. When these animals, plants, and bacteria die, the biological debris is subsequently decomposed and mineralized by microbes. During this process, numerous nonbiodegradable quinone compounds remain, leading to their wide distribution in natural environments (including almost all soils, waters and sediments). In fact, these quinoid compounds are not only widely found in nature but are also widely involved in environmental processes.
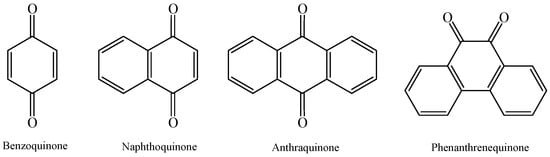
Figure 1.
Structures of benzoquinone, naphthoquinone, anthraquinone, and phenanthrenequinone.
Since the 20th century (1970s), the environmental importance of these quinoid compounds has received increasing attention. In 1967, Roxon et al. [1] reported that riboflavin, a quinone-like compound, can accelerate the transformation of some organic contaminants. Subsequently, an increasing number of researchers began to focus on the application of quinone compounds (e.g., anthraquinone-2,6-disulfonate, anthraquinone-2-sulfonate, humus, carbon materials, etc.) for the transformation of environmental contaminants [2]. A mechanistic study found that the quinone group can be reversibly oxidized and reduced between quinone and hydroquinone due to its electron affinity, which enables it to serve as an electron shuttle in multiple redox reactions. In addition, the presence of quinoid compounds can accelerate reactions by lowering the activation energy of the total reaction and may even be a prerequisite for the reaction to take place under some situations. To date, many kinds of quinoid compounds have been proven to be capable of facilitating electron transfer in redox reactions between diverse inorganic or organic compounds, including organic matter, metals, and minerals [1][2][1,2].
Over the past twenty years, quinoid compounds have attracted more attention than before because of their significant interactions with microbes, which may further increase their environmental importance. Since the 1990s, extensive studies have been conducted in this field [2][3][4][5][6][2,3,4,5,6]. Researchers have found that the reduction and reoxidation of quinone groups can be coupled to the anaerobic respiration of electrochemically active bacteria in the presence of various electron donors, such as saccharides, short-chain fatty acids, alcohol, H2, and even some organic pollutants. During this process, quinone is first reduced to hydroquinone; subsequently, hydroquinone can either directly reduce various electron acceptors, such as azo compounds [7][8][7,8], nitroaromatics [9], polyhalogenated pollutants [10][11][10,11], Fe(III) [12], Cr(VI) [13], Se(IV), and Te(IV) [14], or serve as electron donors to support the bioreduction of NO3−/NO2− [15], As(V) [16], and perchlorate [17] with their reoxidation. Overall, the presence of quinoid compounds considerably enhances the electron transfer in many anaerobic biotransformation processes.
Structurally, all compounds containing quinone groups have the potential to act as redox mediators to accelerate electron transfer. However, things do not work this way in real reactions. In addition to the functional groups, the redox potential (E′0) of effective quinoid redox mediators (QRMs) must also be between those of the terminal electron acceptor and electron donor to reduce the activation energy of reactions. In general, E′0 is an indication of the catalytic capacity of QRMs and should be in the range of −440 mV to −50 mV [18][19][18,19]. Vitamin B12 (−530 mV) was reported to be a very poor redox mediator due to its low E′0 [20]. In this research, taking the source and availability into consideration, the common QRMs are classified into four primary categories, including natural substances, artificially synthesized quinone compounds, microbiologically excreted small molecules, and immobilized QRMs.
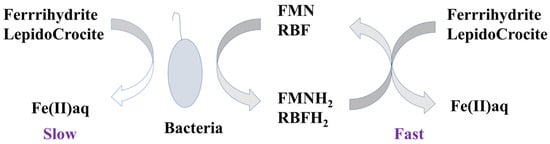
2. Natural Substances
Humic substances, which widely exist in various environments, can be defined as some of the most important natural quinone mediators. Humic substances primarily originate from the degradation of plants and mainly include three types: humic acid, fulvic acid, and humin. In 1996, Lovley et al. [3] first reported that humic substances can participate in some microbial respiration processes. Subsequently, an increasing number of studies have confirmed that humic substances are important redox mediators in nature, especially for some microbe-driven redox reactions (for instance, dissimilatory metal reduction bacteria, sulfate reducing bacteria, and methanogens) [5][21][22][5,21,22]. Humic substances have complex structures; however, quinone groups are considered to be the most important functional groups that are directly involved in electron transport since they can flexibly interconvert between hydroquinone and semiquinone via a reversible electron transfer reaction [23]. It has been reported that some non-quinone groups in humic substances are also able to transfer electrons, but the capacity of some specific non-quinone groups is much lower than that of quinone groups [23][24][23,24]. Accordingly, considering their ubiquity in the environment and efficient conductive capacity, humic substances may play a significant role in the biogeochemical cycles of matter and elements on a global scale.3. Artificially Synthesized Quinone Compounds
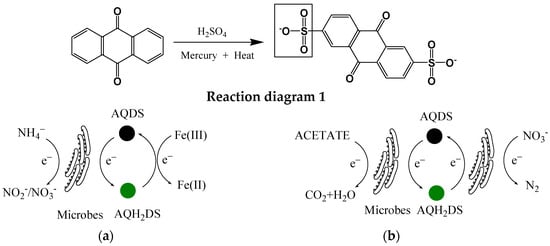
In addition to natural quinone mediators, there are also many artificially synthesized quinones, including 9,10-anthraquinone-2-carboxylic acid, anthraquinone-2,6-disulfonate (AQDS), anthraquinone-2-sulfonate (AQS), etc. Therein, AQDS and AQS are the most studied artificially synthesized quinone compounds. Both AQDS and AQS can be prepared via a chemical synthesis method: reacting anthraquinone (a natural raw material) first with sulfuric acid in the presence of mercury and then with sodium hydroxide (Reaction diagram 1). They were reported to play similar roles; however, their catalytic performance and mechanism during the electron transfer process vary greatly depending on their molecular structures and polarities, as well as the presence of different microorganisms and pollutants [7][18][25][7,18,25]. AQS is suggested to be a more powerful mediator than AQDS. This is because the redox potential of AQS is lower than that of AQDS. Moreover, the additional sulfonate group of AQDS diminishes the ease with which the compound approaches the functional enzyme. In addition, the roles of these artificially synthesized quinones change as reaction conditions change. For example, Zhou et al. [26] demonstrated that AQDS could first act as an electron acceptor during the anammox denitrification process in the presence of iron-reducing bacteria; then, the reduced AQDS can chemically react with Fe(III) (Figure 2a). In addition, for microbes, AQDS can simultaneously be used as an electron donor and an electron acceptor (Figure 2b). Wang et al. [27] reported that AQDS can enhance the coupled biotransformation of phenol and nitrate: AQDS is first reduced to AQH2DS (electron acceptor) by Shewanella sp. XB in the presence of phenol, and then AQH2DS (electron donor) provides electrons to strain XB for its further bioreduction of nitrate. On the whole, artificially synthesized quinone compounds are more targeted and efficient than humic substances.
Figure 2.
The role of AQDS in different reactions. (
a
) Anammox reaction; (
b
) denitrification reaction.