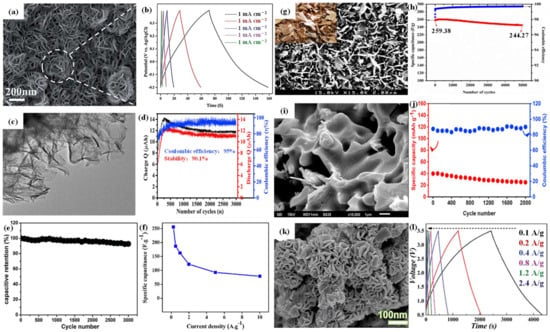
Figure 4. (
a) SEM image of MoS
2 nanoflowers; (
b) GCD curves of MoS
2 nanoflowers at different current densities. (
c) TEM image of MoS
2 nanoflakes; (
d) cyclic performance of MoS
2 nanoflakes at 3 mA cm
−2. (
e) Cyclic performance of 1T-MoS
2 nanosheets at 5 A g
−1. (
f) Specific capacitance of MoS
2 nanoflowers at different current densities. (
g) SEM image of mesoporous MoSe
2; (
h) cyclic performance of mesoporous MoSe
2 at 1 A g
−1. (
i) SEM image of 2H-MoSe
2; (
j) cyclic performance of 2H-MoSe
2 at 5 A g
−1. (
k) SEM image of MoSe
2 nanoflowers; (
l) CV curves of MoSe
2 nanoflowers at different current densities.
The atomic structure of MoSe
2 closely resembles that of MoS
2, comprising three atomic layers held together by weak van der Waals interactions. Consequently, MoSe
2 has attracted considerable interest as a potential electrode material in supercapacitors
[56][59].
MoSe
2 with a mesoporous structure shows a large specific surface area, providing significant benefits for ion transport. Vattikuti et al.
[57][56] successfully synthesized a uniform dry leaf-like mesoporous MoSe
2 nanostructure using a microwave-assisted method, as illustrated in
Figure 4g. The as-prepared leaf-like perforated MoSe
2 exhibited remarkable capacitance of 257.38 F g
−1 at 1 A g
−1 with a capacitance retention of almost 95% after 5000 cycles, see
Figure 4h. In comparison to the mesoporous configuration, MoSe
2 with a nanosheet structure further increases the specific surface area, shortens the ion diffusion path, and improves the electrochemical performance. Upadhyay et al.
[58][57] reported the synthesis of layered 2H-MoSe
2 nanosheets via an in situ selenization route. The SEM image is shown in
Figure 4i. The MoSe
2 nanosheet exhibits a specific capacitance of 46.22 mA h g
−1 at 2 A g
−1. Remarkably, even after 2000 cycles at a current density of 5 A g
−1, a capacitance retention of 64% was observed (
Figure 4j). Additionally, the nanoflower structure would offer ample channels for electrolyte diffusion during the electrochemical processes. Zhang et al.
[59][58] synthesized smooth and irregular pleated flower-like MoSe
2 using a facile hydrothermal method. The SEM image is displayed in
Figure 4k. Furthermore,
Figure 4l illustrates the CV curves of MoSe
2 at different current densities. Notably, the specific capacitance reaches 641.5 mA h g
−1 at a current density of 0.1 A g
−1. The assembled hybrid MoSe
2//AC capacitors displayed a high energy density of 78.75 W h kg
−1 and a high power density of 3600 W kg
−1. In addition, the capacity retention rate is 70.28% after 5000 cycles with a potential window of 0.5–3.5 V.
2.2. Ternary Mo-Based Materials
Ternary Mo-based materials are composed of three elements, including metal molybdates and MXenes. The synthesis pathway of metal molybdate is simple and low cost while exhibiting remarkable physical and chemical properties
[60]. MXenes exhibit a characteristic two-dimensional layered structure, offering a high specific surface area and exceptional electrical conductivity
[61]. Notably, ternary Mo-based materials have been widely researched for supercapacitors in recent years.
2.2.1. Metal Molybdates
Metal molybdates, for example MMoO
4 (M = Cu
[62], Zn
[63], Bi
[64], Ni
[65], Mn
[66], Sn
[67], Co
[68], etc.), constitute a significant category in inorganic materials.
Farahpour et al.
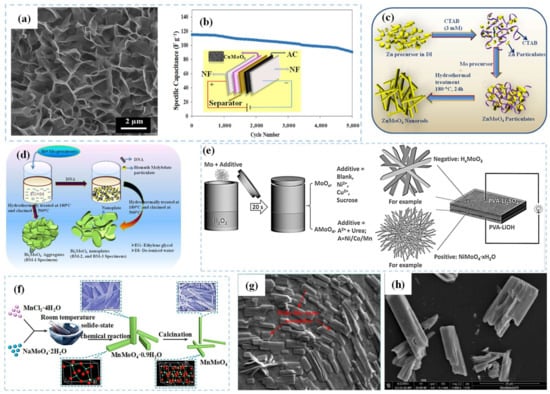
[62] conducted a single-pot hydrothermal method to grow CuMoO
4 nanosheets on nickel foam. In
Figure 5a, the prepared CuMoO
4 nanosheets are uniformly distributed with grass-like morphology. The specific capacitance of CuMoO
4 reached 2259.55 F g
−1 at 1 A g
−1. The cyclic stability analysis showed a capacitance retention of 90.08% at 16 A g
−1 after 5000 cycles. Moreover, the CuMoO
4//AC supercapacitor device displayed a high energy density of 52.51 W h kg
−1 at 600 W kg
−1. In addition, the device exhibited a capacitance retention of 78.6% after 5000 cycles at 4 A g
−1 (
Figure 5b). Gurusamy et al.
[63] produced a series of rod-shaped ZnMoO
4 using a template method by optimizing the concentration of CTAB. The schematic diagram of the synthesis process is presented in
Figure 5c. The rod-like ZnMoO
4 material showed an impressive specific capacitance of 779 F g
−1 at 5 mV s
−1 and retained 90% of the initial capacitance even after 3000 cycles at 100 mV s
−1. Additionally, Yesuraj et al.
[64] employed the hydrothermal method with a DNA template to synthesize Bi
2MoO
6 nanoplates, as depicted in
Figure 5d. The Bi
2MoO
6 nanoplates with a large number of small nanoparticles (5–7 nm) on their surface result in an increased surface area, which facilitated charge transport and ion diffusion. The Bi
2MoO
6 exhibited a high specific capacitance of 698 F g
−1 at 5 mV s
−1, along with a capacitance retention of 86% even after 3000 cycles at a high scan rate of 100 mV s
−1 in 1 M NaOH electrolyte. Qu et al.
[65] presented a rapid and zero-energy consumption method to obtain metal molybdate nanowires in supercapacitors (
Figure 5e). The synthesized NiMoO
4 nanowires exhibited an impressive specific capacitance of 549 C g
−1 at 1 A g
−1. Furthermore, the assembled supercapacitor device demonstrated a specific capacitance of 156 F g
−1 at 0.8 A g
−1, along with an energy density of 55.6 W h kg
−1 at 640 W kg
−1. Additionally, Sheng et al.
[66] employed a solid-state chemical synthesis approach to produce 1D MnMoO
4 0.9H
2O and MnMoO
4 nanorods (
Figure 5f), which exhibited a specific capacitance of 210.2 F g
−1 at 1 A g
−1. Notably, the MnMoO
4 nanorods displayed remarkable cycle stability, maintaining a cycle life of 112.6% even after 10,000 cycles. Furthermore, the electrochemical performance of MnMoO
4 underwent substantial enhancement upon the removal of crystal water from MnMoO
4·0.9H
2O, leading to a noteworthy 2.4-fold increase in specific capacitance. Remarkably, the asymmetric supercapacitor device achieved a high energy density of 23.5 W h kg
−1 at 187.4 W kg
−1. This remarkable electrochemical performance is attributed to the elevated electrical conductivity from the 1D nanostructure after the removal of crystallization water. Sakthikumar et al.
[67] optimized the ratio of CTAB to metal salt and reaction conditions to synthesize sheet-like Sn(MoO
4)
2, as shown in the SEM image in
Figure 5g. The specific capacitance of flake Sn(MoO
4)
2 is 109 F g
−1 at 5 mV s
−1 and the capacitance retention reaches 70% after 4000 cycles at 8 mV s
−1. Li et al.
[68] synthesized CoMoO
4 material in situ on nickel foam using a hydrothermal method (
Figure 5h). The prepared CoMoO
4 shows a cuboid rod-like structure with loose folds on the periphery, enhancing the contact between the electrode and electrolyte, and thereby facilitating ion diffusion and transmission. At a current density of 3 mA cm
−2, the discharge capacitance of CoMoO
4 reaches 11.112 F cm
−2.
Figure 5. (
a) SEM image of CuMoO
4 nanosheets; (
b) cyclic performance of CuMoO
4//AC at 4 A g
−1. (
c) Schematic diagram of the synthesis of ZnMoO
4 nanorods. (
d) Schematic diagram of the synthesis of Bi
2MoO
6 nanoplates. (
e) Schematic diagram of the synthesis of NiMoO
4·xH
2O nanowires. (
f) Schematic diagram of the synthesis of MnMoO
4 nanorods. (
g) SEM image of Sn(MoO
4)
2 nanosheets. (
h) SEM image of CoMoO
4 nanorods.
2.2.2. Mo-MXenes
In recent years, two-dimensional transition metal carbonitride (MXene) materials have attracted extensive attention in the energy storage field, owing to their unique physical and chemical characters
[69]. The MAX phase is classified as a layered carbide or nitride and is characterized by the chemical formula M
n+1AX
n (
n = 1~3). Here, M represents various transition metal elements, including Sc, Ti, Zr, Nb, Ta, Cr, Mo, etc. X stands for carbon and/or nitrogen, while A refers to a main group element. Through etching, A can be removed from the MAX phase, leading to the formation of a graphene-like MXene structure. The structural chemical formula of MXenes is M
n+1X
nT
x (
n = 1~3), where T represents a functional group such as O, F, or OH
[70]. The distinctive properties of MXene materials have sparked interest from researchers in supercapacitors.
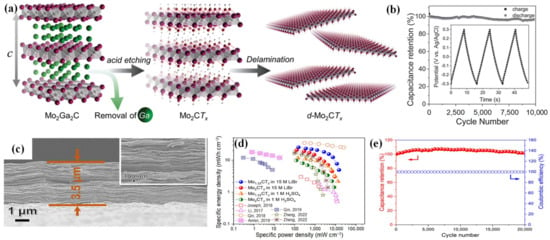
Halim et al.
[71] firstly put forward a large-scale synthesis strategy for 2D Mo
2CT
x flakes. LiF/HCl acts as an etchant to selectively etch Ga from Mo
2Ga
2C powder, leading to a delamination process, as depicted in
Figure 6a. The obtained Mo
2CT
x flakes exhibit high conductivity and effective intersheet conduction due to their dense stacking. At a scan rate of 2 mV s
−1, the specific capacitance reaches 700 F cm
−3, while the capacitance retention is nearly 100% even after 10,000 cycles at 10 A g
−1 (
Figure 6b). Das et al.
[72] conducted a theoretical analysis on the structure of Mo
2CO
2 to predict the electronic structure and investigate its capacitive behavior. As a result, the functionalized MXenes induce a change in charge transfer dynamics. Therefore, H inclines to form covalent bonds with O, leading to the sharing of electrons. Impressively, the theoretically calculated capacitance of Mo
2CO
2 is closely consistent with the experimental results. Zheng et al.
[73] prepared Mo
1.33CT
z i-MXene films with a vacancy structure by etching (Mo
0.66Sc
0.33)
2AlC, as illustrated in
Figure 6c. The inclusion of vacancies notably optimizes the ion transport. Notably, the Mo
1.33CT
z i-MXene attained an energy density of 25.4 mW h cm
−3 at a power density of 152.4 mW cm
−3 in a 15 M LiBr electrolyte, as depicted in
Figure 6d. Even after 20,000 cycles at 100 mV s
−1, 99.4% of the initial specific capacitance is retained (
Figure 6e).
Figure 6. (
a) Schematic diagram of the synthesis of Mo
2CT
x; (
b) cyclic performance of Mo
2CT
x at 10 A g
−1. (
c) SEM image of Mo
1.33CT
z; (
d) Ragone diagram of Mo
1.33CT
z in 1 M H
2SO
4 and 15 M LiBr compared to different Mxene; (
e) cyclic performance of Mo
1.33CT
z at 100 mV s
−1.