The extreme toxicity of nerve agents and the broad spectrum of their physical and chemical properties, enabling the use of these agents in a variety of tactical situations, is a continuing challenge in maintaining the knowledge and capability to detect them, as well as in finding new effective methods. With the military standardization of V-series agents (VX), the sensitivity of traditional chemical methods was no longer satisfactory; therefore, increased attention was paid to cholinesterase methods. The reduction of enzyme activity after contact with NAs allows for the detection of these cholinesterase inhibitors with high sensitivity since, in this case, one and the same enzyme acts as both a sensitive element and an amplifier of the analytical signal.
- nerve agents
- chemosensors
- biosensors
- colour reactions
- fluorescence
- cholinesterase reaction
1. Enzyme, Substrate, and Acid–Base Indicator
2. Enzyme, Thio-Substrate, and Redox Indicator
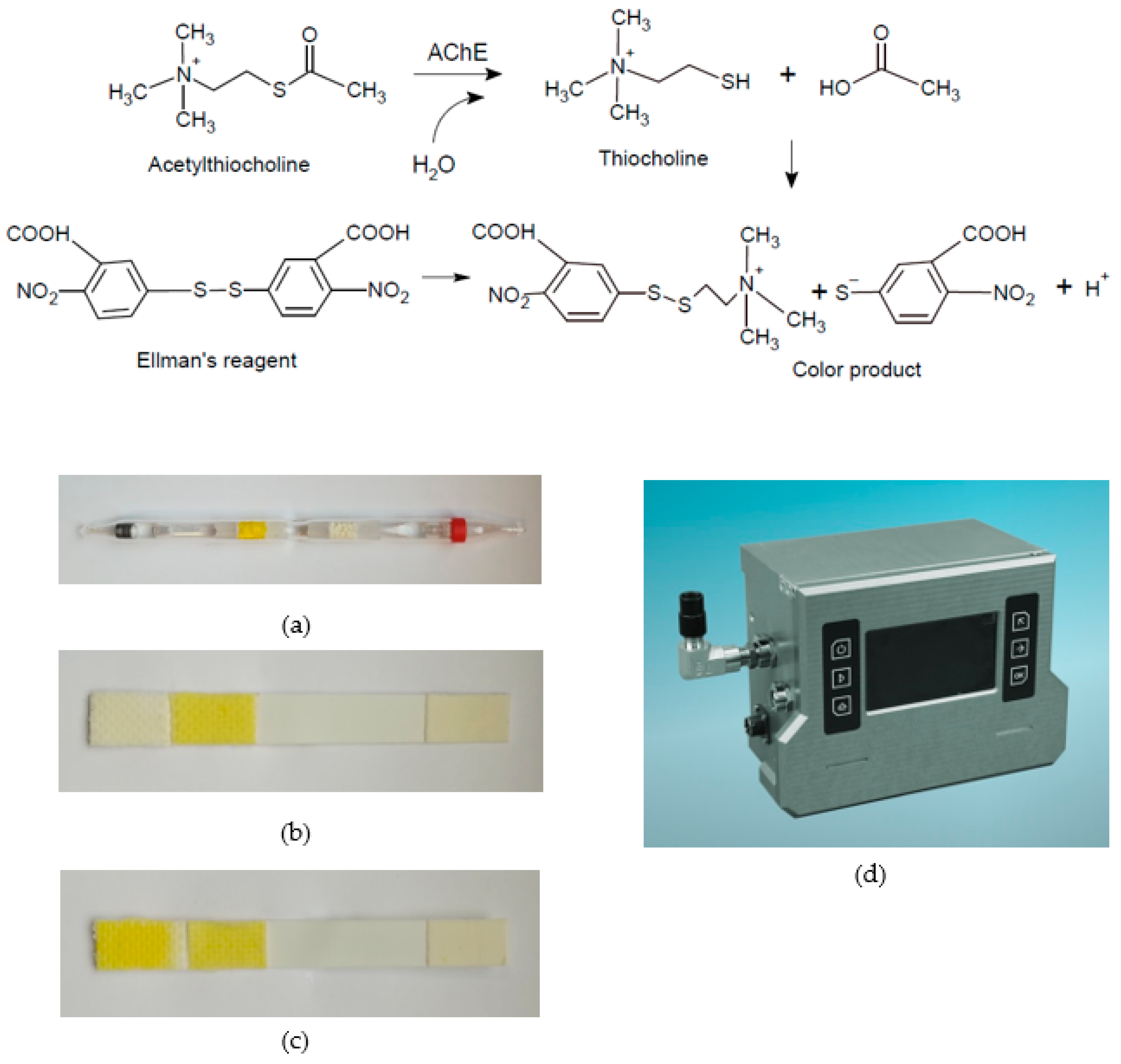
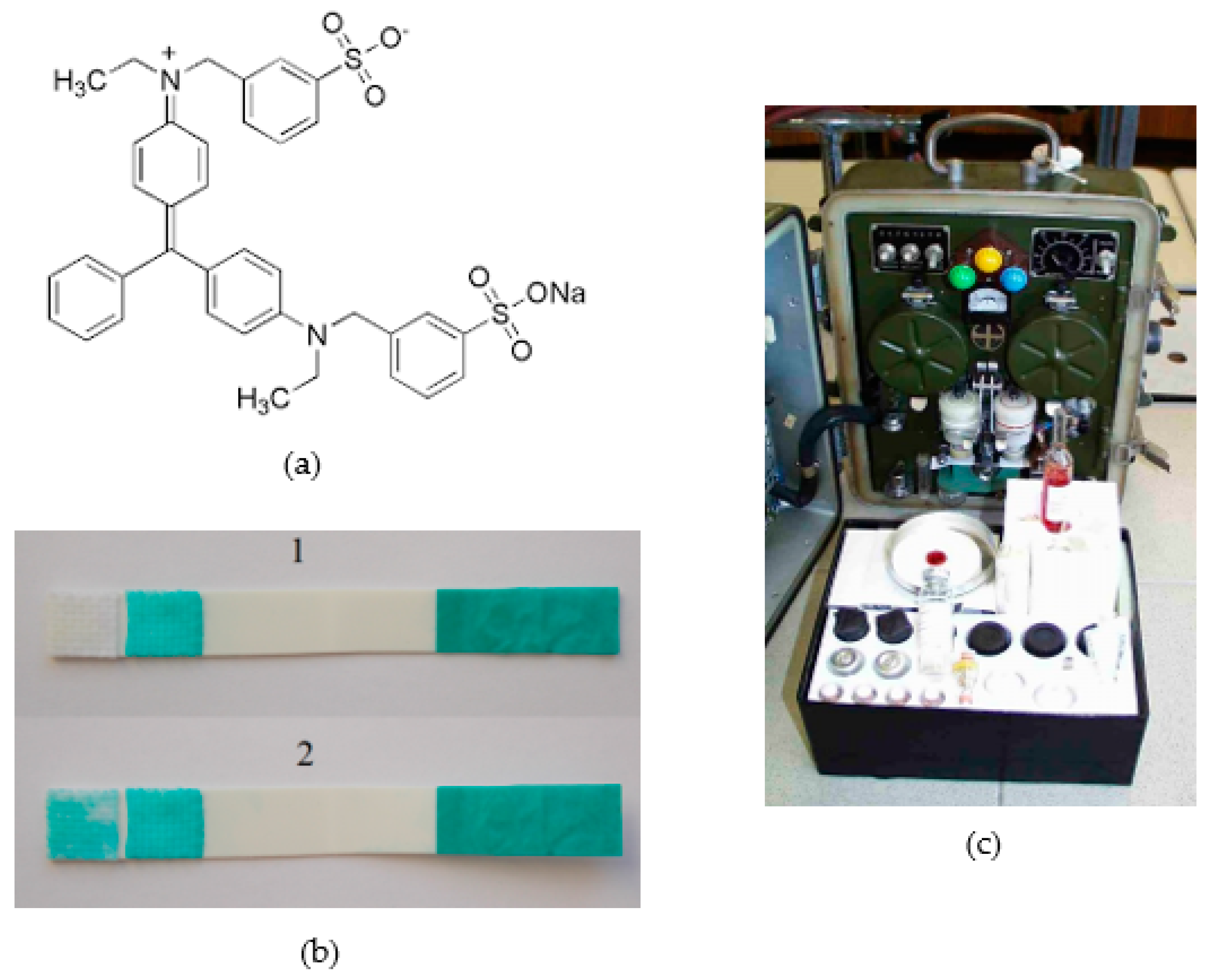
3. Enzyme and Chromogenic Substrate
References
- Schmaltz, F. Kampfstoff-Forschung im Nationalsozialismus ; Wallstein: Göttingen, Germany, 2005; p. 510.
- Limperos, G.; Ranta, K.E. A rapid screening test for the determination of the approximate cholinesterase activity of human blood. Science 1953, 117, 453–455.
- Zacks, S.I.; Blumberg, J.M. Simple and inexpensive anti-cholinesterase detectors for field use. Mil. Med. 1964, 129, 1084–1086.
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–90.
- Vymazalová, K.; Halámek, E.; Kadlčák, J. Cholinesterase biosensor for detection of nerve agents. Chem. Listy 2016, 110, 645–650.
- Pohanka, M.; Hrabinova, M.; Kuca, K. Diagnosis of intoxication by the organophosphate VX: Comparison between an electrochemical sensors and Ellman’s photometric method. Sensors 2008, 8, 5229–5237.
- Wu, J.; Zhu, Y.; Liu, Y.; Chen, J.; Guo, L.; Xie, J. A novel approach for in-site screening of organophosphorus nerve agents based on DTNB modified AgNPs using surface-enhanced Raman spectrometry. Anal. Methods 2022, 14, 4263–4291.
- Zhu, J.; Dhimitruka, I.; Pei, D. 5-(2-Aminoethyl)dithio-2-nitrobenzoate as a more base-stable alternative to Ellman’s reagent. Org. Lett. 2004, 6, 3809–3812.
- Bissbort, S.H.; Vermaak, W.J.H.; Elias, J.; Bester, M.J.; Dhatt, G.S.; Pum, J.K.W. Novel test and its automation for determination of erythrocyte acetylcholinesterase and its application to organophosphate exposure. Clin. Chem. Acta 2001, 303, 139–145.
- Pitschmann, V.; Matějovský, L.; Dymák, M.; Dropa, T.; Urban, M.; Vošahlíková, I. Cholinesterase inhibitor biosensors. Ecol. Saf. 2017, 11, 18–23.
- Pitschmann, V.; Matějovský, L.; Vetchý, D.; Kobliha, Z. Enzymatic determination of anticholinesterases using a composite carrier. Anal. Lett. 2016, 49, 2418–2426.
- Pitschmann, V.; Matějovský, L.; Lobotka, M.; Dědič, J.; Urban, M.; Dymák, M. Modified biosensor for cholinesterase inhibitors with Guinea Green B as the color indicator. Biosensors 2018, 8, 81.
- Matějovský, L.; Pitschmann, V. A strip biosensor with Guinea Green B and Fuchsin Basic color indicators on a glass nanofiber carrier for the cholinesterase detection of nerve agents. ACS Omega 2019, 4, 20978–20986.
- Barrnett, R.J.; Seligman, A.M. Histochemical demonstration of esterase by production of indigo. Science 1951, 114, 579–582.
- Guilbault, G.G.; Kramer, D.N. Resorufin butyrate and indoxyl acetate as fluorogenic substrates for cholinesterase. Anal. Chem. 1965, 31, 120–123.
- Matoušek, J.; Fischer, J.; Cerman, J. Nová fluorimetrická metoda stanovení submikrogramových kvant inhibitorů cholinesterázy . Chemické Zvesti 1968, 22, 184–189.
- Pohanka, M.; Vlcek, V. Preparation and performance of a colorimetric biosensor using acetylcholinesterase and indoxylacetate for assay of nerve agents and drugs. Interdiscip. Toxicol. 2014, 7, 215–218.
- Gelman, C.; Kramer, D.N. Enyzymatic Method for Detection of Anticholinesterases. U.S. Patent 3,049,411, 14 August 1962.
- Kramer, D.N.; Gamson, R.M. Colorimetric determination of acetylcholinesterase activity. Anal. Chem. 1958, 30, 251–254.
- Fu, Q.; Zhang, C.; Xie, J.; Li, Z.; Qu, L.; Cai, X.; Ouyang, H.; Song, Y.; Du, D.; Lin, Y.; et al. Ambient light sensor based colorimetric dipstick reader for rapid monitoring organophosphate pesticides on a smartphone. Anal. Chim. Acta 2019, 1092, 126–131.
- Barendsz, A.W. A detection tube for cholinesterase inhibiting compounds. Int. J. Environ. Anal. Chem. 1979, 6, 89–94.
- Brletich, N.R.; Waters, M.J.; Bowen, G.W.; Tracy, M.F. Worldwide Chemical Detection Equipment Handbook; Chemical and Biological Defense Information Analysis Center: Aberdeen, MD, USA, 1955.
- Halámek, E.; Kobliha, Z.; Pitschmann, V. Analysis of Chemical Warfare Agents; University of Defence: Brno, Czech Republic, 2009.
- Chowdhary, S.; Bhattacharyya, R.; Banerjee, D. A novel fluorescence based assay for the detection of organophosphorus pesticide exposed cholinesterase activity using 1-naphtyl acetate. Biochimie 2019, 160, 100–112.
