2. Psychiatric Diseases and Vitamin D
Vitamin D exhibits a remarkable ability to traverse the blood-brain barrier, activate vitamin D receptors within the central nervous system, and exert influence over human behavior regulation, as elucidated by studies such as Farhangi et al. (2017)
[13][20]. Its role extends to the intricate modulation of neurotransmitter release and the synthesis of neurotrophic factors, pivotal mechanisms contributing to the improvement of mood and behavioral outcomes in individuals, as highlighted in the research conducted by Macova et al. (2017)
[14][21]. This multifaceted influence can also be attributed to vitamin D’s neuroprotective properties, with evidence indicating its potential to reduce plasma C-reactive protein levels in psychiatric disorder patients and its capacity to modulate inflammation through the suppression of proinflammatory cytokines, as observed in studies like Jamilian et al. (2019) and Barker et al. (2013)
[15][22]. These findings collectively underscore the pivotal role of vitamin D in neurological and psychiatric well-being, shedding light on its potential therapeutic implications.
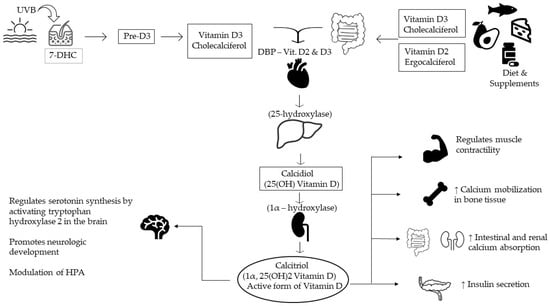
After crossing the blood-brain barrier, vitamin D has implications for many processes: cell differentiation, neurotransmitter synthesis, neurotrophic production and release, calcium homeostasis, cognitive function, oxidative damage prevention, and neuronal structure-function. Therefore, it is not surprising that low vitamin levels have been associated with various psychiatric disorders (depression, schizophrenia, autism spectrum disorders, and Alzheimer’s disease
[16][23].
Some data suggest that the psychiatric population has a higher rate of vitamin D deficiency than the general population
[16][17][18][19][20][21][22][23][24][25][17,23,24,25,26,27,28,29,30,31]. Patients from a psychiatric ward in Serbia have been included in a cross-sectional design study, and a significant number of patients had a lower vitamin D level than expected
[24][30]. Even in an equatorial nation, despite the geographic location and outdoor activities, levels of vitamin D were still low among psychiatric hospitalized subjects
[23][29].
Vitamin D supplementation therapy in psychiatric patients had beneficial effects on depressive symptoms, biomarkers of inflammation, and even oxidative stress
[26][27][28][32,33,34] C-reactive protein and total antioxidant levels have been improved
[28][34].
Patients with alexithymia have been included in a study for measuring serum levels of vitamin D and genotyping vitamin D binding protein, and their results suggest that lower vitamin levels could be involved in the pathophysiology of the disease
[29][35].
4. Neurodevelopmental Disorders
The majority of people with intellectual disabilities were reported to have deficient or insufficient vitamin D levels, with a higher prevalence in women
[30][36]. The level of vitamin D deficiency in patients with intellectual disabilities was not correlated with seasonality, type of admission, or associated treatment
[31][37]. Deficient levels of maternal vitamin D were not correlated with a higher risk of intellectual disability or autism spectrum disorders
[32][38].
Deficient levels of vitamin D in newborns were not correlated with a higher risk of autism spectrum disorders
[33][39]. Autistic children had a significantly lower level of vitamin D when compared to healthy children
[34][40]. Vitamin D had a positive effect on irritability symptoms in autistic children
[35][41].
Vitamin D levels were significantly lower in ADHD children and adolescents
[36][42]. Vitamin D supplementation could improve mental health and behavioral symptoms in children with ADHD and low vitamin D levels
[37][43]. Another study found that vitamin D supplementation in ADHD patients improved outcomes such as cognitive function, hyperactivity, and inattention.
5. Psychotic Spectrum Diseases
Epidemiologic evidence suggests that the etiology of schizophrenia includes genetic and environmental factors and common risk factors for vitamin D, including low prenatal vitamin D, children born in winter and early spring, high-altitude residents or urban residents, and dark-skinned migrants who move to cold climates
[38][44]. Also, schizophrenia patients have poorer general health; they are less active, have a poor diet, and have an increased risk of medical conditions
[39][45].
Recent studies have initiated vitamin D supplementation therapy as an augmented treatment to the standard regimen for schizophrenia
[40][41][42][50,51,52]. Some of them found a negative, but not significant, association between vitamin D and different subscale scores (Positive and negative syndrome scale—PANSS), and other studies have reported improved cognition but not psychotic symptoms
[40][41][50,51]. Later on, in 2021, the data obtained suggested that both antipsychotic medication and vitamin D treatment may improve positive and negative symptoms and also improve attention span
[41][51].
6. Bipolar Disorders
Recent studies have shown that there might be a link between bipolar disorder and vitamin D deficiency
[43][53]. There is little information concerning vitamin D levels and supplementation effects on the course of bipolar disorder; however, there has been a noted increase in 25OHD synthesis during BD decompensation
[44][54]. Other studies have shown a lower level of vitamin D in people with bipolar disorders, but supplementation was not correlated with an improvement in depressive symptoms
[45][55].
7. Depression
Antidepressants, as indicated by Cipriani et al. in 2018
[46][56], can indeed serve as a viable remedy for depression. Nevertheless, their effectiveness in addressing the condition may fall short in certain cases. Additionally, it’s not uncommon for individuals to experience a recurrence of symptoms during treatment, often necessitating multiple trials with different antidepressants before achieving a satisfactory response, as noted by Rush et al. in 2006
[47][57]. Therefore, it is imperative to explore supplementary treatment alternatives for individuals grappling with depression.
While the pathophysiology of depression is not yet fully understood, a deficit in vitamin D serum levels is linked to a higher likelihood of having depression, even after adjusting for confounding variables such as time of the year, socioeconomic status, and lifestyle
[48][58]. Depression is also associated with low-grade inflammation, elevated cytokine levels, and stress-induced disruptions in lipid and glucose metabolism. Vitamin D has been proposed to act as a glucocorticoid antagonist, potentially protecting the hippocampus during HPA axis dysregulation
[49][59]. Additionally, it may influence hippocampal neuron development, serotonin and dopamine release, and synaptic plasticity mechanisms through various pathways
[50][51][60,61]. Furthermore, circulating 25(OH)D levels have been linked to the body’s immune responses
[52][62].
The serum levels of vitamin D were correlated with the degree of depression as measured by scores such as the Hospital Anxiety and Depression Scale, with a stronger correlation in male patients as opposed to female patients
[53][54][63,64]. While the correlation between vitamin D levels and depression has been studied more and more, depressive symptoms do not appear in all patients suffering from vitamin D deficiency, and not all patients with vitamin D deficiency suffer from depression, indicating a more complex pathophysiological pathway.
Vitamin D supplementation was correlated with a decrease in depressive symptoms in a variety of populations, such as elderly depressed people
[55][65], patients with metastatic pulmonary cancer and comorbid depression
[56][66], adolescent girls suffering from depression, and patients with acute stroke and comorbid depression
[57][67]. Vitamin D supplementation was not correlated with a significant difference in neurotransmitters such as serotonin or oxytocin, indicating a different pathophysiological mechanism
[55][65]. Several clinical trials have shown vitamin D supplementation to be an effective way of treating depression
[58][68], even though when antidepressant treatment and psychotherapy are applied in an adequate scenario, the effect of vitamin D supplementation can be negligible
[59][60][69,70] or at most moderate
[61][71].
The outcomes of a comprehensive meta-analysis present compelling evidence pointing to a significant association between low circulating vitamin D levels and a notable three-fold increase in the susceptibility to post-stroke depression. Furthermore, the study underscores several other risk factors, including female gender, hyperlipidemia, and higher NIHSS scores, which were also identified as contributors to an augmented risk or occurrence of post-stroke depression. These findings illuminate the importance of considering routine screening for circulating vitamin D concentrations, not only in individuals recovering from stroke but also in other high-risk populations. Such proactive measures may aid in early identification and intervention, potentially improving mental health outcomes in these vulnerable groups, as documented in the study by Hung et al. (2023)
[62][72].
8. Anxiety Disorder
Since epidemiological research has evidenced a relationship between vitamin D and depression, and given the usual association of depressive symptoms in patients suffering from anxiety, studies have aimed to research the therapeutic effect of vitamin D supplementation in such patients
[38][63][64][65][44,73,74,75].
This current evidence shows several potential mechanisms for improvement after vitamin D supplementation, such as the role in calcium homeostasis or the interference in serotonin synthesis by expression of the serotonin synthesizing gene, therefore maintaining normal levels. Another function is the mediation of several pathways for insulin or serotonin, which are associated with mood disorders
[66][76].
In an experiment conducted by Zhu, C. et al., approximately 160 Chinese individuals suffering from depression were subjected to supplementation with vitamin D for 6 months. There was no perceivable impact on depressive symptoms in these patients, but an improvement in anxiety symptoms
[38][44].
In 2019, Eid, A. et al. investigated the effects of vitamin D supplementation on generalized anxiety disorder (GAD), clinically and biologically, on 30 diagnosed patients from Saudi Arabia. The individuals had serum vitamin D, serotonin, and neopterin (a mediator for cellular immunity and a biomarker of oxidative stress) measured and were divided into 2 groups (with or without vitamin D supplementation). The researchers found significant improvement in GAD patient scores in the vitamin D-treated group
[63][73].
On the other hand, Casseb G.A.S. et al. and De Koning E.J. et al. have described ambiguous results on this matter with little association between vitamin D therapy and anxiety, independently from depression. The results showed an association between anxiety symptoms and serum vitamin D or depressive symptoms. However, the relationship between vitamin D levels and anxiety symptoms was explained by demographic and lifestyle factors such as sunlight, food, or supplements
[64][65][74,75].
9. Obsessive-Compulsive Disorder
Recent investigations have shown an association between reduced vitamin D levels and obsessive-compulsive disorder (OCD)
[67][68][77,78]. It is noteworthy that certain studies, despite not discerning significant differences in vitamin D levels between individuals diagnosed with OCD and healthy controls, have reported that even the healthy participants displayed vitamin D levels within the range considered a deficiency
[69][79]. Although a pathophysiological connection between vitamin D deficiency and OCD has been posited, the precise mechanisms underlying this relationship remain elusive
[70][80].
10. Trauma and Stress-Related Disorders
While the incidence rate for traumatic events during one’s lifetime varies between 25% and 80%, only a small group of trauma victims develop post-traumatic stress disorder (PTSD). This disease is associated with cardiovascular complications, depression, and anxiety
[71][81]. Vitamin D may be implicated through three different mechanisms in this pathogenesis: Firstly, its neuro-inflammatory and neuro-immunological regulation may be involved with psychiatric disorders from PTSD
[72][82]. Secondly, brain regions with altered activity in patients with PTSD express vitamin D receptors (prefrontal cortex, cingulate cortex, hypothalamus)
[9]. Thirdly, vitamin D is implicated in the regulation of serotonin and catecholamine
[73][83].
Another study investigated vitamin D supplementation during winter and the effect on biological markers of stress resilience (serotonin, cortisol, psychophysiological activity) in a randomized clinical trial. In this group, resilience to stress varied with seasonal changes in vitamin D levels (vitamin supplementation during the winter influenced resilience to stress in the spring)
[74][84].
11. Eating Disorders
Patients suffering from eating disorders, such as anorexia nervosa (AD), have been found to present deficient levels of vitamin D
[75][85], with some studies indicating that over half the patients have vitamin D deficiency
[76][86]. While vitamin D deficiency is more of a result than a cause of AD, it has been suggested as a potential cause for the depressive symptoms seen in such patients
[77][87], though this effect has been contested
[78][88]. Vitamin D supplementation for eating disorders should be considered more on the grounds of the comorbid pathologies, such as osteoporosis, than for the eating disorder itself
[79][89].
12. Elimination Disorders
The evaluation of urinary incontinence has led to an association between vitamin D and conditions that increase the risk for elimination disorders. Vitamin D receptors can be found in the bladder and pelvic floor muscles. Additionally, prostatic cells can express a hydroxylase that can synthesize the active form of vitamin D
[80][90].
A recent study evaluated the relationship between vitamin D and urinary incontinence in a longitudinal observation of a cohort of older adults with a previous assessment of daily activities and cognitive and depression screening tests and found that more than half of the participants were deficient in vitamin D, and among them, 38% developed urinary incontinence. This study demonstrates the association between vitamin D and optimal pelvic floor function
[81][91].
13. Sleep-Wake Disorders
Recent studies have shown that vitamin D levels play a role in regulating sleep patterns and, consequently, in sleep disorders
[82][92]. Higher vitamin D levels were significantly associated with a shorter time required to fall asleep
[83][93]. It is worth mentioning that differences in sleep patterns and duration related to vitamin D levels were only observed in daytime workers and not in nighttime workers
[84][94]. Vitamin D deficiency has been associated with lower sleep duration and worse sleep quality in both adult and pediatric populations, with a subsequent increase in the time of sleep onset in pediatric populations, suggesting an effect of vitamin D on circadian rhythm regulation
[85][95].
There appears to be an inverse relationship between excessive daytime sleepiness and vitamin D levels
[86][96]. Lower levels of vitamin D have been reported in people suffering from narcolepsy with cataplexy
[87][97]. Patients with chronic insomnia had significantly lower levels of vitamin D when compared to healthy controls
[88][98]. Patients who were not responding to classical treatment had even lower levels of serum vitamin D when compared with treatment responders
[88][98].
Vitamin D deficiency was present in a large proportion of patients with obstructive sleep apnea (OSA)
[89][99]. Reduced vitamin D levels were found in pediatric patients with obstructive sleep apnea, with lower levels also observed in African American children and obese children, these factors having a cumulative effect
[90][100]. Subjects with OSA have a higher risk of vitamin D deficiency, which is in turn independently correlated with insulin resistance in these patients
[91][101]. Vitamin D levels are inversely correlated with OSA severity
[92][102]. Continuous positive airway pressure (CPAP) treatment improved the clinical manifestations of OSA and vitamin D levels in obese patients
[93][103].
14. Sexual Disorders
Among the organic causes of erectile dysfunction, vascular diseases are the most common (predominantly atherosclerosis). Low levels of vitamin D are associated with increased risk for atherosclerotic events through inflammation, endothelial dysfunction, atherosclerosis, and impaired glucose homeostasis
[93][103].
Apart from the well-known calcium effects, several studies have conducted further analysis of the role of vitamin D levels and sexual function
[94][95][96][97][98][104,105,106,107,108].
In a randomized, double-blind study with female participants diagnosed with sexual dysfunction and serum vitamin D deficiency, after intramuscular administration of vitamin D and evaluation of depressive symptoms, the results showed an improvement in sexual function. Furthermore, the effect of this treatment was independent of the effect on depression
[95][105].
In comparison, another study aimed to investigate the effect of lower vitamin D levels and sexual function in men and concluded that, compared to the healthy participants, the subjects with low vitamin D obtained lower scores in erectile function, sexual desire, and orgasmic function, suggesting that low vitamin D impairs male sexual functioning, and also concluded that the degree of hypovitaminosis correlates with the severity of sexual dysfunction
[96][106]. Canguven O. et al. conducted research with 102 male participants with vitamin D deficiency who received treatment for 12 months while being monitored. The results suggested that the treatment improved testosterone function, erectile function, and metabolic syndrome in middle-aged men
[94][104]. Another large representative sample of U.S. men was included in a cross-sectional study where vitamin D deficiency has been associated with erectile dysfunction but is independent of atherosclerosis
[97][107].
15. Neurocognitive Diseases
In the last 25 years, vitamin D has become a candidate for the development and function of the nervous system and a therapeutic tool
[98][108]. Vitamin D receptors are found in the hippocampus and, therefore, play an important role in memory formation. Patients with vitamin D deficiency present with small hippocampal volumes. The active form of vitamin D supports neurotransmission, neuroprotection, and synaptic plasticity
[99][109].
Studies have shown that patients with Alzheimer’s disease have lower concentrations of vitamin D
[35][100][101][102][103][104][105][41,110,111,112,113,114,115]. Moreover, low serum levels of vitamin D are associated with a higher degree of cognitive impairment
[106][116]. A 1 nmol/L decrease in serum vitamin D was associated with a 6% risk of Alzheimer’s disease
[107][117].
Digging deeper and comparing visual with verbal memory, Kuzma E. et al. studied severe vitamin D deficiency in dementia patients and found an association with only visual memory decline
[108][118]. In a case-control study with geriatric patients, serum vitamin D levels were tested, and a lower concentration was linked to delirium
[109][119].
On the other hand, vitamin D has shown no protective effect on cognitive function in a 5-year follow-up study. Over the course of 5 years, 661 dementia-free patients were included in the study, and only 141 subjects developed dementia. The authors found that, among women, a 50% increase in vitamin D concentration was associated with a higher risk of dementia. In comparison, in a study with 180 patients with already-diagnosed mild cognitive impairment, a 12-month vitamin D supplementation improved cognition by reducing oxidative stress
[107][117]. Another study showed that black participants benefited from vitamin supplementation and showed cognitive improvement in executive and attention scores
[110][120].