Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by José Luis Vilas and Version 2 by Jessie Wu.
In 1987, the European Society for Biomaterials coined the term “biomaterial”, defining it as a non-biological material used in medical devices with the specific purpose of interacting with biological systems. Over time, this definition of biomaterial has evolved, adapting to various contexts. Currently, biomaterials are described as materials that actively interact with biological system to assess, treat, promote healing or even replace any tissue or body function.
- biocompatibility
- biomaterial
- coatings
1. Introduction
The main characteristic of a biomaterial is its biocompatibility, which refers to the ability of the material to elicit an appropriate response from the host in a specific situation [1][2][3][4,5,6]. Again, the interpretation of biocompatibility varies based on the required performance or function of the material. Chen et al. [4][7] defined biocompatibility as a factor that can be assessed through parameters such as cell viability, tissue response, tumor formation, genetic integrity, immune reaction, and blood clotting potential. Acknowledging this wide spectrum of considerations, the Food and Drug Administration (FDA) agency has stipulated that to consider a material biocompatible [5][8], it must not cause harm to the patient. Consequently, evaluating the biocompatibility of a medical device involves considering not only the biological compatibility of the materials used, but also other factors such as its design, including geometry, electric control, and mechanical performance [4][6][7,9]. A comprehensive assessment of these aspects ensures the safety and efficacy of the medical device in its intended application, prioritizing patient well-being.
Beside biocompatibility, as described by Reinwald and collaborators, every biomaterial device must fulfil some functional requirements: safety, which is the most crucial aspect of a medical device; durability, in order to minimize the number of surgical interventions; and bio-functionality, as the biomaterial should be functionally optimized for its intended purpose, ensuring seamless performance without any interferences that could compromise its efficacy [7][10]. Biodegradable biomaterials naturally break down over time, potentially eliminating the need for device removal. Thus, in certain applications, biodegradability can offer significant benefits by enhancing biocompatibility and reducing negative immune responses in the patient.
2. Complications
Biomedical implants encompassing prosthetics, catheters, and an array of other devices, have undoubtedly revolutionized modern medicine, significantly improving the quality of life for countless patients. However, their integration with the human body does come with an inherent risk, which is an increased susceptibility to infections [8][9][10][13,14,15]. In fact, implant-related infections and the lack of biointegration represent the most prevalent and severe complications associated with the utilization of biomaterials. Infections can lead to various complications, ranging from localized discomfort to systemic health issues, potentially needing additional medical interventions and compromising patient outcomes [11][16].
When any biomaterial is implanted in the body, it induces a response from the host tissue, known as the host response [12][17]. This response occurs regardless of the method used to introduce the biomaterial, whether by injection or through surgery. The presence of a foreign biomaterial disrupts the local host tissue environment [12][17]. The magnitude of the host response depends on the extent to which the normal state of the equilibrium, known as homeostasis, is disturbed by the injury caused during implantation. This disruption, along with the introduction of the foreign object, determines the biocompatibility of the material. While numerous biomaterials and medical devices have been successfully implanted in humans, there is currently no material that can completely evade the highly efficient surveillance system of the human body. The host response is initiated by the adsorption of proteins on the surface of the material, leading to the formation of a dense collagenous capsule around the implant [13][14][11,18]. This encapsulation impedes further interaction of the implant with the surrounding tissue, a process often referred to as biofouling [12][15][17,19].
The various stages of foreign body response (FBR) constitute a dynamic process involving multiple intricate events. These stages include injury, blood–material interactions, provisional matrix formation, acute inflammation, chronic inflammation, granular tissue development, and fibrous capsule development (Figure 1) [12][17]. Blood is often the first body fluid to come into contact with implanted devices. Blood compatibility or hemocompatibility refers to a material’s ability to regulate the thrombotic and inflammatory responses induced by the foreign surface upon contact with blood. This attribute is an essential requirement for materials designed for blood–contact applications [16][20]. Such interactions between blood and medical devices trigger a complex series of events, including protein adsorption, platelet adhesion and activation, coagulation and thrombosis. The rapid absorption of plasma protein into the surface of biomaterial represents the initial event in blood–material interaction. This adsorption results in activated proteins that can catalyze, mediate, or moderate subsequent biological response to the biomaterial [15][19]. Surface-induced thrombosis is the main problem impeding the development of long-term blood contacting devices. Thrombus formation on device surfaces is a consequence of two key factors: platelet-mediated reactions and coagulation of blood plasma [16][20].
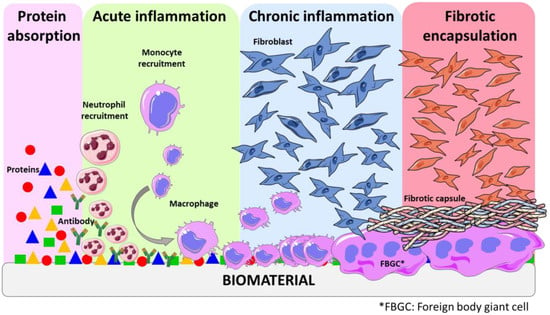
Figure 1.
Schematic representation of some stages of the host tissue response.
Throughout this process, a complex interactions of inflammatory cells, mitogens, chemo-attractants, cytokines, and other bioactive agents also play a key role in orchestrating the response [13][11]. Understanding each of these events is essential as they contribute significantly to the overall outcome of FBR. The delicate interaction between the immune system and the foreign material leads to the formation of provisional matrices, which triggers acute inflammation. This initial inflammatory response paves the way for chronic inflammation and subsequent granular tissue development. Ultimately, a fibrous capsule is formed to completely cover the foreign material, isolating and protecting the surrounding tissue from potential harm [17][21]. It is noteworthy that this late state of FBR is also influenced by the surface properties of the biomaterial. Some studies confirmed that all material classes elicited a comparable inflammatory response, suggesting that the material’s chemical composition plays a secondary role in this process. However, the roughness of the surface has great impact on the FBR—in fact, switching from a flat surface to a microstructured surface using the same material resulted in a notable decrease in the FBR [18][19][22,23].
Over the years, the concept of a “race to the surface” has been proposed to describe the competition between host cells and contaminating bacteria for occupying biomaterial surfaces [20][24]. The successful integration of biomaterials into host tissues is crucial for the effectiveness of many implants. Moreover, most studies conclude that rapid integration is also essential for preventing bacterial adhesion and colonization. In the particular case of orthopedics, the healing of bone tissue around the implant leads to the apposition of bone, facilitating the integration of the implant into the bone tissue, a process known as osseointegration [21][25]. In vitro studies with osteosarcoma cells demonstrate that pre-colonizing bacteria significantly alter and compromise host cell adhesion to material surfaces. It is important to note that if bacterial adhesion occurs before tissue repair takes place, the defense mechanism of the host may not be able to prevent surface colonization and subsequent biofilm formation [22][26].
The primary focus of this resviearchw will be orthopedic implants, as the prosthetics that remain in the body are particularly susceptible to thrombosis, inflammation, and infections, presenting significant challenges. In fact, these complications associated with implants frequently lead to device failure, requiring replacement in some cases, and can even result in chronic diseases [23][24][27,28]. Identifying and diagnosing orthopedic implant infections and inflammation, including determining the infectious agent and its antimicrobial sensitivity, pose significant difficulties. Moreover, treating these infections can be complicated due to various factors, such as antimicrobial resistance, tolerance, and/or persistence. Although the most widely recognized bacterial defense mechanism against antibiotics is resistance, which is based on the release of hydrolases to break down antibiotics and eject the antibiotics from cytosol, persistence stands as another fundamental mechanism that causes antibiotic treatment failure [25][29]. In contrast to resistant cells, persistent cells are genetically susceptible to antibiotics, yet they exhibit phenotypic tolerance, allowing them to endure antibiotic exposure. This phenomenon seems to be an ancestral trait, inherited from predecessor cells, as it is commonly observed in a variety of bacterial species, encompassing both Gram-negative and Gram-positive bacteria. During exposure to antibiotics, these species tend to develop a persister subpopulation as part of their adaptive survival strategy [26][30]. Besides Staphylococcus aureus being a common bacteria around orthopedic implant infection, it is essential to recognize that many other pathogens can also be responsible for causing such infections [10][27][15,31].
Implant infections are complex processes involving interactions among the pathogens, biomaterial, and the response of the host immune system. In the absence of foreign bodies, opportunistic pathogens are typically cleared by the defenses of the immune system. However, as commented previously, in the case of implant-associated infections, the biomaterial triggers a localized tissue response, leading to acute and chronic inflammation, foreign body reaction, granulation tissue formation, and eventual fibrous encapsulation. This unique environment creates a niche of immune depression, known as a locus minoris resistentiae, which makes the implant more susceptible to microbial colonization and infection. Furthermore, the biomaterial serves as a substrate for bacterial adhesion and biofilm formation [10][15]. Bacterial adhesion is the initial step in biomaterial-related infections and serves as a foundation for subsequent implant colonization. Once attached, pathogens form micro-colonies and develop protective biofilms, allowing them to persist in the hostile host environment. Thus, adhesion and biofilm formation are critical processes that enable pathogens to establish and maintain infections in implant sites. Understanding these complex interactions is essential for developing effective strategies to prevent and treat implant-associated infections [28][29][32,33].
Bacterial adhesion is a multi-stage process that can be divided into two main phases (Figure 2). The first stage involves the primary unspecific reversible attachment, while the second stage comprises specific irreversible attachment. When bacteria initially adhere to abiotic surfaces, such as those found in implants, the attachment is typically unspecific [10][15]. However, when they attach to living tissues, it involves specific interactions facilitated by lectins or adhesins. When a bare material surface comes into contact with physiological fluids such as blood and interstitial fluids, it rapidly becomes covered by extracellular matrix (ECM) proteins and immune components within nanoseconds [30][31][34,35]. This process is influenced by the surface chemistry and wettability of the implant surface. Hence, adhesins play a crucial role as the primary mechanism for bacterial attachment to the implant surface within the body. Both Staphylococcus aureus and Staphylococcus epidermis possess multiple mechanism for attachment and biofilm formation, significantly contributing to their virulence in chronic implant infections. The process of biofilm formation encompasses several stages (Figure 2): (I) adhesion, which is the initial stage; (II) micro-colony, where bacterial cells form aggregations and extracellular polymeric substances (EPS) are produced; (III) macro-colony formation, which undergoes further remodeling and maturation, resulting in the development of macro-colonies that appear as towers within the biofilm structure; and (IV) biofilm dispersal, which is the final stage, wherein some bacteria revert to a planktonic lifestyle, potentially colonizing new areas and initiating the biofilm formation process elsewhere [10][15].
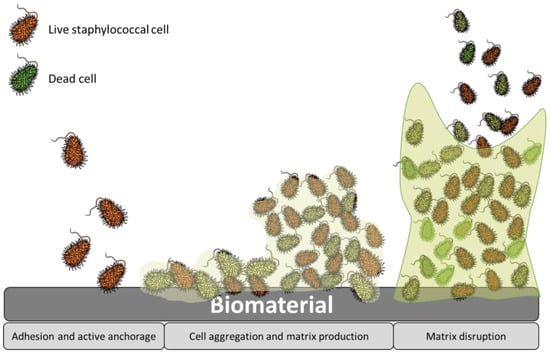
Figure 2.
Stages of staphylococcal biofilm formation.
Biological responses and bacterial adhesion are intricate processes influenced by numerous factors, but it is widely accepted that these responses are significantly affected by the surface properties [32][36]. In fact, various surface characteristics including chemistry, topography, surface free energy, elasticity, and charge play essential roles in modulating protein and cell interactions, and, consequently, host response.
Regarding surface topography and roughness, they play a crucial role in determining the biological responses to foreign materials and bacterial adhesion. Extensive research has shown that surfaces with micro- and nanoscale structures significantly impact various cells and bacteria behaviors. Surface patterning serves as a key determinant influencing both the contact area and the adhesion force between bacteria, proteins or cells, and the substrate. Indeed, these surface features can modulate cell orientation, morphology, adhesion, proliferation, and even regulate cellular functions and gene expression [33][37]. For instance, Yang et al. [34][38] compared the adhesion of both Gram-positive and Gram-negative bacteria on different patterned surfaces (Figure 3). Factors such as the geometry, size, and the height of the patterned surface impact on the interaction of bacterial and surfaces. Nanostructures with a high aspect-ratio, such as nanopillars and nanospikes, exhibited exceptional bactericidal activity. Indeed, when bacterial attachment occurs, the cell membrane of the bacteria lies within these nanostructured patterns cavities until the membrane breaks. On the other hand, both nanotubes and nano ripples have demonstrated efficacy in diminishing bacterial adhesion. Furthermore, enhanced bacterial reduction is obtained with larger diameters for nanotubes and reduced contact within the structure array for both nanotubes and nano ripples. Similarly microscale patterned surfaces including microwells, sub micro pillars, micro pillars, and micro protrusions present significant bacterial growth and colonization inhibition [35][39]. In fact, they trap bacteria within deep valleys, shielding them from the shear force of fluid, while a smooth surface facilitates the movement of attached bacteria, thereby increasing the probability of bacterial adhesion [34][38].
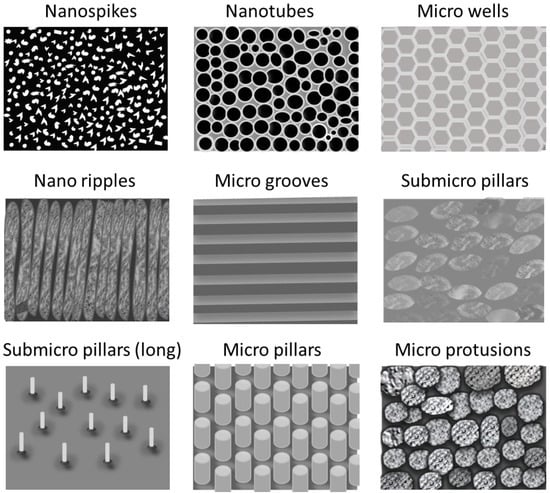
Figure 3.
Schematic representation of the different patterned surfaces.
As commented, bacteria are not the only compounds that are influenced by the topography. Surface texturing serves as a strategic approach to modulate protein uptake on surfaces as well. This technique requires precise control over total protein adsorption levels, influencing the ratio of various proteins, spatial distribution, protein conformation, and surface binding affinity. The impact of nanoscale topographies on protein adsorption is particularly significant when the surface features align with the dimensions of the proteins. Conversely, interactions with topographies significantly larger than dimensions of proteins, such as micrometer-scale patterning, are generally perceived by proteins as a flat surface [36][40]. Moreover, smooth and flat implant surfaces have shown to induce the adhesion of foreign body giant cell (FBGC), which provoke the fibrotic capsule formation [18][22].
Concerning roughness, under static culture conditions, bacteria exhibit preference for smoother surfaces when the average roughness (Ra) value is low, ranging between 0.23 and 6.13 nm. Conversely, as these values increase within the range of 6–30 nm, bacteria tend to adhere to rougher surfaces [34][37][38,41]. This roughness adaptability was studied by Mu et al. [38][42], who prepared quartz surfaces with different roughness and treated with Salmonella enterica culture. When the roughness is low (root-mean-square (RMS) > 10 nm), isolated microcolonies form, hosting a relatively sparse population of adherent bacteria with a low overall areal density. Progressing to intermediate roughness values (RMS between 10 and 40 nm), a substantial increase in adherent bacteria is observed, replacing isolated microclines with loosely connected bacterial monolayers. Additionally, the bacteria exhibited a more pronounced deformation/flattening ratio on these surfaces, suggesting a heightened attraction between bacteria and the surfaces. Conversely, at high roughness values (RMS < 45 nm), the areal density of adhering bacteria is exceeding low, and no microcolonies are observed. Bacteria predominantly exist as individual isolated organisms on these surfaces with a small fraction forming dimeric and trimeric aggregates.
Surface wettability is governed by both roughness and the chemistry of the surface jointly influencing its capability. It must be noted that the water contact angle (WCA) of rough surfaces (known as “apparent” WCA) differs from smooth surfaces (called “intrinsic” WCA). According to the Wenzel model, a rough hydrophilic surface exhibits an apparent WCA value lower than its intrinsic WCA value. Conversely, a rough hydrophobic surface displays an apparent WCA higher that it inherent WCA [39][43]. Some studies concluded that bacteria prefer to adhere to hydrophobic surfaces rather than hydrophilic ones. However, both superhydrophilic and superhydrophobic surfaces have demonstrated antibacterial behavior [40][41][44,45]. In fact, superhydrophobic surfaces, characterized by an apparent WCA exceeding 150°, require the entrapment of air bubbles within nanostructures or microstructures, as outlined by the Cassie and Bexter model [41][45]. Regarding proteins and macrophages, hydrophobic materials exhibit increased protein adsorption but also enhanced macrophage adhesion [42][43][46,47], potentially contributing to the initiation of fibrotic encapsulation. Conversely, in the case of hydrophilic materials, macrophages demonstrate heightened adhesion to positively charged implants in comparison to anionic or nonionic alternatives [18][22].
To address this critical challenge, extensive research and advancements in material science and implant design are continuously pursued.
