Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Murat Yildirim and Version 2 by Rita Xu.
Multiphoton microscopy (MPM) has emerged as a vital tool in neuroscience, enabling deeper imaging with a broader field of view, as well as faster and sub-cellular resolution.
- ultrafast fiber lasers
- multiphoton microscopy
- neuroscience
1. Introduction
Multi-photon microscopy (MPM) is a powerful technique for the minimally invasive imaging of live brains with subcellular resolution and for performing three-dimensional imaging with depths ranging from a few hundred microns to a few millimeters using two- and three-photon excitation processes [1][2][3][4][5][6][1,2,3,4,5,6]. Because of the nonlinear interaction between ultrashort pulses and the brain at the near-infrared wavelengths, MPM can provide the high-resolution imaging, as well as deeper penetration depth. The distinctive advantages of MPM adoption in neuroscience include the ability to visualize the brain activity and structure with subcellular resolution (even imaging fine structures such as spine and axons) in vivo at millimeter depths with or without labeling the different cell types in the brain. Although confocal and light sheet microscopy also provides subcellular resolution, they are only capable of imaging shallower depths compared to multiphoton microscopy. Because it is critical to understand the connectivity between neurons at distinct cortical layers when they process sensory and other kinds of information, multiphoton microscopy is particularly useful in neuroscience to address these types of questions.
A femtosecond pulsed laser is used as a laser source for the MPM. Generally, the ultrashort laser pulses can be generated either by using bulk or fiber-type laser systems. Titanium-doped Sapphire (Ti:S) lasers, which provide stability, short pulse widths in the femtosecond region, and extended wavelength tunability, are the most frequent conventional lasers used in MPM. Ti-S lasers produce wavelengths ranging from 700 to 1300 nm at a repetition rate of 80 MHz. In general, femtosecond laser pulses with this spectral range and repetition rate are appropriate for exciting most fluorophores for structural and functional brain imaging via two-photon interactions [7]. When performing three-photon imaging of the brain, the laser characteristics differ dramatically from those used for two-photon microscopy. For three-photon microscopy, the number of photons that are absorbed during this process can be described as follows [5][6][5,6]:
where the terms are explained as follows: n: number of excited photons, 𝛿: excitation cross-section, P: average power, 𝜏: pulse width, R: repetition rate, NA: numerical aperture, h: Planck ‘s constant, c: speed of light, and 𝜆: wavelength.
Therefore, it is important to increase the laser power and lower the repetition rate and the pulse width in order to increase the number of photons excited in a three-photon process while keeping the numerical aperture and the wavelength constant. Specifically, the wavelength of the laser has been chosen at longer wavelength ranges, such as 1300 and 1700 nm. The laser average power is the key player in three-photon microscopy for brain imaging, because, the high average power can result in (a) tissue heating through linear absorption of longer excitation wavelengths, (b) nonlinear damage at the focal plane due to high peak power/intensity, (c) saturation of fluorophores due to the high pulse energy. Due to these effects, it is crucial to consider laser parameters and perform control experiments to make sure that it is safe to perform 3p imaging in the living brain [6][8][6,8]. Also, the repetition rates must be decreased to 0.5 to 2 MHz, and the pulse widths must be less than 50 fs [5][8][9][5,8,9]. Significant progress has recently been made in the development of optical parametric amplifiers (OPAs) that can produce longer excitation wavelengths with moderate repetition rates at short pulse widths. Although these commercial lasers offer several multiphoton imaging choices in the brain [10], they have several drawbacks, including high costs and sophisticated and bulky equipment. As a result, democratizing these imaging techniques in neuroscience labs is extremely difficult.
As an alternative to conventional ultrafast lasers, great progress has been made in fiber lasers, which can overcome various disadvantages of conventional lasers. In particular, fiber lasers are very small and compact, can be operated with air cooling, have a high pump-to-signal conversion efficiency, have a lower overall cost than conventional solid-state lasers, and can provide unconventional laser characteristics such as longer wavelengths, high repetition rates, variable pulse energy, and narrow pulse widths. Thus, in the recent decade, the use of fiber lasers for neuroscience applications has emerged. The simplest way to generate multiple wavelengths in a fiber laser is to adapt various types of gain fibers to achieve the required wavelength of light [11][12][11,12]. The ytterbium (Yb) fiber is employed to generate 1000 nm wavelengths [13]. Similarly, the laser wavelength spectrum of 1550 nm is achieved by using Erbium (Er)-doped fiber as the gain medium [14][15][14,15] and the thulium (Tm)-doped fiber emits a laser wavelength spectrum of 1700 nm [16]. Recent advancements in femtosecond fiber lasers have been reported in fiber laser oscillators as well as fiber amplifiers. In laser oscillators, important advancements have focused on the development of novel saturable absorbers (SAs), various cavity designs, similaritons, all-normal dispersion (ANDi) configurations, dual-wavelength generation, variable repetition rates, and multimode fiber laser oscillators [17]. Similarly, innovative fiber amplifiers concentrate on chirped pulse amplification and direct amplification approaches for increasing the energy of ultra-short laser pulses. Eventually, the average power of the laser pulses increased to over 1 Watts [18] at MHz repetition rates, which is essential for achieving multi-photon effects such as two- and three-photon fluorescence, as well as label-free imaging in the brain [19].
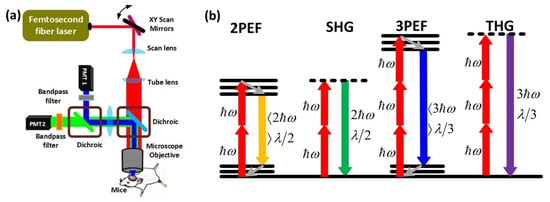 A typical MPM setup is shown in Figure 1a. In a typical multiphoton microscope, a femtosecond pulsed fiber or a conventional laser is used as a laser source. The collimated light is directed to the pair of Galvo mirrors for scanning the beam in the two-dimensional space. Then, the light beam is passed through a scan and tube lens, which magnifies the beam to fill the back aperture of the microscope objective. Finally, the laser beam is transmitted by the dichroic and focused on the tissue through the objective with a beam size in the range of micrometers. The reflected light is directed to the same dichroic mirror which sends the beam to the highly sensitive photon multiplier tubes (PMTs). In front of each PMT, there are band-pass filters to select an appropriate emission wavelength for different kinds of MPM techniques (Figure 1a). There are numerous varieties of MPM based on the degree of the nonlinear excitation process and the type of contrast mechanism. Two-photon excited fluorescence (2PEF), second-harmonic generation (SHG), three-photon excited fluorescence (3PEF), and third-harmonic generation (THG) microscopy techniques are described in Figure 1b. When two lower energy photons are absorbed at the same time, electrons shift from the ground to the excitation energy state, resulting in two-photon excited fluorescence (2PEF). The electrons then lose some energy as they travel to a lower energy ground state level and produce another photon with a wavelength more than half that of the incident photons (Figure 1b). Second-harmonic generation (SHG) occurs when two photons of identical energy excite an electron to a virtual energy state. When the electrons return to the ground state, they emit a photon with half the wavelength of the incident photons (Figure 1b). In terms of three-photon interactions, three-photon excited fluorescence (3PEF) occurs when electrons absorb three photons simultaneously and move to the excitation state. The electrons then lose energy as they migrate from the higher energy excitation states to the ground state, releasing another photon with a wavelength greater than one-third of the excitation of the incident photons. On the other hand, third-harmonic generation (THG) is a parametric harmonic generation process similar to SHG in which three photons of the same energy are involved in the excitation process of electrons to a virtual energy state, and when the excited electrons return to the ground state, they emit photons at one-third of the excitation wavelength of incident photons. The important challenges of 2PEF, 3PEF, SHG, and THG microscopy for brain imaging include developing fluorophores with greater absorption peaks at longer excitation wavelengths and developing PMT modules that can have higher sensitivity outside the visible range of light to perform deeper brain imaging. Besides, femtosecond laser sources should possess versatile characteristics in order to enhance the MPM performance for neuroimaging applications.
A typical MPM setup is shown in Figure 1a. In a typical multiphoton microscope, a femtosecond pulsed fiber or a conventional laser is used as a laser source. The collimated light is directed to the pair of Galvo mirrors for scanning the beam in the two-dimensional space. Then, the light beam is passed through a scan and tube lens, which magnifies the beam to fill the back aperture of the microscope objective. Finally, the laser beam is transmitted by the dichroic and focused on the tissue through the objective with a beam size in the range of micrometers. The reflected light is directed to the same dichroic mirror which sends the beam to the highly sensitive photon multiplier tubes (PMTs). In front of each PMT, there are band-pass filters to select an appropriate emission wavelength for different kinds of MPM techniques (Figure 1a). There are numerous varieties of MPM based on the degree of the nonlinear excitation process and the type of contrast mechanism. Two-photon excited fluorescence (2PEF), second-harmonic generation (SHG), three-photon excited fluorescence (3PEF), and third-harmonic generation (THG) microscopy techniques are described in Figure 1b. When two lower energy photons are absorbed at the same time, electrons shift from the ground to the excitation energy state, resulting in two-photon excited fluorescence (2PEF). The electrons then lose some energy as they travel to a lower energy ground state level and produce another photon with a wavelength more than half that of the incident photons (Figure 1b). Second-harmonic generation (SHG) occurs when two photons of identical energy excite an electron to a virtual energy state. When the electrons return to the ground state, they emit a photon with half the wavelength of the incident photons (Figure 1b). In terms of three-photon interactions, three-photon excited fluorescence (3PEF) occurs when electrons absorb three photons simultaneously and move to the excitation state. The electrons then lose energy as they migrate from the higher energy excitation states to the ground state, releasing another photon with a wavelength greater than one-third of the excitation of the incident photons. On the other hand, third-harmonic generation (THG) is a parametric harmonic generation process similar to SHG in which three photons of the same energy are involved in the excitation process of electrons to a virtual energy state, and when the excited electrons return to the ground state, they emit photons at one-third of the excitation wavelength of incident photons. The important challenges of 2PEF, 3PEF, SHG, and THG microscopy for brain imaging include developing fluorophores with greater absorption peaks at longer excitation wavelengths and developing PMT modules that can have higher sensitivity outside the visible range of light to perform deeper brain imaging. Besides, femtosecond laser sources should possess versatile characteristics in order to enhance the MPM performance for neuroimaging applications.
2. Multiphoton Microscopy (MPM) for Neuronal Imaging
Conventionally, several imaging techniques, including positron emission tomography (PET), ultrasonography, and functional magnetic resonance imaging (fMRI), have been proposed for structural and functional brain imaging. However, they are limited in terms of spatial and temporal resolution [20]. These disadvantages can be solved by optical imaging techniques, such as confocal, light-sheet, two-photon (2P), and three-photon (3P) microscopes. The main advantage of these technologies is to have the capability of imaging the structure and function of the brain at sub-cellular and greater temporal resolutions. When rwesearchers compare the spatial and temporal resolution capabilities of multiphoton, light sheet, and confocal microscopy techniques, they all exhibit almost the same performance. On the other hand, light sheet and confocal microscopy enable brain imaging at shorter depths, but 2P and 3P microscopy techniques can image all cortical layers, white matter, and even subcortical regions in intact brains [5][6][21][5,6,21]. There are several advantages and challenges of 2P and 3P microscopy for performing structural and functional brain imaging. In minimally invasive 2P microscopy for functional brain imaging, it is very feasible to perform high-speed (kHz rate) and larger field of view (several millimeters) functional imaging mostly at superficial cortical layers [22][23][24][25][22,23,24,25]. However, it is challenging to perform 2P microscopy in deeper regions of the brain due to high out-of-focus signal generation. When compared to 2P microscopy, 3P microscopy may readily minimize the challenge of deep brain functional imaging by utilizing longer excitation wavelengths (1300, and 1700 nm) at moderate imaging speeds (5–10 Hz) and a smaller field of view [4][5][6][4,5,6]. Therefore, the challenges of functional brain imaging with 3P microscopy are (a) to increase the imaging speed to kHz range at deeper brain regions, (b) to increase the imaging depth to subcortical regions, (c) to increase the field of view to image multiple brain regions simultaneously. For structural brain imaging, two-photon fluorescence microscopy (2PEF), three-photon fluorescence microscopy (3PEF), second-harmonic generation (SHG), and third-harmonic generation (THG) microscopy are utilized. The challenges of 2PEF, 3PEF, SHG, and THG microscopy for brain imaging include developing fluorophores with greater absorption peaks at longer excitation wavelengths and developing PMT modules that can have higher sensitivity outside the visible range of light to perform deeper brain imaging. A typical MPM experimental setup and different kinds of MPM modalities are explained in Figure 1.
Figure 1. Fundamentals and types of multiphoton microscopy (MPM). (a) A femtosecond laser generates ultrashort pulses, which are sent to galvanometric (xy) scanning mirrors to scan the beam on the back aperture of the objective with the help of scan and tube lenses. The dichroic mirror helps to transmit the excitation wavelength and reflects the emitted light from the mouse brain. Since the beam fills most of the back aperture of the objective lens, the beam is focused to a micrometer level beam size on the focal plane of the objective. The emitted light is directed to the highly sensitive photon multiplier tubes (PMTs) by using two dichroic mirrors and two band-pass filters depending on the type of MPM. (b) Energy-level diagrams of different kinds of MPMs, such as 2PEF, SHG, 3PEF, and THG. ℎ𝜔 is the excitation photon energy, 𝜆 denotes wavelength.
