You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Alberto Palazzuoli.
Patients with heart failure are conventionally stratified into phenotypic groups based on their ejection fraction. The aim of this stratification is to improve disease management with a more targeted therapeutic approach. A further subdivision based on patient gender is justified. It is recognized that women are underrepresented in randomized controlled clinical trials, resulting in limited clinical and molecular differentiation between males and females. However, many observational studies show that the onset, development, and clinical course of the disease may substantially differ between the two sexes.
- heart failure phenotypes
- sex differences
- sex hormones
- biomarkers
- risk factors
1. Introduction
Heart failure is one of the most common diseases of this century. Due to the increase in average survival and the growing incidence of cardiovascular risk factors and comorbidities, the number of people living with chronic heart failure (CHF) is increasing [1]. Therefore, it is mandatory to achieve a better understanding of this condition in order to define the optimal therapeutic management and diagnostic timeframe. It is essential to recognize elements related to sex differences, e.g., the incidence of particular risk factors and the related pathophysiological mechanisms [2]. The identification of specific patterns may lead to a more targeted and effective therapeutic approach. Indeed, the phenotypic manifestations of heart failure are different in men and women due to the different hormonal patterns, molecular signals, and cardiac structures in the two sexes. Although these mechanisms are well known in men, in women they are currently underestimated due to the underrepresentation of females in clinical trials and the lack of detailed sex-matching analysis [3,4][3][4].
2. Epidemiology
Based on the data analysis from the National Health and Nutritional Examination Surveys conducted between 2015 and 2018, the prevalence of heart failure in the population aged over 20 years is estimated to be around 6 million individuals. According to this registry, in the age range between 20 and 79 years, the prevalence of heart failure is higher in men than in women. However, in the older population, particularly among women, the prevalence increases [5,6][5][6]. Between 2014 and 2018, there was a comparable increase in the hospitalization rate for heart failure in both men and women [4]. Currently, the overall risk of developing heart failure is roughly similar for both sexes. In the Framingham Heart Study, it is estimated to be 21% in men and 20% in women at the age of 40, while in the Rotterdam Study, it is 33% in men and 29% in women at the age of 55 [7,8][7][8]. Despite a similar overall risk, women with heart failure, as identified by the Kansas City Cardiomyopathy Questionnaire, experience a greater burden of symptoms, an overall poorer health status, and lower quality of life [9]. Furthermore, significant epidemiological differences emerge when examining heart failure phenotypes. The Southwestern European community-based Epidemiology of Heart Failure and Learning (EPICA) study revealed that heart failure with preserved ejection fraction (HFpEF) is more prevalent in the female population, with a prevalence that increases with age (0% in men and 1% in women in the age group 25–49 years, increasing to 4–6% in men and 8–10% in women for individuals aged 80 or older) [10] (Table 1).Table 1.
Summary of epidemiological data on heart failure in men and women from major studies in the scientific literature.
| Epidemiological Data | Men | Women |
|---|---|---|
| Framingham Heart Study | The risk of developing heart failure is 21% at age 40 years | The risk of developing heart failure is 20% at age 40 years |
| Rotterdam Study | The risk of developing heart failure is 33% at age 55 years | The risk of developing heart failure is 29% at age 55 years |
| Southwestern European community-based Epidemiology of Heart Failure and Learning (EPICA) study | The prevalence of heart failure with preserved ejection fraction is 0% in the 25–49 age group, and increases to 4–6% in men aged 80 or older | The prevalence of heart failure with preserved ejection fraction is 1% in the 25–49 age group, and increases to 8–10% in women aged 80 or older |
3. Risk Factors
Significant differences in the incidence and distribution of risk factors between men and women are well documented. This gap determines different characteristics in the frequency and types of heart failure (HF) in each sex. Furthermore, there are specific risk factors associated with each sex that deserve a detailed description. These differences in timing and prevalence contribute to the different phenotypic patterns that define the development and evolution of the disease, and the response to therapy (Table 2).Table 2.
Differences in main risk factors for heart failure between sexes.
| Risk Factor | Men | Women |
|---|---|---|
| Diabetes mellitus | 2.4-fold increased risk of HF | 5-fold increased risk of HF |
| Hypertension | 2-fold increased risk of HF | 3-fold increased risk oh HF |
| Obesity | Lower association with male sex and HFrEF | Greater association with female sex and HFpEF |
| Tobacco smoke | Higher consumption but lower correlation (45% increased risk) | Lower consumption (increasing) but higher correlation (88% increased risk) |
| Coronary artery disease | Macrovascular disease more associated with both HFrEF and HFpEF | Less frequent, more often microvascular dysfunction |
| Anemia and iron deficiency | Less frequent | More frequent and associated with HFpEF |
| Vitamin D deficiency | Less frequent | More frequent and associated with higher cardiovascular mortality |
| Anorexia nervosa | Higher association with cardiovascular events | More frequent |
3.1. Diabetes Mellitus
Diabetes is a traditional risk factor for heart failure and has a stronger impact on women. Diabetes is associated with an approximately 5-fold increase in the risk of developing heart failure in women, compared to a 2.4-fold increased risk in men [19]. Furthermore, imaging techniques have revealed, in women, a higher rate of left ventricular remodeling characterized by increased left ventricular wall thickness and an elevated left ventricular mass index [20]. Diabetes appears to be implicated in the etiopathogenetic mechanisms of heart failure with preserved ejection fraction (HFpEF) through systemic vascular inflammation and endothelial dysfunction [21,22][21][22]. The production of free radicals and the reduced bioavailability of nitric oxide (NO), stemming from the biochemical and metabolic changes that occur in individuals with diabetes, lead to a decrease in the activity of guanylate monophosphate cyclase (GMPc) in cardiomyocytes. This results in reduced myocardial relaxation, an increased stimulus for hypertrophy, and increased wall stiffness [23,24][23][24]. The association between diabetes and HFpEF, as well as the relevance of diabetes as a risk factor in women, aligns with the greater prevalence of this phenotype in women.3.2. Hypertension
Hypertension is a traditional risk factor associated with HF. Although the prevalence of arterial hypertension is similar in both sexes, according to the Framingham Heart Study, it appears to be more closely related to a greater incidence of heart failure in women (3-fold increased risk) than in men (2-fold increased risk) [25]. According to the results of the PARAMOUNT study (Prospective comparative of ARNI with ARB on Management Of heart failUre with preserved ejectioN section), women have greater arterial stiffness than men. This stiffness seems to be responsible for the development of cardiac hypertrophy and an increase in vascular resistance [26]. Additionally, increased blood pressure may lead to an increase in cardiac afterload, predisposing to the development of diastolic dysfunction. In addition to the structural changes in the heart muscle, hypertension can also lead to increased stiffness of the heart chambers, further contributing to diastolic dysfunction. Hypertension also determines a phenomenon of microvascular inflammation underlying the pathogenesis of HFpEF [21]. Interestingly, it has been suggested that there is a common pathophysiological pathway connecting preeclampsia, fetal growth retardation (FGR), and the subsequent development of heart failure with preserved ejection fraction (HFpEF) later in life [27]. This pathway involves various interconnected mechanisms. Preeclampsia increases chronic inflammation that may persist beyond pregnancy and contribute to the development of cardiovascular problems later in life. Preeclampsia is also associated with increased oxidative stress, potentially setting the stage for HFpEF. Some authors suggest that preeclampsia may accelerate vascular biological aging contributing to HFpEF [28]. These interconnected mechanisms collectively contribute to the development of HFpEF in individuals who have a history of preeclampsia and fetal growth retardation during pregnancy. This pathway is being investigated as a potential link; the exact mechanisms and the extent of their contribution to the development of HFpEF vary among individuals.3.3. Obesity
Obesity is an important risk factor associated with the development of cardiovascular disease. Recently, the role of obesity in heart failure has been analyzed in depth, revealing that it is more frequently associated with HFpEF than with HFrEF. Furthermore, obesity has an important role as a greater risk factor in women than in men because it is associated with a sedentary lifestyle and reduced physical activity and also because of the inflammatory response associated with the visceral obesity of menopause [29,30,31][29][30][31]. The reason for the relationship between obesity and HFpEF appears to be due to some enhancement in its metabolic processes: insulin resistance is responsible for a systemic inflammatory state that leads to concentric cardiac remodeling [31,32][31][32]. Increased LDL deposition and oxidized fatty acid increase vascular oxidative stress, causing an endothelial damage drive leading to an increased vascular tone and microcirculatory vasoconstriction. Pre-menopausal women are relatively protected from these mechanisms thanks to the anti-inflammatory and antioxidant action of estrogens. However, with the decrease in circulating estrogen levels following menopause, there is a reduction of these protective mechanisms as well as a significant increase in visceral fat responsible for the increased risk of HFpEF [33,34,35][33][34][35]. Fat distribution is also crucial in determining cardiovascular risk; it is known that a visceral, central, or android-type distribution, more typical of men and post-menopausal women, is associated with a higher risk. Recent studies show that this type of adiposity is more harmful in women than in men, with a closer correlation and a higher cardiometabolic risk. Similarly, the gynoid distribution is more protective in men than in women, with a more significant decrease in risk in the former (90–84% lower in men versus 80–78% in women) [36]. Leptin and adiponectin are both hormones produced by adipose tissue, but they have different functions and effects on the body. Leptin is often referred to as the “satiety hormone” because its primary role is to regulate appetite and energy balance. Leptin also plays a role in regulating metabolism and energy expenditure. Adiponectin has various functions related to insulin sensitivity and anti-inflammatory effects. Adiponectin levels are generally higher in lean individuals and lower in those with obesity. Higher levels of adiponectin are associated with improved insulin sensitivity and metabolic health. Adiponectin levels tend to decrease with increasing body fat [30,33,35][30][33][35].3.4. Tobacco Smoke
According to the First National Health and Nutrition Examination Survey (NHANES I) study, the risk of heart failure associated with cigarette smoking is higher for women than for men [37]. This higher risk to date has been offset by lower consumption by women, but this trend is currently changing, especially in industrialized countries [38,39][38][39]. A plausible reason is a change in the lifestyle of women who have taken on leadership positions, with the consequent stress due to the burden of responsibility. Moreover, women have a low perception of their cardiovascular risk and are more easily exposed to risk factors. Furthermore, with the widespread use of electronic smoking devices, the associated risk is likely to be underestimated, since little is known about the effects of new electronic and non-electronic smoking devices. This potential new risk factor must be further investigated [40].3.5. Coronary Artery Disease
Coronary heart disease is the major risk factor for the development of HF, especially in industrialized countries [41]. It is now established that the incidence of macrovascular coronary artery disease is higher in men, leading more frequently to an HFrEF phenotype [42]. In recent studies, a close correlation has been seen between CAD and HFpEF, since it equally determines both systolic and diastolic dysfunction; the incidence of HFrEF and HFpEF related to CAD seems to be comparable [43]. This association appears to be stronger in males than in females for HFpEF; indeed, in the I-PRESERVE study, the cause of HFpEF was more frequently ischemic in men than in women (34% vs. 19%) [44]. A recent study examined disparities in all-cause mortality between women and men with HFrEF and the impact of the presence of CAD. Despite an overall lower mortality rate among female patients than among men, women without CAD had the lowest mortality, while women and men with CAD had a similar 10-year mortality risk. This study highlights that the presence of CAD has a significantly greater prognostic impact in women than in men with newly diagnosed HFrEF. These findings highlight the importance of implementing individualized risk assessment strategies for patients with newly diagnosed HFrEF, with particular attention to the increased mortality risk associated with the coexistence of CAD and HFrEF in women [45].3.6. Anemia and Iron Deficiency
Anemia is associated with an increased risk of death and hospitalization in heart failure [46,47][46][47]. This is related to the activation of neurohormonal signals, implicated in oxidative metabolism and leading to systemic inflammation [44]. This hypothesis correlates well with the evidence from studies demonstrating a higher association between anemia and HFpEF [47,48,49][47][48][49]. It has been hypothesized that anemia and iron deficiency, known to be more common in women, could play a role in the increased predisposition for HFpEF [49,50][49][50].3.7. Vitamin D Deficiency
Low levels of vitamin D (<20 ng/mL) seem to be predictive of an increase in cardiovascular mortality [51]. Although a direct correlation between vitamin D deficiency and the onset of heart failure has not yet been demonstrated, it has been ascertained that this molecule plays a key role in regulating many mechanisms associated with it. First, the VDRs regulate blood pressure by acting on the renin–angiotensin–aldosterone system; some studies on mice have shown how silencing of the VDR genes leads to an activation of the RAAS system with an increase in the onset of arterial hypertension and ventricular hypertrophy [52]. Furthermore, vitamin D deficiency seems to be implicated in the genesis of endothelial dysfunction through the reduction of nitric oxide production [53]. Adequate levels of vitamin D prevent the activation of the mechanisms that lead to the destabilization of atherosclerotic plaques: the downregulation of metalloproteinases MMP-2 and MMP-9 leads to a decrease in the levels of inflammatory cytokines and chemokines (IL-6, IL-12, interferon-γ, and TNF-α) and an increase in anti-inflammatory factors such as IL-10 [51,54,55][51][54][55]. Vitamin D appears to have an antithrombotic action through the regulation of thrombomodulin and tissue factor expression [56]. A large proportion of postmenopausal women (50–80%) is affected by vitamin D deficiency [57]. After the menopause transition, the cutaneous and hepatic production of vitamin D is reduced, as is its intestinal absorption [58,59][58][59]. In addition, estrogens regulate the hepatic production of vitamin D-binding protein (DBP) and albumin, to which vitamin D binds within the bloodstream [60]. In the post-menopausal period, the drop in estrogen levels is associated with a reduction in circulating vitamin D. The correlation between a lack of vitamin D and a drop in estrogen levels therefore makes post-menopausal women more exposed to cardiovascular alterations.3.8. Anorexia
Anorexia nervosa (AN) is characterized by severe malnutrition and electrolyte imbalances and may be associated with different types of cardiovascular complications according to gender. In a nationwide registry, anorexia was associated with worse outcomes in males than in females: CV events, arrhythmia, and heart failure complications were more severe in males [61]. Most reports included small samples of women showing alterations linked to electrolyte imbalance, dysprotidemia, and iron deficiency. This condition facilitates arrhythmic complications, conduction abnormalities, autonomic dysfunction, and hypotension [62]. Additionally, interstitial fibrosis and loss of cross-striations and myofibrils occur in protein-calorie malnutrition, reducing myocardial mass and contractility. Finally, increased QT prolongation can occur as a consequence of electrolyte abnormalities [63]. QT prolongation is recognized as the main cause of sudden cardiac death in the AN population. Although anorexia is a condition much more frequent in females, cross-sectional studies comparing the two genders may contribute to a better understanding of the difference in the impact of this disease on males and females.3.9. Sex-Specific Risk Factors in Women
Adverse outcomes of pregnancy that involve both the mother and the fetus have been identified as risk factors for cardiovascular disease [64]. Hypertension disorders during pregnancy, i.e., gestational hypertension, preeclampsia, eclampsia, as well as chronic hypertension, increase the risk of developing arterial hypertension, coronary artery disease, and heart failure up to 40 years later [64,65,66][64][65][66]. A persistent vascular dysfunction was found in women who had a hypertension-related disease during pregnancy [67]. Cardiovascular health before pregnancy has an impact on both adverse outcomes and future CV risk. A recent AHA statement highlighted that it is essential to assess cardiovascular health in young women and promote primary prevention by following the “8 essential” guidelines [27,68][27][68]. The “8 essential” framework includes eight key health factors: diet, physical activity, no smoking, body mass index, blood pressure, lipids, sleep health, and blood sugar. This framework provides a comprehensive approach to assessing and promoting cardiovascular health [69]. Gestational diabetes increases the risk of developing type 2 diabetes later in life and increases the risk of myocardial infarction and diabetic cardiomyopathy [31]. A large cohort study showed that women with gestational diabetes had a higher risk of developing heart failure (62% higher) [70,71][70][71]. Peripartum cardiomyopathy develops during the last months of pregnancy or in the months following delivery in women who have no other known cause of HF; it is a significant life-threatening cause of HF in women [72]. Breast cancer is associated with an increased risk of heart failure (HF). The radiotherapy and chemotherapy treatments commonly used after diagnosis are the primary causes [73]. Takotsubo syndrome is more frequent in women (female-male ratio of 9:1) due to the greater impact of emotional stress on cardiovascular events [42]. The hypothesized etiopathogenetic mechanism includes a dysfunction of the microcirculation of neurogenic origin [74].4. Different Heart Failure Profiles between Sexes
The higher frequency of HFrEF in men and of HFpEF in women is a mirror of the different pathophysiological mechanisms underlying these conditions. Men are more predisposed to macrovascular alterations of the coronary arteries, a known etiological factor of HFrEF [2,4][2][4]. Women frequently present microcirculatory anomalies that seem to be at the basis of the development of HFpEF. As a result of a recent prospective multinational study, microvascular dysfunction was present in 75% of cases of HFpEF and was proportionally correlated to the increase in NT-proBNP [14]. On the basis of microvascular dysfunction, there is an alteration of the signal mediated by nitric oxide (NO). In women, the proinflammatory state induced by the comorbidities leads to a reduction in the activity of eNO synthase, a reduction in NO synthesis, and consequent dysregulation of arteriolar tone and an increase in blood pressure [75]. Furthermore, in post-menopause, the reduction in the level of estrogen leads to a further reduction in the production of NO, enhancing the aforementioned mechanism [11]. Women have a microvascular proinflammatory state due to a different immune pattern compared to men. Women present a higher level of proinflammatory cytokines and greater activity of CD4 and CD8 T cells, which support the onset of autoimmune diseases, often related to diastolic dysfunction [76,77][76][77]. This proinflammatory status is confirmed by higher levels of inflammatory biomarkers in women. In an analysis of healthy subjects from the Framingham Heart Study, women had a higher expression of markers such as CRP (C-reactive protein), hemopexin, and C2 [3]. Similarly, the Dallas Heart Study found higher levels of hsCRP (high-sensitivity CRP), D-dimer, and osteoprotegerin, and lower levels of IL (interleukin)-18 and phospholipase A2 [77] in women. These differences appear to be primarily X-linked: the human X chromosome includes multiple immune-related genes, such as IL-2 receptor-γ chain, IL-3 receptor-α chain, IL-9 receptor, IL-13 receptor-α chains, Toll-like receptor 7 (TLR7), TLR8, and IL-1 receptor-associated kinase [1,20,21][1][20][21]. Aside from a genetic basis, the proinflammatory state of women is influenced by the effects of estrogens on innate immunity and on adipose tissue deposition at the liver and abdominal levels [42,71][42][71]. Other differences between the two sexes are evident in ventricular and vascular mechanics. Women have anatomically smaller heart chambers than men, even after indexing by body surface area, with consequently lower stroke volumes; however, cardiac output is usually comparable in men and women thanks to a higher heart rate. Women are also affected by an increase in left ventricular wall stiffness, which increases with age [78]. With exercise, the lower chronotropic and contractile reserve of women, associated with the previously mentioned reduced stroke volumes, and higher ventricular rigidity, determine a reduced physical exercise tolerance, associated with a lower peak of oxygen consumption [79,80][79][80]. In women, vessels are smaller in size and have a greater wall stiffness, responsible for earlier wave reflection leading to an increase in afterload and blood pressure, diastolic dysfunction, and a concentric-type remodeling of the left ventricle [21,81,82][21][81][82]. The vessel wall thickness, not counterbalanced by an appropriate cardiac output, leads to an earlier loss of ventriculovascular coupling reserve during physical exercise in women, another key element of HFpEF [83,84][83][84]. Further, the pathophysiological feature of HFpEF is the association with pulmonary hypertension. Pulmonary hypertension is more frequent in women, due to a higher prevalence of mitral regurgitation and a greater susceptibility to idiopathic pulmonary hypertension [85,86][85][86].5. Differences in Cellular and Endocrine Patterns
The differences in the regulation of the cardiovascular system and the greater predisposition of the two sexes to the clinical manifestations described above can be justified by the action of sex hormones. Both estrogens and androgens play specific roles in some protective or predisposing pathways. The drop in the levels of these hormones that occurs with age determines alterations responsible for cardiovascular changes as well as for the development of chronic disease [87]. The action of sex hormones is exerted on various cellular targets through the interaction with different types of receptors, with the activation of intracellular patterns determining biochemical, electrolytic, genetic, or epigenetic modifications. A particular key role seems to be played through the expression of certain types of microRNAs regulated by sex hormones (Figure 1).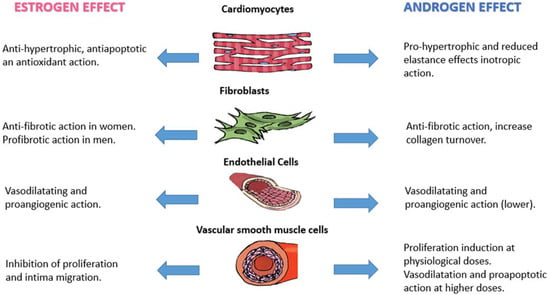
Figure 1.
Effect of sex hormones on different cell types of the cardiovascular system.
References
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc. Res. 2023, 118, 3272–3287, Erratum in Cardiovasc. Res. 2023, 119, 1453.
- Arata, A.; Ricci, F.; Khanji, M.Y.; Mantini, C.; Angeli, F.; Aquilani, R.; Di Baldassarre, A.; Renda, G.; Mattioli, A.V.; Nodari, S.; et al. Sex Differences in Heart Failure: What Do We Know? J. Cardiovasc. Dev. Dis. 2023, 10, 277.
- Bierer, B.E.; Meloney, L.G.; Ahmed, H.R.; White, S.A. Advancing the inclusion of underrepresented women in clinical research. Cell Rep. Med. 2022, 3, 100553.
- Khan, S.S.; Beach, L.B.; Yancy, C.W. Sex-Based Differences in Heart Failure: JACC Focus Seminar 7/7. J. Am. Coll. Cardiol. 2022, 79, 1530–1541.
- Dunlay, S.M.; Roger, V.L.; Redfield, M.M. Epidemiology of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2017, 14, 591–602.
- Agarwal, M.A.; Fonarow, G.C.; Ziaeian, B. National trends in heart failure hospitalizations and readmissions from 2010 to 2017. JAMA Cardiol. 2022, 7, 115.
- Lloyd-Jones, D.M.; Larson, M.G.; Leip, E.P.; Beiser, A.; D’Agostino, R.B.; Kannel, W.B.; Murabito, J.M.; Vasan, R.S.; Benjamin, E.J.; Levy, D.; et al. Lifetime risk for developing congestive heart failure: The Framingham Heart Study. Circulation 2002, 106, 3068–3072.
- Bleumink, G.S.; Knetsch, A.M.; Sturkenboom, M.C.; Straus, S.M.; Hofman, A.; Deckers, J.W.; Witteman, J.C.; Stricker, B.H. Quantifying the heart failure epidemic: Prevalence, incidence rate, lifetime risk and prognosis of heart failure The Rotterdam Study. Eur. Heart J. 2004, 25, 1614–1619.
- Rosendale, N.; Albert, M.A. The intersection of sexual orientation, gender identity, and race/ethnicity on cardiovascular health: A review of the literature and needed research. Curr. Cardiovasc. Risk Rep. 2020, 14, 17.
- Teia, F.; Fonseca, C.; Mota, T.; Morais, H.; Matias, F.; de Sousa, A.; Oliveira, A.; EPICA Investigators. Prevalence of chronic heart failure in Southwestern Europe: The EPICA study. Eur. J. Heart Fail. 2002, 4, 531–539.
- Beale, A.L.; Meyer, P.; Marwick, T.H.; Lam, C.S.; Kaye, D.M. Sex differences in cardiovascular pathophysiology: Why women are overrepresented in heart failure with preserved ejection fraction. Circulation 2018, 138, 198–205.
- Triposkiadis, F.; Xanthopoulos, A.; Butler, J. Cardiovascular aging and heart failure: JACC review topic of the week. J. Am. Coll. Cardiol. 2019, 74, 804–813.
- Shim, C.Y.; Park, S.; Choi, D.; Yang, W.I.; Cho, I.J.; Choi, E.Y.; Chung, N.; Ha, J.W. Sex differences in central hemodynamics and their relationship to left ventricular diastolic function. J. Am. Coll. Cardiol. 2011, 57, 1226–1233.
- Shah, S.J.; Lam, C.S.; Svedlund, S.; Saraste, A.; Hage, C.; Tan, R.S.; Beussink-Nelson, N.; Ljung Faxen, U.; Lagerstrom Fermer, M.; Broberg, M.A.; et al. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur. Heart J. 2018, 39, 3439–3450.
- Vasan, R.S.; Larson, M.G.; Benjamin, E.J.; Evans, J.C.; Reiss, C.K.; Levy, D. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: Prevalence and mortality in a population-based cohort. J. Am. Coll. Cardiol. 1999, 33, 1948–1955.
- Hsich, E.M.; Grau-Sepulveda, M.V.; Hernandez, A.F.; Eapen, Z.J.; Xian, Y.; Schwamm, L.H.; Bhatt, D.L.; Fonarow, G.C. Relationship between sex, ejection fraction, and B-type natriuretic peptide levels in patients hospitalized with heart failure and associations with inhospital outcomes: Findings from the Get with The Guideline-Heart Failure Registry. Am. Heart J. 2013, 166, 1063–1071.e3.
- Johnstone, D.; Limacher, M.; Rousseau, M.; Liang, C.S.; Ekelund, L.; Herman, M.; Stewart, D.; Guillotte, M.; Bjerken, G.; Gaasch, W.; et al. Clinical characteristics of patients in Studies of Left Ventricular Dysfunction (SOLVD). Am. J. Cardiol. 1992, 70, 894–900.
- Hsich, E.M.; Grau-Sepulveda, M.V.; Hernandez, A.F.; Peterson, E.D.; Schwamm, L.H.; Bhatt, D.L.; Fonarow, G.C. Sex differences in in-hospital mortality in acute decompensated heart failure with reduced and preserved ejection fraction. Am. Heart J. 2012, 163, 430–437.e3.
- Kannel, W.B.; Hjortland, M.; Castelli, W.P. Role of diabetes in congestive heart failure: The Framingham study. Am. J. Cardiol. 1974, 34, 29–34.
- Galderisi, M.; Anderson, K.M.; Wilson, P.W.; Levy, D. Echocardiographic evidence for the existence of a distinct diabetic cardiomyopathy (the Framingham Heart Study). Am. J. Cardiol. 1991, 68, 85–89.
- Paulus, W.J.; Tschöpe, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271.
- Cocchi, C.; Coppi, F.; Farinetti, A.; Mattioli, A.V. Cardiovascular disease prevention and therapy in women with Type 2 diabetes. Future Cardiol. 2021, 17, 487–496.
- Palazzuoli, A.; Ruocco, G.; Gronda, E. Noncardiac comorbidity clustering in heart failure: An overlooked aspect with potential therapeutic door. Heart Fail. Rev. 2022, 27, 767–778.
- Van Heerebeek, L.; Hamdani, N.; Falcão-Pires, I.; Leite-Moreira, A.F.; Begieneman, M.P.; Bronzwaer, J.G.; van der Velden, J.; Stienen, G.J.M.; Gerrit, J.L.; Somsen, A.; et al. Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation 2012, 126, 830–839.
- Levy, D.; Larson, M.G.; Vasan, R.S.; Kannel, W.B.; Ho, K.K.L. The progression from hypertension to congestive heart failure. JAMA 1996, 275, 1557–1562.
- Gori, M.; Lam, C.S.P.; Gupta, D.K.; Santos, A.B.S.; Cheng, S.; Shah, A.M.; Claggett, B.; Zile, M.R.; Kraigher-Krainer, E.; Pieske, B.; et al. Sex-specific cardiovascular structure and function in heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2014, 16, 535–542.
- Mattioli, A.V.; Coppi, F.; Bucciarelli, V.; Gallina, S. Cardiovascular risk stratification in young women: The pivotal role of pregnancy. J. Cardiovasc. Med. 2023, 24, 793–797.
- Cadeddu, C.; Franconi, F.; Cassisa, L.; Campesi, I.; Pepe, A.; Cugusi, L.; Maffei, S.; Gallina, S.; Sciomer, S.; Mercuro, G.; et al. Working Group of Gender Medicine of Italian Society of Cardiology. Arterial hypertension in the female world: Pathophysiology and therapy. J. Cardiovasc. Med. 2016, 17, 229–236.
- Eaton, C.B.; Pettinger, M.; Rossouw, J.; Martin, L.W.; Foraker, R.; Quddus, A.; Liu, S.; Wampler, N.S.; Hank Wu, W.C.; Manson, J.E.; et al. Risk factors for incident hospitalized heart failure with preserved versus reduced ejection fraction in a multiracial cohort of postmenopausal women. Circ. Heart Fail. 2016, 9, e002883.
- Savji, N.; Meijers, W.C.; Bartz, T.M.; Bhambhani, V.; Cushman, M.; Nayor, M.; Kizer, J.R.; Sarma, A.; Blaha, M.J.; Gansevoort, R.T.; et al. The association of obesity and cardiometabolic traits with incident HFpEF and HFrEF. JACC Heart Fail. 2018, 6, 701–709.
- Bucciarelli, V.; Mattioli, A.V.; Sciomer, S.; Moscucci, F.; Renda, G.; Gallina, S. The Impact of Physical Activity and Inactivity on Cardiovascular Risk across Women’s Lifespan: An Updated Review. J. Clin. Med. 2023, 12, 4347.
- Palazzuoli, A.; Beltrami, M. Are HFpEF and HFmrEF So Different? The Need to Understand Distinct Phenotypes. Front. Cardiovasc. Med. 2021, 8, 676658.
- Ambikairajah, A.; Walsh, E.; Tabatabaei-Jafari, H.; Cherbuin, N. Fat mass changes during menopause: A metaanalysis. Am. J. Obstet. Gynecol. 2019, 221, 393–409.e50.
- Florijn, B.W.; Bijkerk, R.; van der Veer, E.P.; van Zonneveld, A.J. Gender and cardiovascular disease: Are sex-biased microRNA networks a driving force behind heart failure with preserved ejection fraction in women? Cardiovasc. Res. 2018, 114, 210–225.
- Mattioli, A.V.; Selleri, V.; Zanini, G.; Nasi, M.; Pinti, M.; Stefanelli, C.; Fedele, F.; Gallina, S. Physical Activity and Diet in Older Women: A Narrative Review. J. Clin. Med. 2023, 12, 81.
- Yang, Y.; Xie, M.; Yuan, S.; Zeng, Y.; Dong, Y.; Wang, Z.; Xiao, Q.; Dong, B.; Ma, J.; Hu, J. Sex differences in the associations between adiposity distribution and cardiometabolic risk factors in overweight or obese individuals: A cross-sectional study. BMC Public Health 2021, 21, 1232.
- He, J.; Ogden, L.G.; Bazzano, L.A.; Vupputuri, S.; Loria, C.; Whelton, P.K. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch. Intern. Med. 2001, 161, 996–1002.
- Hitchman, S.C.; Fong, G.T. Gender empowerment and female-to-male smoking prevalence ratios. Bull. World Health Organ. 2011, 89, 195–202.
- Peters, S.A.; Huxley, R.R.; Woodward, M. Do smoking habits differ between women and men in contemporary Western populations? Evidence from half a million people in the UK Biobank study. BMJ Open 2014, 4, e005663.
- Rose, J.J.; Krishnan-Sarin, S.; Exil, V.J.; Hamburg, N.M.; Fetterman, J.L.; Ichinose, F.; Perez-Pinzon, M.A.; Rezk-Hanna, M.; Williamson, E. Cardiopulmonary Impact of Electronic Cigarettes and Vaping Products: A Scientific Statement from the American Heart Association. Circulation 2023, 148, 703–728.
- Khatibzadeh, S.; Farzadfar, F.; Oliver, J.; Ezzati, M.; Moran, A. Worldwide risk factors for heart failure: A systematic review and pooled analysis. Int. J. Cardiol. 2012, 168, 1186–1194.
- Lam, C.S.P.; Arnott, C.; Beale, A.L.; Chandramouli, C.; Hilfiker-Kleiner, D.; Kaye, D.M.; Ky, B.; Santema, B.T.; Sliwa, K.; Voors, A.A. Sex differences in heart failure. Eur. Heart J. 2019, 40, 3859–3868.
- Go, A.S.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.J.; Berry, J.D.; Blaha, M.J.; Dai, S.; Ford, E.S.; Fox, C.S.; Franco, S.; et al. Heart disease and stroke statistics–2013 update: A report from the American Heart Association. Circulation 2013, 127, e6–e245.
- Lam, C.S.; Carson, P.E.; Anand, I.S.; Rector, T.S.; Kuskowski, M.; Komajda, M.; McKelvie, R.S.; McMurray, J.J.; Zile, M.R.; Massie, B.M.; et al. Sex differences in clinical characteristics and outcomes in elderly patients with heart failure and preserved ejection fraction: The Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial. Circ. Heart Fail. 2012, 5, 571–578.
- Nielsen, R.R.; Anker, N.; Stødkilde-Jørgensen, N.; Thrane, P.G.; Hansen, M.K.; Pryds, K.; Mortensen, M.B.; Olesen, K.K.W.; Maeng, M. Impact of Coronary Artery Disease in Women with Newly Diagnosed Heart Failure and Reduced Ejection Fraction. JACC Heart Fail. 2023; in press.
- Berry, C.; Poppe, K.; Gamble, G.; Earle, N.; Ezekowitz, J.; Squire, I.; McMurray, J.; McAlister, F.; Komajda, M.; Swedberg, K.; et al. Prognostic significance of anaemia in patients with heart failure with preserved and reduced ejection fraction: Results from the MAGGIC individual patient data meta-analysis. QJM 2016, 109, 377–382.
- Anand, I.S.; Gupta, P. Anemia and iron deficiency in heart failure: Current concepts and emerging therapies. Circulation 2018, 138, 80–98.
- Lund, L.H.; Donal, E.; Oger, E.; Hage, C.; Persson, H.; Haugen-Löfman, I.; Ennezat, P.-V.; Sportouch-Dukhan, C.; Drouet, E.; Daubert, J.-C.; et al. Association between cardiovascular vs. non-cardiovascular co-morbidities and outcomes in heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2014, 16, 992–1001.
- Steinberg, B.A.; Zhao, X.; Heidenreich, P.A.; Peterson, E.D.; Bhatt, D.L.; Cannon, C.P.; Hernandez, A.F.; Fonarow, G.C. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: Prevalence, therapies, and outcomes. Circulation 2012, 126, 65–75.
- Macdougall, I.C.; Canaud, B.; de Francisco, A.L.; Filippatos, G.; Ponikowski, P.; Silverberg, D.; van Veldhiesen, D.J.; Anker, S.D. Beyond the cardiorenal anaemia syndrome: Recognizing the role of iron deficiency. Eur. J. Heart Fail. 2012, 14, 882–886.
- Roffe-Vazquez, D.N.; Huerta-Delgado, A.S.; Castillo, E.C.; Villarreal-Calderón, J.R.; Gonzalez-Gil, A.M.; Enriquez, C.; GarciaRivas, G.; Elizondo-Montemayor, L. Correlation of Vitamin D with Inflammatory Cytokines, Atherosclerotic Parameters, and Lifestyle Factors in the Setting of Heart Failure: A 12-Month Follow-Up Study. Int. J. Mol. Sci. 2019, 20, 5811.
- Li, Y.C.; Kong, J.; Wei, M.; Chen, Z.F.; Liu, S.Q.; Cao, L.P. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J. Clin. Investig. 2002, 110, 229–238.
- Andrukhova, O.; Slavic, S.; Zeitz, U.; Riesen, S.C.; Heppelmann, M.S.; Ambrisko, T.D.; Markovic, M.; Kuebler, W.M.; Erben, R.G. Vitamin D Is a Regulator of Endothelial Nitric Oxide Synthase and Arterial Stiffness in Mice. Mol. Endocrinol. 2014, 28, 53–64.
- Zhu, Y.; Mahon, B.D.; Froicu, M.; Cantorna, M.T. Calcium and 1 alpha,25-dihydroxyvitamin D3 target the TNF-alpha pathway to suppress experimental inflammatory bowel disease. Eur. J. Immunol. 2005, 35, 217–224.
- Mathieu, C.; Adorini, L. The coming of age of 1,25-dihydroxyvitamin D(3) analogs as immunomodulatory agents. Trends Mol. Med. 2002, 8, 174–179.
- Koyama, T.; Shibakura, M.; Ohsawa, M.; Kamiyama, R.; Hirosawa, S. Anticoagulant effects of 1alpha,25-dihydroxyvitamin D3 on human myelogenous leukemia cells and monocytes. Blood 1998, 92, 160–167.
- Kennel, K.A.; Drake, M.T.; Hurley, D.L. Vitamin D deficiency in adults: When to test and how to treat. Mayo Clin. Proc. 2010, 85, 752–757.
- Holick, M.F.; Matsuoka, L.Y.; Wortsman, J. Age, vitamin D, and solar ultraviolet. Lancent 1989, 334, 1104–1105.
- Mei, Z.; Hu, H.; Zou, Y.; Li, D. The role of vitamin D in menopausal women’s health. Front. Physiol. 2023, 14, 1211896.
- Marino, R.; Misra, M. Extra-Skeletal Effects of Vitamin D. Nutrients 2019, 11, 1460.
- Kalla, A.; Krishnamoorthy, P.; Gopalakrishnan, A.; Garg, J.; Patel, N.C.; Figueredo, V.M. Gender and age differences in cardiovascular complications in anorexia nervosa patients. Int. J. Cardiol. 2017, 227, 55–57.
- Arcelus, J.; Mitchell, A.J.; Wales, J.; Nielsen, S. Mortality rates in patients with anorexia nervosa and other eating disorders. A meta-analysis of 36 studies. Arch. Gen. Psychiatry 2011, 68, 724–731.
- Vaurs, C.; Rollin, A.; Bérard, E.; Valet, M.; Saulnier, A.; Hazane, F.; Ritz, P.; Maury, P. QT interval is not prolonged in patients with eating disorders. Int. J. Cardiol. 2014, 177, 134–135.
- Mattioli, A.V.; Moscucci, F.; Sciomer, S.; Maffei, S.; Nasi, M.; Pinti, M.; Bucciarelli, V.; Dei Cas, A.; Parati, G.; Ciccone, M.; et al. Cardiovascular prevention in women: Un update By the Italian Society of Cardiology Working Group On “Prevention, Hypertension and peripheral disease”. J. Cardiovasc. Med. 2023, 24 (Suppl. S2), e147–e155.
- Daubert, M.A.; Douglas, P.S. Primary prevention of heart failure in women. JACC Heart Fail. 2019, 7, 181–191.
- Männistö, T.; Mendola, P.; Vääräsmäki, M.; Järvelin, M.R.; Hartikainen, A.L.; Pouta, A.; Suvanto, E. Elevated blood pressure in pregnancy and subsequent chronic disease risk. Circulation 2013, 127, 681–690.
- Grand’Maison, S.; Pilote, L.; Okano, M.; Landry, T.; Dayan, N. Markers of vascular dysfunction after hypertensive disorders of pregnancy: A systematic review and meta-analysis. Hypertension 2016, 68, 1447–1458.
- Khan, S.S.; Brewer, L.C.; Canobbio, M.M.; Cipolla, M.J.; Grobman, W.A.; Lewey, J.; Michos, E.D.; Miller, E.C.; Perak, A.M.; Wei, G.S.; et al. Optimizing Prepregnancy Cardiovascular Health to Improve Outcomes in Pregnant and Postpartum Individuals and Offspring: A Scientific Statement From the American Heart Association. Circulation 2023, 147, e76–e91.
- Mattioli, A.V.; Gallina, S. Early cardiovascular prevention: The crucial role of nurse-led intervention. BMC Nurs. 2023, 22, 347.
- Echouffo-Tcheugui, J.B.; Guan, J.; Retnakaran, R.; Shah, B.R. Gestational diabetes and incident heart failure: A cohort study. Diabet. Care 2021, 44, 2346–2352.
- Yusuf, S.; Hawken, S.; Ôunpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Lisheng, L. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 2004, 364, 937–952.
- Sliwa, K.; Hilfiker-Kleiner, D.; Petrie, M.C.; Mebazaa, A.; Pieske, B.; Buchmann, E.; Regitz-Zagrosek, V.; Schaufelberger, M.; Tavazzi, L.; van Veldhuisen, D.J.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: A position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur. J. Heart Fail. 2010, 12, 767–778.
- Abdel-Qadir, H.; Austin, P.C.; Lee, D.S.; Amir, E.; Tu, J.V.; Thavendiranathan, P.; Fung, K.; Anderson, G.M. A population-based study of cardiovascular mortality following early-stage breast cancer. JAMA Cardiol. 2017, 2, 88–93.
- Pelliccia, F.; Kaski, J.C.; Crea, F.; Camici, P.G. Pathophysiology of Takotsubo syndrome. Circulation 2017, 135, 2426–2441.
- Schiattarella, G.G.; Altamirano, F.; Tong, D.; French, K.M.; Villalobos, E.; Kim, S.Y.; Luo, X.; Jiang, N.; May, H.I.; Wang, Z.V.; et al. Nitrosative stress drives heart failure with preserved ejection fraction. Nature 2019, 568, 351–356.
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638.
- Aslam, F.; Bandeali, S.J.; Khan, N.A.; Alam, M. Diastolic dysfunction in rheumatoid arthritis: A meta-analysis and systematic review. Arthritis Care Res. 2013, 65, 534–543.
- Redfield, M.M.; Jacobsen, S.J.; Borlaug, B.A.; Rodeheffer, R.J.; Kass, D.A. Age- and gender-related ventricular-vascular stiffening: A community-based study. Circulation 2005, 112, 2254–2262.
- Ha, J.W.; Lee, H.C.; Park, S.; Choi, E.Y.; Seo, H.S.; Shim, C.Y.; Kim, J.M.; Ahn, J.A.; Lee, S.W.; Rim, S.J.; et al. Gender-related difference in left ventricular diastolic elastance during exercise in patients with diabetes mellitus. Circ. J. 2008, 72, 1443–1448.
- Palazzuoli, A.; Del Buono, M.; Ruocco, G.; Caravita, S.; Abbate, A.; Lavie, C.J. The Conundrum of HFpEF Definition: Non-Invasive Assessment Uncertainties and Alternative Diagnostic Strategies. Curr. Probl. Cardiol. 2023, 48, 101433.
- Mohammed, S.F.; Hussain, S.; Mirzoyev, S.A.; Edwards, W.D.; Maleszewski, J.J.; Redfield, M.M. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation 2015, 131, 550–559.
- Russo, C.; Jin, Z.; Palmieri, V.; Homma, S.; Rundek, T.; Elkind, M.S.; Sacco, R.L.; Di Tullio, M.R. Arterial stiffness and wave reflection: Sex differences and relationship with left ventricular diastolic function. Hypertension 2012, 60, 362–368.
- Borlaug, B.A.; Olson, T.P.; Lam, C.S.; Flood, K.S.; Lerman, A.; Johnson, B.D.; Redfield, M.M. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J. Am. Coll. Cardiol. 2010, 56, 845–854.
- Palazzuoli, A.; Caravita, S.; Paolillo, S.; Ghio, S.; Tocchetti, C.G.; Ruocco, G.; Correale, M.; Ambrosio, G.; Perrone Filardi, P.; Senni, M.; et al. Current gaps in HFpEF trials: Time to reconsider patients’ selection and to target phenotypes. Prog. Cardiovasc. Dis. 2021, 67, 89–97.
- Barilli, M.; Tavera, M.C.; Valente, S.; Palazzuoli, A. Structural and Hemodynamic Changes of the Right Ventricle in PH-HFpEF. Int. J. Mol. Sci. 2022, 23, 4554.
- Badesch, D.B.; Raskob, G.E.; Elliott, C.G.; Krichman, A.M.; Farber, H.W.; Frost, A.E.; Barst, R.J.; Benza, R.L.; Liou, T.G.; Turner, M.; et al. Pulmonary arterial hypertension: Baseline characteristics from the REVEAL Registry. Chest 2010, 137, 376–387.
- Pedram, A.; Razandi, M.; Lubahn, D.; Liu, J.; Vannan, M.; Levin, E.R. Estrogen Inhibits Cardiac Hypertrophy: Role of Estrogen Receptor-β to Inhibit Calcineurin. Endocrinology 2008, 149, 3361–3369.
More
